Abstract
Regulatory T-cells (Tregs), known for their immune suppressive function, have been reported in higher numbers, with activated phenotypes and greater potency, in hepatitis B virus (HBV)-related liver diseases than in normal conditions. The numbers, phenotypes, and function of intrahepatic and/or tumor-infiltrating Tregs in HBV-related liver diseases also differ from those of Tregs in the peripheral blood. By inhibiting the function of effector T-cells (Teffs), Tregs play a substantial role in the formation and maintenance of the liver's suppressive microenvironment, which might account for the progression of HBV-related hepatitis and hepatocellular carcinoma (HCC). In acute hepatitis B virus infection, Tregs can safeguard the liver from damage at the cost of prolonged antiviral processes, which results in chronic HBV infection in the liver. Furthermore, Tregs play a role in the development of cirrhosis, the transformation of cirrhosis to HCC, and the progression and metastasis of HCC. Higher levels of Tregs in the peripheral blood and/or tumor sites signify a poorer prognosis in HBV-related liver conditions, and observational data from mouse models and human patients support the theory that depleting Tregs may be therapeutic in HBV-related liver diseases by inducing antiviral and antitumor immunity.
Keywords: Regulatory T-cells, Hepatitis B virus, Hepatocellular carcinoma
Introduction
Regulatory T-cells (Tregs), comprising 5–10% of cluster of differentiation (CD) 4+ T-cells, can be divided into two subsets: natural regulatory T-cells (nTregs) and induced regulatory T-cells (iTregs).1 The former subset originates in the thymus in response to strong T-cell receptor (TCR) engagement with self-peptides, and the latter, which exerts suppressive functions comparable to nTregs, is induced from naive CD4+ T-cell precursors in the periphery.2 Constitutively expressed on the surface of nTregs, CD25 was the first surface marker discovered to identify Tregs. CD4+CD25high T-cells constitute a clear Treg population, whereas CD4+CD25+ T-cells also comprise activated T-cells.3 However, other markers can be used to differentiate the Treg population.4 Forkhead box protein 3 (Foxp3) is a widely used marker for Tregs and a definitive marker to define Tregs in patients with cancer and autoimmune diseases, although it appears to define conventional activated T-cells, more broadly, in vitro.5, 6 Foxp3 is critical for the development and function of Tregs in both mice and humans.7, 8, 9 Specifically, the expression of Foxp3 in Tregs leads to functional and phenotypic differences between Tregs and effector T-cells (Teffs).10 In addition to CD25 and Foxp3, Tregs express cytotoxic T-lymphocyte antigen (CTLA)-4, lymphocyte activation antigen-3 (LAG-3), interleukin (IL)-7 receptor alpha-chain (CD127), glucocorticoid induced tumor necrosis factor receptor (GITR), and T-cell immunoglobulin and mucin domain 3 (Tim-3).10, 11, 12, 13, 14 Some of these molecular markers are presently used as markers of activated Tregs.11
Tregs encompass a large population of lymphocytes that play pivotal roles in maintaining immune homeostasis. These cells play a substantial role in the development and maintenance of immunological tolerance by suppressing many cell types, including CD4+ and CD8+ T-cells, B-cells, dendritic cells (DC), natural killer (NK) cells, and natural killer T (NKT) cells.15, 16 Tregs mediate allergy suppression, autoimmune diseases, immune-mediated transplant rejection, and pathogen-induced immunopathologies.17 Nonetheless, in addition to these advantageous immunoregulatory functions of Tregs in the immune system, they also limit beneficial immune responses by blocking antigen-specific immunity to specific pathogenic agents such as hepatitis B virus (HBV) and by limiting anti-tumor immunity.18 The suppressive functions of Tregs are clearly antigen dependent in vivo.11 Antigen-specific Tregs tend to be more effective in modifying disease than polyclonal Treg populations.3 Tregs at various stages of diseases and Tregs in the peripheral blood vs. tumor sites also display distinct functions.19
Numerous reports have described, in detail, probable mechanisms for Treg regulation of immune responses.3, 7, 20, 21, 22, 23 Four primary mechanisms are involved in the suppressive function of Tregs. First, Tregs suppress immune responses by secreting inhibitory cytokines such as transforming growth factor-β (TGF-β), IL-10, and IL-35. Second, Tregs regulate the maturation and function of dendritic cells (DCs). Third, Tregs produce metabolites including nucleotides that likely inhibit Teffs. Lastly, Tregs show direct cytolytic action via granzyme and perforin, which is probably the mechanism underlying cell contact-mediated suppression.24
China shows the highest incidence of HBV in the world. HBV infection and hepatocellular carcinoma (HCC) are also significant health problems worldwide.25 In China, HCC often develops secondary to HBV infection. The long-term survival of patients with HCC is unsatisfactory, even when surgical treatments, including liver resection and transplantation, are performed. The molecular pathogenesis of HCC secondary to HBV infection is not well understood. In adults, HBV infection mostly leads to self-limiting, acute hepatitis, resulting in long-lasting protection against re-infection. However, in 10% of infected adults and 90% of infected children, HBV is established as a chronic infection.26 HBV is not cytotoxic and does not injure the liver directly. Host immunity, therefore, plays a crucial role in the pathogenesis of HBV infection and HCC, as well as the host's response to antiviral and antitumor therapies.21 Considering the substantial role of Tregs in immune responses against HBV and cancer cells, understanding the associations between Tregs and HBV-related liver diseases is essential.
Tregs in acute HBV infection
Characteristics of the intrahepatic virus-specific T-cell response, including Teffs and Tregs in patients with acute HBV infection, have seldom been studied because of the potential for complications related to standard liver biopsies. However, in the studies that have been performed, the frequency of Tregs in patients with acute HBV was lower or comparable to that of healthy controls during the early acute phase of infection; Treg levels are then elevated appreciably throughout the convalescent phase, returning to normal levels with resolution of the infection.10, 27, 28, 29, 30 These fluctuations in the Treg population may be important marker for patients with HBV infection.
The mechanisms behind the recruitment, activation, and differentiation of Tregs are under investigation. Research has shown that CXC chemokine receptor 3 (CXCR3) mediates the recruitment of Tregs to inflamed human liver tissue via the hepatic sinusoidal endothelium.31 Upregulation of CC chemokine receptor (CCR) 5, CCR4, and CCR8 signifies the activation and differentiation of Tregs.27
The immunopathological mechanism of acute hepatitis associated with HBV infection is not well understood. The role of Tregs in acute HBV infection is just beginning to emerge, with adaptive immune responses in the liver found to be associated with the resolution of the acute HBV infection.32, 33 The accumulation of Teffs plays a significant role in liver damage and necro-inflammation during the acute phase.27 A study by Sprengers et al33 showed a correlation between the levels of intrahepatic CD8+ T-cells and the degree of liver damage. They observed that three months after anti-hepatitis B surface antigen (HBsAg) seroconversion, the levels of intrahepatic HBV-specific CD8+ T-cells remained high. Another analysis showed that the induction and expansion of Tregs could limit excessive immune-mediated damage in response to HBV infection by downregulating critical effector cells such as CD8+ T-cells, which results in viral persistence.34 Stross et al35 revealed the complex regulatory function of Tregs during acute infection by depleting Tregs in the initial stage of adenovirus (Ad) HBV infection, an infection initiated by an Ad-vectored HBV genome, in a mouse model. They found that the numbers of CD4+Foxp3+ Tregs in livers increased rapidly—the typical reduction in Tregs during the early acute phase of infection was not observed—after the initiation of HBV replication. Perhaps surprisingly, initial transient depletion of Tregs failed to enhance the proliferation of HBV-specific Teffs, but it did limit cytokine production and cytotoxicity of Teffs, alleviating the liver damage. In this study, depletion of Tregs increased immune control of acute HBV early in infection; hepatitis B envelope antigen (HBeAg) and HBsAg were cleared considerably faster in the serum of Treg-depleted mice than in that of controls. Furthermore, early elimination of Tregs improved recruitment of macrophages and dendritic cells into HBV-infected livers. Therefore, to some extent, Tregs alleviate immunopathological liver damage by downregulating the antiviral activity of Teffs at the cost of prolonged virus clearance.
