Abstract
Objective
The continuous uninterrupted feedback system is the essential part of any well-organized system. We propose aLYNX concept that is a possibility to use an artificial intelligence algorithm or a neural network model in decision-making system so as to avoid possible mistakes and to remind the doctors to review tactics once more in selected cases.
Method
aLYNX system includes: registry with significant factors, decisions and results; machine learning process based on this registry data; the use of the machine learning results as the adviser. We show a possibility to build a computer adviser with a neural network model for making a choice between coronary aortic bypass surgery (CABG) and percutaneous coronary intervention (PCI) in order to achieve a higher 5-year survival rate in patients with angina based on the experience of 5107 patients.
Results
The neural network was trained by 4679 patients who achieved 5-year survival. Among them, 2390 patients underwent PCI and 2289 CABG. After training, the correlation coefficient (r) of the network was 0.74 for training, 0.67 for validation, 0.71 for test and 0.73 for total. Simulation of the neural network function has been performed after training in the two groups of patients with known 5-year outcome. The disagreement rate was significantly higher in the dead patient group than that in the survivor group between neural network model and heart team [16.8% (787/4679) vs. 20.3% (87/428), P = 0.065)].
Conclusion
The study shows the possibility to build a computer adviser with a neural network model for making a choice between CABG and PCI in order to achieve a higher 5-year survival rate in patients with angina.
Keywords: Coronary artery bypass grafting, Percutaneous coronary intervention, Artificial intelligence, Decision making
Introduction
Contemporary guidelines of European Society of Cardiology have approved the stable angina management algorithm.1 The main alternatives are coronary aortic bypass surgery (CABG) and percutaneous coronary intervention (PCI). In case CABG is feasible and there is anatomic possibility to perform PCI, there are three groups of patients. The first group will benefit from PCI because of good coronary anatomy and perfect late results. These criteria are one- or two-vessel disease without proximal left anterior descending artery stenosis, one-vessel disease with proximal left anterior descending artery stenosis, two-vessel disease with proximal left anterior descending artery stenosis, left main disease with a SYNTAX score ≤22, and three-vessel disease with a SYNTAX score ≤22. The second group with left main disease with a SYNTAX score >32 and three-vessel disease with a SYNTAX score >22 will benefit from CABG because of better late results despite the surgical trauma. The third group seems to have balanced risks between the late results of CABG and minimal invasion of PCI with perfect immediate effect of PCI. This group is quite large and accounts for more than half of all patients after coronary angiography. For these people, decision has to be made by a multidisciplinary heart team including a doctor in charge, cardiologist, interventional cardiologist and cardiovascular surgeon.
The first key point of this algorithm is SYNTAX score. Although it is a useful and evidence-based tool, some pitfalls do exist in its application. First, it is based on the first-generation Taxus stent. Modern materials can yield significantly better results, and parameters have to be corrected in a real-time manner in a changing world.2 Second, the SYNTAX score is the mean result of a multicentre trial. Results can differ from place to place in terms of skills, equipment and resources. Third, genetics and treatment adherence vary from place to place.3
Multidisciplinary approach is required in a multivessel disease and it is recommended for patients who are not covered by the institutional protocol discussed by the heart team or when decision-making is complex.1
Heart team is the second key point and another weakness of this algorithm. Every member solves the problems based on his knowledge and experiences. Cardiovascular surgeon usually sees more often stent restenosis than shunt failure because of the preliminary selection at the coronary angiography stage by an interventional cardiologist. Repeated CABG is rare and difficult. An interventional cardiologist usually tries to solve this problem without a cardiovascular surgeon. An interventional cardiologist usually sees the symptomatic patients and has a higher probability to see the restenosis or shunt occlusion than in the real-world situation. All these doctors are educated by statistics of trials, but they may get misrepresented data which are the reasons for mistakes. These errors can occur mostly in rare and complex cases where a heart team plays a significant role. Correct feedback is the solution.