Tregs in chronic hepatitis B virus infection
Tregs are related to immune dysfunction in chronic HBV infections
The local expression of co-inhibitory receptors and immunosuppressive mediators results in the unique immune regulatory environment of the liver. This hepatic suppressive microenvironment consists primarily of higher numbers of Tregs, upregulated programmed death-1/programmed death ligand-1 (PD-1/PD-L1) signals, low levels of Toll-like receptor (TLR) expression, cytokines such as TGF-β and IL-10, and non-parenchymal liver cells such as dysfunctional DCs.29, 36 The special immune state of the liver is closely associated with the strength of an HBV-specific T-cell response. T-cell exhaustion or dysfunction in patients with chronic HBV infection has been observed in many studies. Previous research findings have indicated that chronic HBV infection is related to an increase in Tregs and defective CD8+ T-cells that fail to produce interferon-γ (IFN-γ).37, 38 Help from CD4+ T-cells is important for the maintenance of CD8+ T-cell function during chronic infections, but in chronic HBV infections, CD4+ T-cells also lose this capacity.39 Apart from Tregs and inhibitory receptors that reduce the functionality of HBV-specific CD8+ T-cells,15 in chronic infections, T-cell dysfunction also occurs through functional exhaustion resulting from a high antigen load and mutations in the virus.39 During most persistent viral infections, the sustained presence of viral antigen renders virus-specific T-cells dysfunctional.40
Based on several reports, it is apparent that innate immunity is deactivated in the immune tolerant phase and that adaptive immunity is exhausted in the apoptotic stage. Consequently, there is no immune-mediated liver damage in the immune-tolerant phase, even with HBV replication.41, 42 Immune tolerance to HBV is maintained in patients with chronic infection but without hepatitis, which is partly controlled by the host's Tregs.43 Acute exacerbation of chronic HBV infection is thought to be related to the loss of immune tolerance.
Features of Tregs in chronic HBV infections
Various markers have been used to identify Tregs in different studies. Treg levels in patients chronically infected with HBV can be affected by the choice of Treg markers.44 Comparisons of Tregs in chronic HBV infection, healthy controls and other HBV-related liver diseases are shown in Table 1. In most studies, the frequency of Tregs in the liver tissues and/or peripheral blood of patients with chronic HBV infection was higher than that of asymptomatic HBV-infected patients, inactive HBsAg carriers, patients acutely infected with HBV, or healthy controls, which might be helpful in preventing extensive liver damage. In addition, intrahepatic Tregs are functionally and phenotypically distinct from peripheral blood Tregs in patients with chronic HBV infections.19 However, some studies have shown that the frequency and/or number of Tregs are not significantly different between individuals with chronic HBV infections and healthy controls. One study reported similar frequencies and suppressive capacities of CD4+CD25+ Tregs in patients with chronic HBV infections and individuals that had recovered from HBV infection.45
Table 1.
Comparisons of Tregs in chronic HBV infection, HC and other HBV-related liver diseases.
| Markers | Positions | Comparisons of Treg frequencies | References |
|---|---|---|---|
| CD4+CD45RA−Foxp3low | PBT and IHT | ACLF > AsC and CHB | 46 |
| CD4+CD25+Foxp3+ | PBT | ACLF > CHB | 47 |
| CD4+CD25+ | PBT | ACLF = AHB | 48 |
| ACLF > CHB and HC | |||
| CD4+CD25+Foxp3+ | PBT | ACLF > CHB and HC | 49, 50 |
| CD4+CD25+ | PBT | ACLF > CHB and HC | 28, 51 |
| TIT | ACLF > CHB and HC | ||
| CD4+CD45RA−Foxp3high | PBT and IHT | CHB > HC | 46 |
| ACLF > AsC | |||
| CD4+CD25high | PBT | ACLF > CHB and HC | 52 |
| CD4+CD25+Foxp3+ | PBT | CHB > HC | 53, 54 |
| CD4+Foxp3+ | PBT and IHT | CHB > HC | 55 |
| CD25+CD127low/− | PBT | CHB > AsC, inactive HBsAg carriers and HC | 44 |
| CD4+CD25+ | PBT | CHB > HC | 42 |
| CD4+CD39+Foxp3+ | PBT | AsC > ACLF, CHB and HC | 56 |
| CD4+CD25+Foxp3+ | IHT | CHB > HC and resolved HBV | 57 |
| IHT | AsC > HC and resolved HBV | ||
| CD4+CD25+Foxp3+ | PBT | AHB > CHB > HC | 27 |
| CD4+CD25high | PBT | CHB > AHB and HC | 9 |
| CD4+CD127low CD25hi-int | PBT | CHB > HC | 58, 59 |
| CD4+CD25+ | PBT | CHB > HC | 60, 61 |
| CD4+CD25high | PBT | CHB > AHB and HC | 28, 30, 62 |
| CD4+CD25high CTLA-4+ | PBT | CHB = HC | 63 |
| CD4+CD25+ | PBT | CHB = HC | 64 |
Tregs: regulatory T-cells; HBV: hepatitis B virus; HC: healthy control; CD: cluster of differentiation; Foxp3: forkhead box protein 3; PBT: peripheral blood Tregs; IHT: intrahepatic Tregs; ACLF: acute-on-chronic liver failure; AsC: asymptomatic carriers; CHB: chronic hepatitis B; TIT: tumor infiltrating Tregs; AHB: acute hepatitis B; CTLA-4: cytotoxic T-lymphocyte antigen-4; >: significantly higher; <: significantly lower; =: no significant difference.
Tregs are associated with the progression of chronic HBV disease
Tregs have not been directly implicated in the progression of hepatitis disease, including chronic infections or late-stage cirrhosis. However, type 1 regulatory T-cells (Tr1) and nTregs apparently perform a crucial role in establishing chronic hepatitis and cirrhosis.65, 66
During chronic HBV infection, inflammatory liver damage is typically not the result of elevated numbers of infiltrating CD8+ T-lymphocytes, but rather a result of Fas ligand (Fas-L) expression by Kupffer cells and increased cytolytic activity of cells in portal areas.67 Within the immune-active phase of chronic HBV infection, an increase in innate immune cells, including DCs, can cause liver damage, but is unable to clear the virus. Nonetheless, adaptive immunity remains impaired.
The question arises: What is the precise relationship between Tregs and liver pathology in patients with chronic HBV infections? Normally, liver inflammation and immune-mediated livery injury can be alleviated by Tregs; there is a study that demonstrates an inverse relationship between Tregs and liver inflammation.15 However, in contrast to this finding, Speletas et al68 indicated that Tregs may regulate apoptosis-induced inflammation. They observed a substantial increase in Foxp3+ expression in diseases associated with inflammation.68 Other studies have confirmed an increase in Tregs in liver tissues of patients chronically infected with HBV with severe hepatitis and suggested that increased Tregs at the site of inflammation are associated with chronicity and degree of liver inflammation.28, 52 Some studies have indicated that the prevalence of CD4+CD25high Tregs in peripheral blood is indicative of disease severity in patients with chronic HBV infections or acute-on-chronic liver failure (ACLF).3, 52
Even in the presence of normal serum transaminase, which may result from an expansion of the Treg population, disease will progress in patients with chronic HBV infection, suggesting that low levels of liver inflammation do not correlate with less severe disease. Fibrogenesis and cirrhosis may be related to decompensation of the immune response.69
This suggests another question: Is there an association between Tregs and liver fibrogenesis or cirrhosis? Many experts have recognized hepatic stellate cells (HSCs) as the principal effectors in liver fibrogenesis, but the mechanism underlying this process remains uncertain. A few reports have suggested that HSCs can promote liver disease progression by enhancing the immunosuppressive function of Tregs. However, this putative association between HSCs and Tregs should be investigated further.29 An imbalance in Tregs and T helper (Th) 17 cells also plays an important role in the occurrence, development, and outcome of chronic HBV infections.70, 71 Several studies have demonstrated that peripheral Treg and Th17 frequencies in patients with HBV-related liver fibrosis were both significantly increased, and their numbers were correlated. The Treg/Th17 balance might affect the progression of fibrosis in HBV-infected patients, especially in those with liver failure resulting from HSC activation and leading to more severe liver injury.42 A lower Treg/Th17 ratio always indicates greater liver injury and fibrosis progression. However, Claassen et al72 did not find any significant correlation between Tregs and fibrosis.
An inefficient immune response—one that fails to clear the virus—leads to chronic inflammation and tissue remodeling through hepatocytes apoptosis, necrosis, and regeneration, and, finally, pseudolobuli take shape. Development of chronic inflammation and the unique liver microenvironment are responsible for the genomic instability and resulting mutations that promote neoplastic transformation.73
Tregs in hepatocellular carcinomas
Recruitment of Tregs to the tumor site
The detailed mechanisms underlying recruitment of Tregs to the tumor microenvironment are not well understood. Tumor-derived macrophages can produce CC-chemokine ligand (CCL) 22, which is strongly associated with the recruitment of Tregs to tumor sites.2, 74, 75 A previous study by Yang et al74 showed that elevated TGF-β activity associated with the persistence of HBV in liver tissue can lead to enhanced production of CCL22 by suppressing the expression of microRNA-34a (miR-34a). Apart from CCL22, tumor hypoxia can promote the recruitment of Tregs by upregulating CCL28.76 The CCR6-CCL20 axis was also found to recruit Tregs to tumor lesions in a study by Chen et al.77 These researchers observed high levels of CCL20-secreting cancer cells and scattered CCL20-secreting Kupffer cells in tumor regions. Circulating CD4+CD25+ Tregs, which express CCR6 highly, selectively migrate to tumors in patients with HCC because of CCL20 recruitment.77 In addition, CCL17 is responsible for the recruitment of Tregs.2, 78
Tregs influence immune dysregulation and tumorigenesis in HCC
IFN-γ-producing CD4+ T helper 1 (Th1) cells and CD8+ T-cells are believed to be the primary immune cells responsible for limiting tumor growth and development by inhibiting and killing tumor cells. However, a complicated regulatory network contributes to immune dysregulation in patients with HCC. Cellular immune suppressive mechanisms in patients with HCC, including those associated with Tregs, Th 17 cells, CD14+ human leukocyte antigen DR (HLA-DR) (low/−) myeloid-derived suppressor cells, neutrophils, and monocytes, promote the development of an immunosuppressive environment in the liver.23, 39, 79, 80 There is an additional factor contributing to T-cell dysfunction—anergy. Anergy occurs early in the course of tumor progression and plays a major part in T-cell impairment in cancers.39, 81 Moreover, high virus antigen loads also induce T-cell functional exhaustion, which likely affects T-cells function in more invasive cancers. In this article, we summarize the role of Tregs in defining the special immune state of patients with HCC.
Many studies have shown that Tregs play important roles in diminishing the anti-tumor effects of tumor-infiltrating lymphocytes.39, 82, 83 Tregs that accumulate in the tumor site can promote disease progression by suppressing tissue-derived CD4+CD25− T-cell activation.84 Chen et al77 showed that Tregs from tumor-infiltrating lymphocytes, non-tumor-infiltrating lymphocytes, and/or peripheral blood inhibit CD4+CD25− T-cell proliferation and INF-γ production in a dose-independent manner. Ormandy et al85 co-cultured Tregs with activated CD4+CD25− T-cells, and Tregs potently suppressed their proliferation and cytokine secretion. Tregs can also inhibit tumor antigen-specific and non-specific CD8+ T-cells. A study by Fu et al86 showed that Tregs in HCC patients inhibited the activation, proliferation, degranulation, and production of granzyme A, granzyme B, and perforin from CD8+ T-cells induced by anti-CD3/CD28 antibodies, resulting in impaired CD8+ T-cell function. Yang et al87 observed that Tregs in the peri-tumoral region play a critical role in the progression of HCC by downregulating CD8+ cytotoxic T-cell activity. Further, the findings of Kobayashi et al88 suggest that the prevalence of CD8+ tumor-infiltrating lymphocytes decreases significantly during hepatocarcinogenesis and is inversely correlated with that of infiltrating Tregs.
The mechanisms underlying hepatocarcinogenesis remain unclear. To a certain extent, Tregs in the tumor microenvironment can increase the frequency of viral mutation by inducing cellular cytidine deaminase, and some immune-escape HBV variants have been associated with hepatocarcinogenesis.89 More importantly, the suppressive function of Tregs is related to chronic inflammation in tumors, and chronic inflammatory pathways contribute to an inflammation-necrosis-regeneration process, which is critical to hepatocarcinogenesis. Chronic inflammation is associated not only with hepatocarcinogenesis but also with the recurrence and metastasis of HCC.89 However, Zamarron et al80 suggested that Tregs might help prevent and/or delay inflammation-mediated tumor development. These conflicting results indicate that further investigation of the role of CD4+Foxp3+ Tregs in initial tumor transformation is needed.
In other kinds of cancers such as breast cancer,90 the accumulation of Tregs at tumor sites correlates with increased microvessel density and biomarkers that can accelerate angiogenesis such as vascular endothelial growth factor (VEGF), which suggests an association between Tregs and angiogenesis.20 Tregs were also found to be associated with angiogenesis in ovarian cancers.76 In HCCs, Huang et al91 discovered that Tregs were positively correlated with microvessel density in tumor sites, illustrating the promotion of HCC progression following angiogenesis fostered by tumor-infiltrating Tregs. Finally, a study by Ye et al81 showed that higher levels of IL-10, TGF-β1, and VEGF were detected in tumors than in non-tumor tissues in HCC because of a decrease in effective immune cells and an increase of suppressor immune cells such as Tregs. However, additional evidence is needed to determine whether Tregs contribute to hepatocarcinogenesis by promoting angiogenesis.
The characteristics of Tregs in HCC
In tumor tissues, most Tregs accumulate in the parenchymal region of the liver, where the Tregs are close to liver tumor cells, whereas in non-tumor tissues, the majority of Foxp3+ cells locate in the mesenchymal region. These results suggest that physical contact between Tregs and tumor cells may be necessary for Tregs to exert their regulatory function.77
The average number of intratumoral Tregs is significantly higher than the number of Tregs in corresponding peritumoral tissues,91, 92, 93 counterparts of non-tumor regions in the liver,94 and peripheral blood.95 Tumor-infiltrating lymphocytes have a higher proportion of Treg infiltration than that observed in non-tumor infiltrating lymphocytes.96 The frequencies of both in HCC, intratumoral and peripheral Tregs, were higher than those in patients with chronic HBV infection and healthy controls.93, 94, 97, 98 Comparisons of Tregs in HCC, healthy controls and other HBV-related liver diseases are shown in Table 2.
Table 2.
Comparisons of Tregs in HCC, HC and other HBV-related liver diseases.
| Markers of Tregs | Positions of Tregs | Comparisons of Treg frequencies | References |
|---|---|---|---|
| CD4+CD25+Foxp3+ | TIT | HCC > HC | 77, 99 |
| CD4+CD25+Foxp3+ | PBT | HCC > HC | 60, 86, 94, 100, 101, 102, 103 |
| CD4+CD25+CD127− | PBT | HCC > HC | 104 |
| CD4+CD25+ | PBT | HCC > CHB > HC | 98, 105 |
| TIT | HCC > CHB | ||
| CD4+CD25high Foxp3+ | PBT | HCC > HC | 77, 93 |
| CD4+Foxp3+ | TIT | Advanced HCC > early stage HCC | 55 |
| CD4+Foxp3+ | TIT | HCC > CHB > HC | 106, 107, 108 |
| CD4+CD25+ | PBT | HCC < HC | 109 |
Tregs: regulatory T-cells; HCC: hepatocellular carcinoma; HC: healthy control; HBV: hepatitis B virus; CD: cluster of differentiation; Foxp3: forkhead box protein 3; TIT: tumor infiltrating Tregs; PBT: peripheral blood Tregs; CHB: chronic hepatitis B; >: significantly higher; <: significantly lower.