Patient-related factors, institutional factors and referral patterns may influence the decision-making process.1 There are various factors to be taken into consideration by the physician, thus producing a lot of uncertainties. Computation intelligence is one of the approaches to dealing with such uncertainty in decision-making.4, 5 In this study, we proposed the concept of artificial intelligence model as an assistant in treatment-decision making and built a machine adviser to make a choice between CABG and PCI for patients with angina.
Methods
The first part of this concept is registry
We built up original software to gather the clinical data. The first program was a database containing medical electronic records of patients6 and software as service on-line registry for follow-up.7 The system collected feedback on health status from the patients who underwent the PCI or CABG in our hospital. General practitioners, doctors in charge or doctors of our hospital who keep track of the patients are responsible for sending the feedback data, which include a number of factors that can be significant in revascularization choice and can be used immediately to adjust decision-making process. The data were expressed in measurable values to reflect the real situation and to make decision-making process more unbiased and precise. Choice of revascularization strategy is based not only on anatomy but also on various factors. These factors can be patient-associated and also dependent of the environment and process of decision-making.
A total of 5107 patients who underwent CABG or PCI during 2006–2010 were included in our registry. The registry contained 40 factors (Table 1) from evidence in current trials and scores and hypothetical ones. The 5-year outcomes were investigated. Death was defined as the primary endpoint, because in multifactor decision-making these factors can not only result in cardiac death. Also the cause of death, stroke, myocardial infarction and revascularization were included in registry.
Table 1.
Learning matrix factors.
| Decision | 1 – PCI 2 – CABG | |
|---|---|---|
| 1 | Unstable angina | 1 – yes, 0 – no |
| 2 | Myocardial infarction at admission | 1 – yes, 0 – no |
| 3 | Stable angina | 1 – yes, 0 – no |
| 4 | Age | Years |
| 5 | Gender | 1 – male, 2 – female |
| 6 | Diabetes | 1 – yes, 0 – no |
| 7 | Left ventricle aneurism | 1 – yes, 0 – no |
| 8 | Akinesis | 1 – yes, 0 – no |
| 9 | Diskinesis | 1 – yes, 0 – no |
| 10 | Hypokinesis | 1 – yes, 0 – no |
| 11 | Thrombus in left ventricle | 1 – yes, 0 – no |
| 12 | Serum ALT more than 50 ЕD/L | 1 – yes, 0 – no |
| 13 | Serum direct bilirubin 3.4 μmol/L | 1 – yes, 0 – no |
| 14 | Serum urea more than 6.4 mmol/L | 1 – yes, 0 – no |
| 15 | Serum creatinine 102 μmol/L | 1 – yes, 0 – no |
| 16 | GFR | ml/min |
| 17 | Disability | 0, 1, 2, 3 |
| 18 | Living place | 0 – capital, 1 – small town, 2 – village |
| 19 | Atrial fibrillation | 1 – yes, 0 – no |
| 20 | Serum glucose max | mmol/L |
| 21 | Maximum carotid stenosis | 0 – no, 1 – < 50%, 2 – 50–75%, 3 – > 75% |
| 22 | Atrial flutter | 1 – yes, 0 – no |
| 23 | Rest heart rate | 1/min |
| 24 | Cardiomyopathy | 1 – yes, 0 – no |
| 25 | Chronic obstructive pulmonary disease | 1 – yes, 0 – no |
| 26 | Echo: right ventrical pressure | mm Hg |
| 27 | Echo: right atrial size | cm |
| 28 | Echo: right ventrical size | cm |
| 29 | Echo: left ventricle end systolic size | cm |
| 30 | Echo: systolic volume | ml |
| 31 | Echo: left ventricle ejection fraction | % |
| 32 | Aortal regurgitation | −, +, ++, +++, ++++ |
| 33 | Mitral regurgitation | −, +, ++, +++, ++++ |
| 34 | Tricuspid regurgitation | −, +, ++, +++, ++++ |
| 35 | Chronical heart failure, NYHA | 0, 1, 2, 3 |
| 36 | SYNTAX | 1 |
| 37 | Coronary angio: number of cardiocycles contrast evacuation | 1 |
| 38 | Echo: left ventricle end diasystolic volume | ml |
| 39 | Echo: left ventricle end systolic volume | ml |
| 40 | Muscle bridging | 1 – yes, 0 – no |
PCI: percutaneous coronary angioplasty, CABG: coronary aortic bypass surgery, ALT: alanine aminotransferase, GFR: glomerular filtration rate, NYHA: New York Heart Association.