Intrahepatic Tregs more commonly display activated phenotypes than circulating Tregs.106 Pedroza-Gonzalez et al106 found that intratumoral Tregs expressed significantly more inducible co-stimulator (ICOS) and GITR than Tregs from tumor-free livers and peripheral blood, indicating a higher state of Treg activation at the tumor site than in surrounding tissues. The expression of Foxp3 and CTLA was also significantly higher in HCC patients compared to patients with chronic HBV infections.98 A study by Chen et al77 showed that, in addition to Foxp3, CD45RO, and CTLA-4, Tregs expressed elevated levels of CD69 and HLA-DR, indicating a terminally differentiated subpopulation of effector Tregs in HCC. Another study found increased numbers of Tregs in the peripheral blood and tumor-infiltrating lymphocytes and also higher levels of HLA-DR, GITR, and CD103 expressed in patients with HCC.110 Ormandy et al85 showed that, in patients with HCC, increased numbers of Tregs in the peripheral blood expressed high levels of HLA-DR and GITR, and low or no CD45RA. Cao et al102 observed that CD45RA, CD45RO, CD69, CD62L, GITR, CTLA-4, Ki67 (a proliferation marker), granzyme A, granzyme B, and Foxp3 expression was upregulated in CD4+CD25+ T-cells after exposure to HCC cell lines in vitro.
The function of Tregs in tumor sites is distinct from that of Tregs in the peripheral blood. Pedroza-Gonzalez et al106 found that tumor-infiltrating Tregs were highly activated and were more potent suppressors of tumor-specific and non-tumor-specific CD4+ T-cell responses. Other researchers have found similar results. In one study, CD4+CD25+CD127low/− CD49d− Tregs were present in higher numbers and more frequently, displaying a more suppressive effect in intratumoral areas than in peritumoral regions and peripheral blood.95 Observations by Cao et al102 strongly suggested that tumor-related factors not only induced and expanded CD4+CD25+ T-cells, but also enhanced their suppressor capacities. Specifically, some results have suggested that Tregs in the peritumoral region in HCCs play a critical role in controlling CD8+ cytotoxic T-cell activity and contribute to the progression of HCC.86 In addition, another study showed that Tregs from tumor sites with a high proportion of Foxp3+ cells were more active and potent than their counterparts from tumor sites with a low proportion of Foxp3+ cells in HCC.111 Thus, Foxp3 expression may be responsible for the different functions of Tregs.
Tregs play a role in the progression and metastasis of HCC
The role of Tregs in the progression and metastasis of human liver cancer is just beginning to emerge. One study showed that intratumoral Tregs accumulated in a stepwise manner—from viral hepatitis, to pre-cirrhosis, liver cirrhosis, and early pathologic lesions such as adenomatous hyperplasia and atypical adenomatous hyperplasia, and to early HCC and advanced HCC, indicating that Treg infiltration is associated with the formation and progression of hepatocarcinogenesis.88 The prevalence of circulating Tregs in the later stages was also found to be higher than in the earlier stages of HCC.112 Moreover, the frequency of tumor-infiltrating Tregs in patients with metastasized tumors was higher than those without metastasis,96 yet a study showed that there were less intratumoral Tregs in the advanced stage of HCC than in the early stage of HCC, whereas the circulating Treg frequency increased with HCC progression.55 Apart from these fluctuations in Treg frequencies, a high Treg density is significantly correlated with clinicopathological features such as the absence of tumor encapsulation and presence of tumor vascular invasion. Thus, Tregs may be associated with HCC invasiveness.113
Portal vein tumor thrombus (PVTT), which is a significant risk factor for reduced HCC survival, severely damages liver function and correlates with poor prognosis in patients with HCC.114, 115 Tregs are significantly associated with PVTT formation through the TGF-β-miR-34a-CCL22 axis, which is associated with tumor progression and metastasis.74
The tolerant immune microenvironment of HCC facilitates an impaired immune response in patients with chronic HBV infections and HCC, and is responsible for the progression and metastasis of HCC. A substantial surge in the activity of TGF-β signaling, which has been linked to the persistence of HBV in a study, might represent the beginning of alterations in the liver microenvironment.116 TGF-β can suppress the expression of miR-34a, a recently discovered micro RNA, resulting in enhanced production of CCL22 and the recruitment of Tregs (CCL22, in combination with CCR4, can recruit Tregs).20, 77 Finally, Tregs can modify HCC cells in ways that potentiate their invasiveness, such as PVTT formation.114
In addition, a higher rate of PVTT formation was found in HBV positive patients than those without the infection. Therefore, HCC initiated by HBV infection predisposes a patient for the development of PVTT.74 We speculate that the progression and metastasis of HCC is a consequence of interactions between many intricate components. The detailed mechanisms underlying these processes are under investigation.
Tregs are associated with prognosis in HBV-related liver diseases
Tregs are related to clinicopathological features that correlate with prognosis in HBV-related liver conditions (Table 3).
Table 3.
Relationships between Tregs and clinicopathological features of HBV-related diseases.
| Markers of Tregs | Tregs positions | Classes | Clinicopathologic features | Relation | References |
|---|---|---|---|---|---|
| CD4+Foxp3+ | TIT | HCC | Liver cirrhosis | (+) | 47 |
| CD4+CD25+ | TIT | HCC | Tumor size | (+) | 47, 56 |
| CD4+Foxp3+ | TIT | HCC | Poorer differentiation | (+) | 53, 88 |
| CD4+CD25+CD127− | PBT | HCC | Decreased circulating leukocytes and ferritin; portal vein thrombosis, heptic vein involvement; advanced clinical stages evaluated by TNM or BCLC scores | (+) | 72 |
| CD4+CD25+Foxp3+ | TIT | HCC | Preoperative serum AFP level | (+) | 117 |
| CD4+Foxp3+ | TIT | HCC | Absence of tumor encapsulation; | (+) | 118 |
| presence of tumor vascular invasion | |||||
| CD4+CD25+Foxp3+ | PBT | HBeAg+ CHB | HBV DNA load | (+) | 11 |
| CD4+CD25+Foxp3+ | PBT | CHB and AsC | HBV DNA load | (+) | 105 |
| CD4+CD25+ | PBT | ACLF | HBV DNA load | (+) | 36, 67 |
| Serumal IL-10 | (+) | ||||
| INR | (+) | ||||
| MELD score | (+) | ||||
| CD4+Foxp3+ | PBT | CHB | HBsAg | (+) | 38 |
| CD4+CD45RA−Foxp3high | PBT | CAH | HBV DNA load | (+) | 61 |
| CD4+CD45RA−Foxp3low | PBT | CAH | HAI score | (+) | 61 |
| CD4+CD25+Foxp3+ | PBT | CHB | Serum ALT, HBsAg, HBeAg | (+) | 30 |
| CD4+CD25high | PBT | CHB | HBV DNA load | (+) | 30, 67, 95 |
| CD4+CD39+Foxp3+ | PBT | AsC | HBV DNA load | (+) | 70 |
| Serum ALT | (−) | ||||
| CD4+CD25high | PBT | CHB | HBV DNA load | (+) | 119 |
| HBeAg | No | ||||
| CD4+Foxp3+IL-10+ | PBT | CHB | HBV DNA load | (+) | 106 |
| CD4+CD25+ | PBT | CHB | HBV DNA load | (+) | 28 |
Tregs: regulatory T-cells; HBV: hepatitis B virus; CD: cluster of differentiation; Foxp3: forkhead box protein 3; TIT: tumor infiltrating Tregs; HCC: hepatocellular carcinoma; PBT: peripheral blood Tregs; TNM: Tumor-Node-Metastasis; BCLC: Barcelona Clinic Liver Cancer; AFP: Alpha fetal protein; HBeAg: Hepatitis B envelope antigen; CHB: chronic hepatitis B; DNA: deoxyribonucleic acid; AsC: asymptomatic carriers; ACLF: acute-on-chronic liver failure; IL-10: interleukin-10; INR: international normalized ratio; MELD: Model for end stage liver disease; HBsAg: hepatitis B surface antigen; CAH: chronic active hepatitis; HAI: histological activity index; ALT: alanine aminotransferase; (+): positively correlated; (−): negatively correlated; No: no correlation.
Recent studies suggest that the proportion of intrahepatic Tregs is higher in patients with a higher chronic HBV load, which might explain the uncontrolled viral replication and indicate a poor prognosis.19, 66 Patients with chronic HBV infection with more than 107 HBV copies/ml had higher level of Tregs than subjects with lower viral loads.120 In HCC, the frequency of peripheral Tregs was found to correlate with clinical features associated with a poor prognosis, including portal vein thrombosis, hepatic vein involvement, and advanced clinical stages determined by Barcelona Clinic Liver Cancer scores or Tumor-Node-Metastasis staging system.104 In addition, an increase in CD4+CD25+ T-cells in the tumor microenvironment positively correlates with tumor sizes,121, 122 absence of tumor encapsulation, and presence of tumor vascular invasion.113
Several studies have indicated that tumor-infiltrating Tregs are increased in HCC and that they can be used as an independent prognostic factor for patients with HCC.88, 113, 123, 124 Specifically, survival analyses have shown that Tregs can indicate HCC prognosis.88, 113 The 5-year survival in patients with higher levels of Tregs in both peripheral blood and tumor tissues was significantly less than that in the patients with lower levels of Tregs.125 Low levels of intratumoral Tregs coupled with high levels of intratumoral activated cytotoxic T-lymphocytes (CTLs) were associated with favorable disease-free survival (DFS) and overall survival (OS) rates. CTLs alone have been reported to be predictors in many cancers, but in HCC they have only been associated with improved OS but not DFS.113 In contrast, a study indicates that CD8+ T-cells have no prognostic value.88 Results of recent survival analyses of patients with HCC are summarized in Table 4.
Table 4.
Relationships between Tregs and survival of patients with HCC.
| Tregs markers | Class | Tregs conditions | OS | DFS | References |
|---|---|---|---|---|---|
| CD4+Foxp3+ | HCC | High TIT and high intratumoral IL-17 (+) T-cells | (−) | (−) | 97 |
| High TIT and high peritumoral IL-17 (+) T-cells | (−) | (−) | |||
| CD4+Foxp3+ | HCC | High TIT and low intratumoral CTLs | (−) | (−) | 113, 121 |
| Low TIT and low peritumoral CTLs | (+) | (+) | |||
| Low TIT and high peritumoral CTLs | (−) | (−) | |||
| High CTLs | (+) | No | |||
| CD4+Foxp3+ | HCC | High TIT | (−) | (−) | 77, 88, 91, 92, 113, 114 |
| CD4+Foxp3+ | Early stage HCC | High PBT and TIT | (−) | No | 55 |
| Balance of CD8+ T-cells and TIT | No | No | |||
| CD4+Foxp3+ | HCC | High ratio of TIT/CD8+ T-cells | (−) | (−) | 123 |
| CD4+CD25+Foxp3+ | HCC | High TIT | No | (−) | 124 |
Tregs: regulatory T-cells; HCC: hepatocellular carcinoma; OS: overall survival; DFS: disease-free survival; CD: cluster of differentiation; Foxp3: forkhead box protein 3; TIT: tumor infiltrating Tregs; IL-17: interleukin-17; CTL: cytotoxic lymphocyte; PBT: peripheral blood Tregs; (+): better prognosis; (−): worse prognosis; No: no correlation.
In contrast, results of a study by Yu et al126 showed that a decreased Tregs/Th17 ratio and increased TGF-β1/IL-17 ratio may be associated with increased survival and decreased disease progression in HBV-associated liver cirrhosis patients. In patients with ACLF, one study indicated that, at the onset of disease, the Treg to Th17 ratio and Th17 frequency were significant predictors of patient survival, with a low Treg/Th17 ratio suggesting poorer prognosis.47
Therapeutic interventions related to Tregs in HBV-related liver diseases
Depletion of Tregs during acute viral infection may prevent viral persistence.10 A report by Stross et al35 noted that Treg depletion accelerates virus clearance. However, the phenotypic diversity of Tregs makes them difficult to identify, and there are currently no specific antibodies against human Tregs to facilitate targeted depletion.127 More importantly, there are side effects: Treg depletion may lead to autoimmune reactions and increased immune-mediated liver damage resulting from tumor necrosis factor-secreting T-cells or innate immune cells migrating to the liver.35
In patients with chronic HBV infection, interventions to restore HBV-specific immunity by inhibiting virus replication with antiviral treatments such as adefovir have only been partially successful, but HBV has not been completely cleared. Chronic HBV infection combined with the establishment of a tolerant immune microenvironment make functional restoration of antiviral immunity extremely difficult. The tolerant immune microenvironment is induced by a variety of elements; therefore, Tregs should be depleted in conjunction with other immune therapies,117 e.g., PD-1 and/or LAG-3 blockade39 or vaccination in combination with administration of cytokines.128 In one study, elimination of Tregs followed by stimulation with HBV-core 18-27 peptide significantly improved anti-virus CTL responses in patients with chronic HBV infections.59
Eliminating immune tolerance and anergy is one of the main purposes of tumor immunotherapy.39 Therapies against chronic HBV infection should also be applied as tumor immunotherapies to rescue T-cells from exhaustion. Treg function can be inhibited by targeting functional molecules with antibodies such as anti-CD25129, 130, 131 and anti-CTLA-4132 and by inhibiting Treg recruitment and/or expansion,35 which can increase the number of tumor-reactive T-cells for a potent anti-tumor response.133, 134, 135, 136
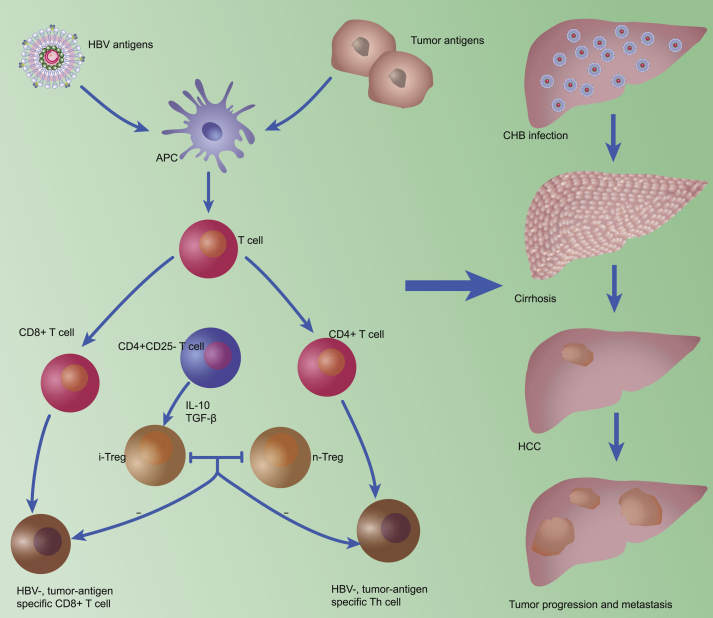
To conclude, Tregs participate in the configuration and maintenance of a suppressive microenvironment in the liver, which allows HBV infection to progress to HCC. The numbers of tumor-infiltrating and/or intrahepatic Tregs increase gradually from the establishment of chronic HBV infection to cirrhosis and HCC. In addition, activated phenotypes and potent Tregs are found in tumor sites. The suppressive environment initiated by Tregs, therefore, is associated with the chronicity of HBV infection, as well as HCC progression, metastasis, and prognosis (Fig. 1). Tregs should be considered a target for HCC therapies. However, the protocols for Treg management remain to be defined.
Fig. 1.
Tregs play a significant role in virus persistence and the formation, progression, and metastasis of HCC. Teffs differentiate in response to HBV and tumor antigens, and IFN-γ-producing CD4+ Th1-cells and CD8+ T-cells are the principle immune cells responsible for inhibiting tumor growth and development. Tregs are mainly induced from CD4+CD25− T-cells in the periphery, with cytokines such as TGF-β and IL-10 contributing to this process. iTregs and nTregs show similar suppression functions, inhibiting Teffs and reducing the anti-viral and anti-tumoral immune response. HBV: hepatitis B virus; APC: antigen-presenting cell; CD: cluster of differentiation; IL-10: interleukin-10; TGF-β: transforming growth factor-β; iTreg: induced regulatory T-cell; nTreg: natural regulatory T cell; Th: T-helper; CHB: chronic hepatitis B; Tregs: regulatory T-cells; HCC: hepatocellular carcinoma; Teffs: effector T-cells; IFN-γ: interferon-γ.