The patient characteristics were shown in Table 2 and Table 3. After coronary angiography, patients underwent CABG or PCI in accordance with the decisions of multidisciplinary heart team. Among these 5107 patients, 428 had reached the primary endpoint. The 5-year survival was taken as the sign of right decision. There were significant differences between PCI and CABG group in age, renal function, urea, serum creatinine, glucose levels, end systolic and end diastolic volume of left ventricle, mitral, aortic and tricuspid regurgitation and SYNTAX score. Patients with low compensation of diabetes mellitus tend to underwent PCI.
Table 2.
Characteristics of 5107 patients who underwent CABG or PCI during 2006–2010.
| PCI |
CABG |
t | P-value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age, years | 57.70 | 9.13 | 58.45 | 8.01 | −3.1396 | 0.0017 |
| Aortic regurgitation, | 0.75 | 2.01 | 0.68 | 0.27 | 1.6161 | 0.1062 |
| Disability level | 1.32 | 1.36 | 1.64 | 1.30 | −8.6949 | 0.0000 |
| Echo right atrium size, cm | 3.96 | 0.37 | 3.99 | 0.40 | −2.4391 | 0.0148 |
| Echo: ejection fraction, % | 56.48 | 6.98 | 56.34 | 7.43 | 0.706 | 0.4803 |
| Echo: left ventricle end diastolic size, cm | 5.21 | 0.41 | 5.24 | 0.43 | −3.1233 | 0.0018 |
| Echo: left ventricle end systolic size, cm | 3.59 | 0.45 | 3.62 | 0.48 | −2.3868 | 0.0170 |
| Echo: right atrium size, cm | 4.64 | 0.38 | 4.65 | 0.43 | −1.4226 | 0.1549 |
| Echo: right ventricle size, cm | 2.34 | 0.23 | 2.34 | 0.24 | −0.2034 | 0.8388 |
| Echo: systolic volume, ml | 74.09 | 8.81 | 74.42 | 9.73 | −1.2965 | 0.1949 |
| GFR, ml · min−1 · 1.73−2 | 83.50 | 42.94 | 83.55 | 30.92 | −0.0494 | 0.9606 |
| Rest heart rate, min−1 | 61.98 | 5.71 | 61.82 | 6.12 | 0.9437 | 0.3454 |
| Mean right ventricle pressure, mm Hg | 28.51 | 3.25 | 28.49 | 3.79 | 0.243 | 0.8080 |
| Serum glucose, mmol/L | 6.06 | 2.07 | 5.95 | 1.59 | 2.2045 | 0.0275 |
| Serum urea, mmol/L | 7.37 | 4.70 | 8.14 | 5.60 | −5.3687 | 0.0000 |
| SYNTAX score | 17.76 | 9.14 | 32.62 | 10.05 | −55.3177 | 0.0000 |
| Tricuspid regurgitation | 1.13 | 0.24 | 1.10 | 0.33 | 3.3898 | 0.0007 |
PCI: percutaneous coronary angioplasty, CABG: coronary aortic bypass surgery, GFR: glomerular filtration rate.
Table 3.