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Zhang H., Kong H., Zeng X., Guo L., Sun X., He S. Subsets of regulatory T cells and their roles in allergy. J Transl Med. 2014;12:125. doi: 10.1186/1479-5876-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15:544–551. doi: 10.1007/s10147-010-0130-1. [DOI] [PubMed] [Google Scholar]
- 3.Schmetterer K.G., Neunkirchner A., Pickl W.F. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–2276. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 4.Kakita N., Kanto T., Itose I. Comparative analyses of regulatory T cell subsets in patients with hepatocellular carcinoma: a crucial role of CD25- FOXP3- T cells. Int J Cancer. 2012;131:2573–2583. doi: 10.1002/ijc.27535. [DOI] [PubMed] [Google Scholar]
- 5.Wang R., Kozhaya L., Mercer F., Khaitan A., Fujii H., Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryczek I., Liu R., Wang G. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 7.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot J.D., Rudensky A.Y. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 9.Peng G., Li S., Wu W., Sun Z., Chen Y., Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123:57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billerbeck E., Bottler T., Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camisaschi C., Casati C., Rini F. LAG-3 expression defines a subset of CD4+CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. J Immunol. 2010;184:6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- 13.Liu W., Putnam A.L., Xu-Yu Z. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J., Zhang Y., Zhang J.P., Liang J., Li L., Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS One. 2013;8:e58006. doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knolle P.A., Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014;146:1193–1207. doi: 10.1053/j.gastro.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S., Setoguchi R., Yagi H., Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Hori S., Carvalho T.L., Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Piao W.H., Jee Y.H., Liu R.L. IL-21 modulates CD4+ CD25+ regulatory T-cell homeostasis in experimental autoimmune encephalomyelitis. Scand J Immunol. 2008;67:37–46. doi: 10.1111/j.1365-3083.2007.02035.x. [DOI] [PubMed] [Google Scholar]
- 19.Stoop J.N., Claassen M.A., Woltman A.M. Intrahepatic regulatory T cells are phenotypically distinct from their peripheral counterparts in chronic HBV patients. Clin Immunol. 2008;129:419–427. doi: 10.1016/j.clim.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Facciabene A., Motz G.T., Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Wang Y., Chen Y. Cellular immune response in patients with chronic hepatitis B virus infection. Microb Pathog. 2014;74:59–62. doi: 10.1016/j.micpath.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang Y.L., Zhang H.G., Peng J.R. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immunother. 2009;58:877–886. doi: 10.1007/s00262-008-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gondek D.C., Lu L.F., Quezada S.A., Sakaguchi S., Noelle R.J. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M., Katayama F., Kato H. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011;21:401–416. doi: 10.2188/jea.JE20100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoop J.N., Woltman A.M., Biesta P.J. Tumor necrosis factor alpha inhibits the suppressive effect of regulatory T cells on the hepatitis B virus-specific immune response. Hepatology. 2007;46:699–705. doi: 10.1002/hep.21761. [DOI] [PubMed] [Google Scholar]
- 27.Trehan Pati N., Geffers R., Sukriti Gene expression signatures of peripheral CD4+ T cells clearly discriminate between patients with acute and chronic hepatitis B infection. Hepatology. 2009;49:781–790. doi: 10.1002/hep.22696. [DOI] [PubMed] [Google Scholar]
- 28.Xu D., Fu J., Jin L. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Zhang J.Y., Wang L.F., Wang F.S. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2012;27:223–230. doi: 10.1111/j.1440-1746.2011.06940.x. [DOI] [PubMed] [Google Scholar]
- 30.Fu J.L., Xu D.P., Zhao P. The characterization of regulatory T cells in peripheral blood of HBV-infected patients. Natl Med J China. 2006;86:1522–1525. [PubMed] [Google Scholar]
- 31.Oo Y.H., Weston C.J., Lalor P.F. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 32.Rehermann B. Chronic infections with hepatotropic viruses: mechanisms of impairment of cellular immune responses. Semin Liver Dis. 2007;27:152–160. doi: 10.1055/s-2007-979468. [DOI] [PubMed] [Google Scholar]
- 33.Sprengers D., van der Molen R.G., Kusters J.G. Analysis of intrahepatic HBV-specific cytotoxic T-cells during and after acute HBV infection in humans. J Hepatol. 2006;45:182–189. doi: 10.1016/j.jhep.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 35.Stross L., Günther J., Gasteiger G. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology. 2012;56:873–883. doi: 10.1002/hep.25765. [DOI] [PubMed] [Google Scholar]
- 36.Bauer T., Sprinzl M., Protzer U. Immune control of hepatitis B virus. Dig Dis. 2011;29:423–433. doi: 10.1159/000329809. [DOI] [PubMed] [Google Scholar]
- 37.Trehanpati N., Hissar S., Shrivastav S., Sarin S.K. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res. 2013;138:700–710. [PMC free article] [PubMed] [Google Scholar]
- 38.Shrivastava S., TrehanPati N., Patra S. Increased regulatory T cells and impaired functions of circulating CD8 T lymphocytes is associated with viral persistence in Hepatitis B virus-positive newborns. J Viral Hepat. 2013;20:582–591. doi: 10.1111/jvh.12078. [DOI] [PubMed] [Google Scholar]
- 39.Kim P.S., Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuse S., Molloy M.J., Usherwood E.J. Immune responses against persistent viral infections: possible avenues for immunotherapeutic interventions. Crit Rev Immunol. 2008;28:159–183. doi: 10.1615/critrevimmunol.v28.i2.40. [DOI] [PubMed] [Google Scholar]
- 41.Ganem D., Prince A.M. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Qiu S.J., She W.M. Significance of the balance between regulatory T (Treg) and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One. 2012;7:e39307. doi: 10.1371/journal.pone.0039307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koay L.B., Feng I.C., Sheu M.J. Hepatitis B virus (HBV) core antigen-specific regulatory T cells confer sustained remission to anti-HBV therapy in chronic hepatitis B with acute exacerbation. Hum Immunol. 2011;72:687–698. doi: 10.1016/j.humimm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Xu H.T., Xing T.J., Li H., Ye J. Association of T regulatory cells with natural course and response to treatment with interferon-alpha in patients with chronic hepatitis B infection. Chin Med J (Engl) 2012;125:1465–1468. [PubMed] [Google Scholar]
- 45.Franzese O., Kennedy P.T., Gehring A.J. Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J Virol. 2005;79:3322–3328. doi: 10.1128/JVI.79.6.3322-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M., Zhou J., Zhao T. Dissection of a circulating and intrahepatic CD4+Foxp3+ T-cell subpopulation in chronic hepatitis B virus (HBV) infection: a highly informative strategy for distinguishing chronic HBV infection states. J Infect Dis. 2012;205:1111–1120. doi: 10.1093/infdis/jis011. [DOI] [PubMed] [Google Scholar]
- 47.Liang X.S., Li C.Z., Zhou Y., Yin W., Liu Y.Y., Fan W.H. Changes in circulating Foxp3+ regulatory T cells and interleukin-17-producing T helper cells during HBV-related acute-on-chronic liver failure. World J Gastroenterol. 2014;20:8558–8571. doi: 10.3748/wjg.v20.i26.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong X., Gong Y., Zeng H. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure. Liver Int. 2013;33:1517–1526. doi: 10.1111/liv.12248. [DOI] [PubMed] [Google Scholar]
- 49.Niu Y.H., Yin D.L., Liu H.L. Restoring the Treg cell to Th17 cell ratio may alleviate HBV-related acute-on-chronic liver failure. World J Gastroenterol. 2013;19:4146–4154. doi: 10.3748/wjg.v19.i26.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhai S., Zhang L., Dang S. The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol. 2011;24:303–310. doi: 10.1089/vim.2010.0135. [DOI] [PubMed] [Google Scholar]
- 51.Shen C., Yan W.Z., Zhao C.Y. Increased CD4+CD25+ regulatory T cells correlate with poor short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure patients. J Microbiol Immunol Infect. 2015;48:137–146. doi: 10.1016/j.jmii.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Yang J., Yi P., Wei L. Phenotypes and clinical significance of circulating CD4+CD25+ regulatory T cells (Tregs) in patients with acute-on-chronic liver failure (ACLF) J Transl Med. 2012;10:193. doi: 10.1186/1479-5876-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Badawy O., Sayed D., Badary M.S., Abd-Alrahman M.E., El-Feky M.A., Thabit A.G. Relations of regulatory T cells with hepatitis markers in chronic hepatitis B virus infection. Hum Immunol. 2012;73:335–341. doi: 10.1016/j.humimm.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Yang G.L., Xu L.M., Yao H.Y. Association between CD4+CD25+Foxp3+ regulatory T cells and serum transforming growth factor beta 1 in patients with chronic hepatitis B. Chin J Hepatol. 2009;17:831–834. [PubMed] [Google Scholar]
- 55.Wang F., Jing X., Li G. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int. 2012;32:644–655. doi: 10.1111/j.1478-3231.2011.02675.x. [DOI] [PubMed] [Google Scholar]
- 56.Tang Y., Jiang L., Zheng Y., Ni B., Wu Y. Expression of CD39 on FoxP3+ T regulatory cells correlates with progression of HBV infection. BMC Immunol. 2012;13:17. doi: 10.1186/1471-2172-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang G., Liu A., Xie Q. Association of CD4+CD25+Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- 58.Miyaaki H., Zhou H., Ichikawa T. Study of liver-targeted regulatory T cells in hepatitis B and C virus in chronically infected patients. Liver Int. 2009;29:702–707. doi: 10.1111/j.1478-3231.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H.H., Guo F., Fei R. Inhibition of CD4+ CD25+ regulatory T cells in chronic hepatitis B patients. Natl Med J China. 2008;88:511–515. [PubMed] [Google Scholar]
- 60.Zhang H.H., Fei R., Mei M.H. The frequency, phenotypes and functions of CD4+ CD25+ regulatory T cells in hepatocellular carcinoma patients. Chin J Hepatol. 2007;15:266–272. [PubMed] [Google Scholar]
- 61.Stoop J.N., van der Molen R.G., Baan C.C. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 62.Fu J.L., Xu D.P., Shi M. The phenotype and function of CD4+ CD25+ regulatory T cells in hepatitis B patients. Chin J Intern Med. 2006;45:642–645. [PubMed] [Google Scholar]
- 63.Kondo Y., Kobayashi K., Ueno Y. Mechanism of T cell hyporesponsiveness to HBcAg is associated with regulatory T cells in chronic hepatitis B. World J Gastroenterol. 2006;12:4310–4317. doi: 10.3748/wjg.v12.i27.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unitt E., Rushbrook S.M., Marshall A. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 65.Miroux C., Vausselin T., Delhem N. Regulatory T cells in HBV and HCV liver diseases: implication of regulatory T lymphocytes in the control of immune response. Expert Opin Biol Ther. 2010;10:1563–1572. doi: 10.1517/14712598.2010.529125. [DOI] [PubMed] [Google Scholar]
- 66.Barboza L., Salmen S., Goncalves L. Antigen-induced regulatory T cells in HBV chronically infected patients. Virology. 2007;368:41–49. doi: 10.1016/j.virol.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 67.Tang T.J., Kwekkeboom J., Laman J.D. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10:159–167. doi: 10.1046/j.1365-2893.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 68.Speletas M., Argentou N., Germanidis G. Foxp3 expression in liver correlates with the degree but not the cause of inflammation. Mediat Inflamm. 2011;2011:827565. doi: 10.1155/2011/827565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferri S., Lalanne C., Lanzoni G. Redistribution of regulatory T-cells across the evolving stages of chronic hepatitis C. Dig Liver Dis. 2011;43:807–813. doi: 10.1016/j.dld.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 70.Su Z.J., Yu X.P., Guo R.Y. Changes in the balance between Treg and Th17 cells in patients with chronic hepatitis B. Diagn Microbiol Infect Dis. 2013;76:437–444. doi: 10.1016/j.diagmicrobio.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 71.Niu Y., Liu H., Yin D. The balance between intrahepatic IL-17+ T cells and Foxp3+ regulatory T cells plays an important role in HBV-related end-stage liver disease. BMC Immunol. 2011;12:47. doi: 10.1186/1471-2172-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claassen M.A., de Knegt R.J., Tilanus H.W., Janssen H.L., Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 2010;52:315–321. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Castello G., Scala S., Palmieri G., Curley S.A., Izzo F. HCV-related hepatocellular carcinoma: from chronic inflammation to cancer. Clin Immunol. 2010;134:237–250. doi: 10.1016/j.clim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Yang P., Li Q.J., Feng Y. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291–303. doi: 10.1016/j.ccr.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curiel T.J., Coukos G., Zou L. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 76.Facciabene A., Peng X., Hagemann I.S. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 77.Chen K.J., Lin S.Z., Zhou L. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riezu-Boj J.I., Larrea E., Aldabe R. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422–431. doi: 10.1016/j.jhep.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Kalathil S., Lugade A.A., Miller A., Iyer R., Thanavala Y. Higher frequencies of GARP+CTLA-4+Foxp3+ T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zamarron B.F., Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye Y.B., Peng F., Li J.Y. Significance of the expression of lymphocytes and cytokines infiltrating in HCC. Chin J Cell Mol Imm. 2011;27:1056–1060. [PubMed] [Google Scholar]
- 82.Shirabe K., Motomura T., Muto J. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. Int J Clin Oncol. 2010;15:552–558. doi: 10.1007/s10147-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 83.Yang M.C., Chang C.P., Lei H.Y. Induction of liver fibrosis in a murine hepatoma model by thioacetamide is associated with enhanced tumor growth and suppressed antitumor immunity. Lab Invest. 2010;90:1782–1793. doi: 10.1038/labinvest.2010.139. [DOI] [PubMed] [Google Scholar]
- 84.Zhou J., Ding T., Pan W., Zhu L.Y., Li L., Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 85.Ormandy L.A., Hillemann T., Wedemeyer H., Manns M.P., Greten T.F., Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 86.Fu J., Xu D., Liu Z. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 87.Yang X.H., Yamagiwa S., Ichida T. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi N., Hiraoka N., Yamagami W. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 89.Han Y.F., Zhao J., Ma L.Y. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol. 2011;17:4258–4270. doi: 10.3748/wjg.v17.i38.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ki M.R., Goo M.J., Park J.K. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β 1- induced inflammatory signaling. Lab Invest. 2010;90:1507–1516. doi: 10.1038/labinvest.2010.109. [DOI] [PubMed] [Google Scholar]
- 91.Huang Y., Wang F.M., Wang T. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion. 2012;86:329–337. doi: 10.1159/000342801. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y., Wang F.M., Wang T. Tumor-infiltrating FoxP3+ Tregs are associated with CD34 expression and prognosis of hepatocellular carcinoma. Chin J Hepatol. 2012;20:25–29. doi: 10.3760/cma.j.issn.1007-3418.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Feng X., Li B., Ye H., Long D. Increased frequency of CD4+CD25highFoxP3+ regulatory T cells in patients with hepatocellular carcinoma. Arch Immunol Ther Exp (Warsz) 2011;59:309–314. doi: 10.1007/s00005-011-0127-0. [DOI] [PubMed] [Google Scholar]
- 94.Wang S.Y., Fu J.L., Lv J.Y., Chen L.M., Lv S., Wang F.S. Increase in peripheral and liver infiltrating regulatory T cells favors development of primary hepatocellular carcinoma. Chin J Cell Mol Imm. 2011;27:668–670. [PubMed] [Google Scholar]
- 95.Wu H., Chen P., Liao R. Intratumoral regulatory T cells with higher prevalence and more suppressive activity in hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2013;28:1555–1564. doi: 10.1111/jgh.12202. [DOI] [PubMed] [Google Scholar]
- 96.Guo C.L., Yang H.C., Yang X.H. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:5909–5913. doi: 10.7314/apjcp.2012.13.11.5909. [DOI] [PubMed] [Google Scholar]
- 97.Huang Y., Wang F., Wang Y. Intrahepatic interleukin-17+ T cells and FoxP3+ regulatory T cells cooperate to promote development and affect the prognosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:851–859. doi: 10.1111/jgh.12418. [DOI] [PubMed] [Google Scholar]