Characteristics of 5107 patients who underwent CABG or PCI during 2006–2010 (Chi-square).
| Factor | PCI (%) | CABG (%) | P-values (Pearson Chi-square) |
|---|---|---|---|
| Unstable angina | 8.65 | 4.47 | 0.0000 |
| Myocardial infarction at admission | 33.65 | 4.39 | 0.0000 |
| Stable angina | 57.7 | 91.14 | 0.0000 |
| Male | 80.91 | 86.15 | 0.0000 |
| Female | 19.09 | 13.85 | 0.0000 |
| Diabetes mellitus type 2 | 32.66 | 32.22 | 0.7366 |
| Left ventricle aneurism | 12.08 | 15.47 | 0.0004 |
| Left ventricle diskinesis | 4.69 | 5.07 | 0.5201 |
| Left ventricle akinesis | 19.09 | 22.43 | 0.0033 |
| Left ventricle hypokinesis | 79.69 | 81.8 | 0.0563 |
| Thrombus in left ventricle | 4.61 | 7.37 | 0.0000 |
| Serum ALT more than 50 ED | 41.24 | 40.93 | 0.8279 |
| Serum direct bilirubin more than 3.4 μmol/L | 7.65 | 7.91 | 0.7373 |
| Serum urea more than 6.4 mmol/L | 51.42 | 65.61 | 0.0000 |
| Serum creatinine more than 102 μmol/L | 18.13 | 18.41 | 0.8012 |
| Disability group 3 | 31.97 | 37.01 | 0.0000 |
| Village | 28.35 | 34.07 | 0.0000 |
| Atrial fibrillation | 8.96 | 8.58 | 0.6337 |
| Atrial flutter | 1.52 | 1.77 | 0.4868 |
| Cardiomyopathy | 1.56 | 3.95 | 0.0000 |
| Chronic obstructive pulmonary disease | 3.43 | 4.31 | 0.1029 |
| Muscle bridging | 4.5 | 1.61 | 0.0000 |
PCI: percutaneous coronary angioplasty, CABG: coronary aortic bypass surgery, ALT: alanine aminotransferase.
The second part is machine learning
To demonstrate the process we used a neural network model. It was made by Neural Networks pattern Recognition component of MATLAB R2014b for MacOS i64.
The third part of approach is the use of the result as an adviser
Data was analysed with Statsoft Statistica 6.0 software (SPSS Inc. Chicago, IL, USA) using Pearson's Chi-square test for 2 × 2 tables.
Results
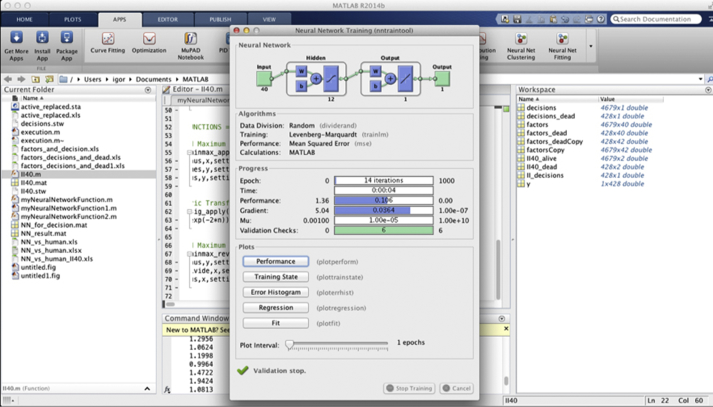
The neural network was trained by 4679 patients who achieved 5-year survival. Among them, 2390 patients underwent PCI and 2289 CABG. Fig. 1 shows the Matlab window at the training stage. At the right part of picture there are 2 matrices, one with the factors and other with decisions.
Fig. 1.
Neural network architecture and education.
Optimal architecture of the neural network has been constructed using a cut-and-try method. The two-layer feed-forward network was used for pattern recognition. Input layer contained 12 neurons, and the second 1 neuron. 70% of patients had been randomly selected for training, 15% for validation and 15% for testing (Fig. 1).