- 98.Thakur S., Singla A., Chawla Y., Rajwanshi A., Kalra N., Arora S.K. Expansion of peripheral and intratumoral regulatory T-cells in hepatocellular carcinoma: a case-control study. Indian J Pathol Microbiol. 2011;54:448–453. doi: 10.4103/0377-4929.85073. [DOI] [PubMed] [Google Scholar]
- 99.Flecken T., Schmidt N., Hild S. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han Y., Yang Y., Chen Z. Human hepatocellular carcinoma-infiltrating CD4+CD69+Foxp3- regulatory T cell suppresses T cell response via membrane-bound TGF-β1. J Mol Med (Berl) 2014;92:539–550. doi: 10.1007/s00109-014-1143-4. [DOI] [PubMed] [Google Scholar]
- 101.Wang Y., Deng B., Tang W., Liu T., Shen X. TGF-β1 secreted by hepatocellular carcinoma induces the expression of the Foxp3 gene and suppresses antitumor immunity in the tumor microenvironment. Dig Dis Sci. 2013;58:1644–1652. doi: 10.1007/s10620-012-2550-4. [DOI] [PubMed] [Google Scholar]
- 102.Cao M., Cabrera R., Xu Y. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4+CD25+ regulatory T cells. Lab Invest. 2007;87:582–590. doi: 10.1038/labinvest.3700540. [DOI] [PubMed] [Google Scholar]
- 103.Peng Q.Q., Li S.P., Xu L., Li J.Q. Clinical significance of the proportion of CD4+CD25+ regulatory T cells in peripheral blood of hepatocellular carcinoma patients: a report of 117 cases. Ai Zheng. 2007;26:748–751. [PubMed] [Google Scholar]
- 104.Chen T., Song D., Min Z. Perioperative dynamic alterations in peripheral regulatory T and B cells in patients with hepatocellular carcinoma. J Transl Med. 2012;10:14. doi: 10.1186/1479-5876-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nan X.P., Zhang Y., Yu H.T. Circulating CD4+CD25high regulatory T cells and expression of PD-1 and BTLA on CD4+ T cells in patients with chronic hepatitis B virus infection. Viral Immunol. 2010;23:63–70. doi: 10.1089/vim.2009.0061. [DOI] [PubMed] [Google Scholar]
- 106.Pedroza-Gonzalez A., Verhoef C., Ijzermans J.N. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology. 2013;57:183–194. doi: 10.1002/hep.26013. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y., Gan J.H., Luo E.P., Wang X.H., Chen L., Yang L. Effect and clinical significance of glucocorticoid on CD4+CD25+ regulatory T cells in patients with hepatitis B virus-related pre-liver failure. Chin J Hepatol. 2014;22:577–579. doi: 10.3760/cma.j.issn.1007-3418.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 108.Gao Y.W., Chen Y.X., Wang Z.M. Increased expression of cyclooxygenase-2 and increased infiltration of regulatory T cells in tumors of patients with hepatocellular carcinoma. Digestion. 2009;79:169–176. doi: 10.1159/000210266. [DOI] [PubMed] [Google Scholar]
- 109.Yang X.H., Liu B.R., Jiang H.C. The presence and the significance of CD4+CD25+ regulatory T cells in livers of patients with hepatocellular carcinoma. Chin J Hepatol. 2007;15:279–282. [PubMed] [Google Scholar]
- 110.Zhang H.H., Mei M.H., Fei R. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int J Oncol. 2010;36:841–848. doi: 10.3892/ijo_00000561. [DOI] [PubMed] [Google Scholar]
- 111.Lin S.Z., Chen K.J., Xu Z.Y. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev Res (Phila) 2013;6:594–602. doi: 10.1158/1940-6207.CAPR-12-0379. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H.H., Mei M.H., Fei R. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17(suppl 1):34–43. doi: 10.1111/j.1365-2893.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 113.Gao Q., Qiu S.J., Fan J. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 114.Shen S.L., Liang L.J., Peng B.G., He Q., Kuang M., Lai J.M. Foxp3+ regulatory T cells and the formation of portal vein tumour thrombus in patients with hepatocellular carcinoma. Can J Surg. 2011;54:89–94. doi: 10.1503/cjs.028009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu L., Xue F., Li Y., Shao M., Sun Y., Wei G. A long-term follow-up and comprehensive observation of risk and prognosis factors of recurrence and survival after resection of hepatocellular carcinoma. Cell Biochem Biophys. 2014;69:421–431. doi: 10.1007/s12013-013-9813-3. [DOI] [PubMed] [Google Scholar]
- 116.Lin G.H., Wang J., Li S.H., Wang J., Xu L., Li S.P. Relationship and clinical significance of TGF-beta1 expression with Treg cell infiltration in hepatocellular carcinoma. Chin J Cancer. 2010;29:403–407. doi: 10.5732/cjc.009.10628. [DOI] [PubMed] [Google Scholar]
- 117.Litzinger M.T., Fernando R., Curiel T.J., Grosenbach D.W., Schlom J., Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stoop J.N., van der Molen R.G., Kuipers E.J., Kusters J.G., Janssen H.L. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology. 2007;361:141–148. doi: 10.1016/j.virol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 119.Li J., Shi J., Ren W., Wu W., Chen Z. Regulatory role of CD4+CD25+Foxp3+ regulatory T cells on IL-17-secreting T cells in chronic hepatitis B patients. Dig Dis Sci. 2014;59:1475–1483. doi: 10.1007/s10620-013-3022-1. [DOI] [PubMed] [Google Scholar]
- 120.Aalaei-Andabili S.H., Alavian S.M. Regulatory T cells are the most important determinant factor of hepatitis B infection prognosis: a systematic review and meta-analysis. Vaccine. 2012;30:5595–5602. doi: 10.1016/j.vaccine.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 121.Chen K.J., Zhou L., Xie H.Y., Ahmed T.E., Feng X.W., Zheng S.S. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29:1817–1826. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 122.Lee W.C., Wu T.J., Chou H.S. The impact of CD4+ CD25+ T cells in the tumor microenvironment of hepatocellular carcinoma. Surgery. 2012;151:213–222. doi: 10.1016/j.surg.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 123.Mathai A.M., Kapadia M.J., Alexander J., Kernochan L.E., Swanson P.E., Yeh M.M. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:980–986. doi: 10.1097/PAS.0b013e31824e9b7c. [DOI] [PubMed] [Google Scholar]
- 124.Sasaki A., Tanaka F., Mimori K. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:173–179. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 125.Li S.P., Peng Q.Q., Ding T. Clinical significance of regulatory T cells proportion in the peripheral blood and tumor tissue in primary hepatocellular carcinoma. Chin J Oncol. 2008;30:523–527. [PubMed] [Google Scholar]
- 126.Yu X., Guo R., Ming D. Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-β1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis. J Gastroenterol Hepatol. 2014;29:1065–1072. doi: 10.1111/jgh.12459. [DOI] [PubMed] [Google Scholar]
- 127.Sutmuller R.P., van Duivenvoorde L.M., van Elsas A. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertoletti A., Gehring A.J. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- 129.Zhou S., Chen L., Qin J. Depletion of CD4+ CD25+ regulatory T cells promotes CCL21-mediated antitumor immunity. Plos One. 2013;8:e73952. doi: 10.1371/journal.pone.0073952. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Chen L., Zhou S., Qin J. Combination of SLC administration and Tregs depletion is an attractive strategy for targeting hepatocellular carcinoma. Mol Cancer. 2013;12:153. doi: 10.1186/1476-4598-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cany J., Tran L., Gauttier V. Immunotherapy of hepatocellular carcinoma: is there a place for regulatory T-lymphocyte depletion? Immunotherapy. 2011;3:32–34. doi: 10.2217/imt.11.29. [DOI] [PubMed] [Google Scholar]
- 132.Phan G.Q., Weber J.S., Sondak V.K. CTLA-4 blockade with monoclonal antibodies in patients with metastatic cancer: surgical issues. Ann Surg Oncol. 2008;15:3014–3021. doi: 10.1245/s10434-008-0104-y. [DOI] [PubMed] [Google Scholar]
- 133.Oo Y.H., Sakaguchi S. Regulatory T-cell directed therapies in liver diseases. J Hepatol. 2013;59:1127–1134. doi: 10.1016/j.jhep.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 134.Alatrakchi N., Koziel M. Regulatory T cells and viral liver disease. J Viral Hepat. 2009;16:223–229. doi: 10.1111/j.1365-2893.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 135.Greten T.F., Ormandy L.A., Fikuart A. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–218. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 136.Nagayama Y., Hase W., Motoyoshi Y., Saitoh O., Sogawa R., Nakao K. Distinct responses of two hepatocellular carcinoma cell lines of a similar origin to immunotherapies targeting regulatory or effector T cells. Oncol Rep. 2007;17:1269–1273. [PubMed] [Google Scholar]