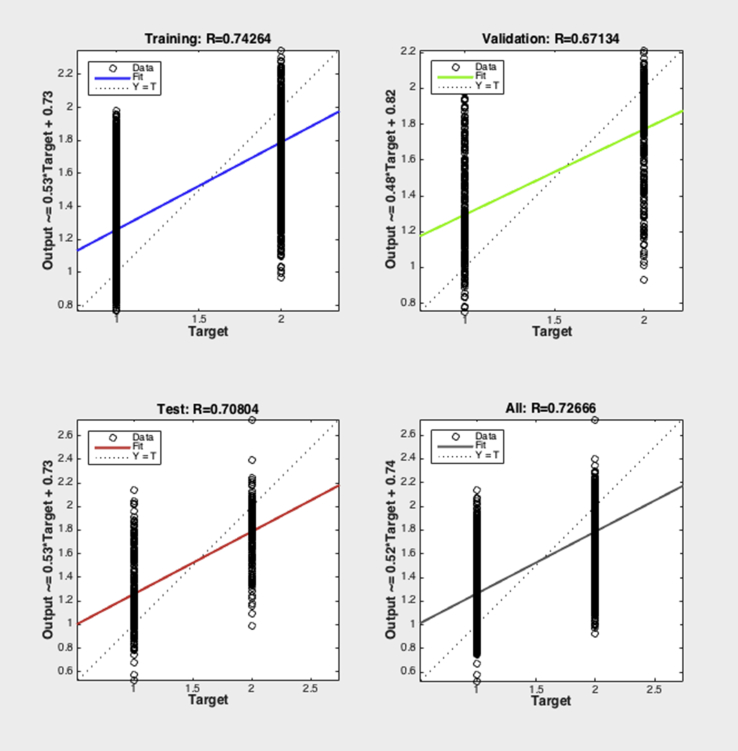
After training, the correlation coefficient (r) of the network was 0.74 for training, 0.67 for validation, 0.71 for test and 0.73 for total (Fig. 2). Simulation of the neural network function has been performed after training in the two groups of patients with known 5-year outcome. The first group survived and second reached primary endpoint (II40_factors_dead = II40 (factors_dead)). II40 is the neural network decision function and II40_factors_dead is a vector with neural network decision results. The network simulation resulted in vector that was converted to table, where the first column was patient identification, the second 5-year survival outcome, the third heart team decision, the fourth neural network model decision (PCI or CABG), the fifth the disagreement between human and neural network model (“1” if yes, “0” if no).
Fig. 2.
Neural network training result.
The disagreement rate was significantly higher in the dead patient group than that in the survivor group between neural network model and heart team [16.8% (787/4679) vs. 20.3% (87/428), P = 0.065].
Discussion
The decision-making between CABG and PCI are very complex and the accuracy of this machine adviser is restricted by some limitations. First, the quality of data collected by any registry that can be used for training the neural network is not ensured. Second, decision-making process in Heart Team lies not only on the suitability for PCI vs. CABG, but also the preferences of surgeons or interventionists. It depends on the institutional factors and reference patterns such as procedure waiting lists, equipment and stent availability.
For establishing an absolute and ideal adviser in the future, the following functions are needed to be developed. (1) Evidence-based guideline algorithm for unambiguous criteria that is defined as absolute in guidelines. It must include absolute or near absolute contraindications that exclude one alternative, absolute or near absolute indications that prefer one option. For example, this function will sort out CABG infeasible patients or type III indications. This function must select cases where at least one option is unacceptable by the evidence or guidelines. (2) Particular context function that suggests a complex multifactor decision-making process when both options seems to be acceptable. To solve this problem, fuzzy methodology is useful. The place of aLYNX algorithm is here. (3) Function that integrate the opinions of a group of experts, aLYNX and patient using a group decision technique. Example of these techniques8 are Supra Decision Maker (SDM),9 MAUT methods of group decision,10 the WINGDSS software method,11 extended Analytic Hierarchy Process,12 PROMETHEE procedure for group decision support,13 ELECTRE methodology.14
In addition, a fully functional artificial intelligence adviser must explain why this decision is optimal.
The study shows the possibility to build a computer adviser with a neural network model for making a choice between CABG and PCI in order to achieve a higher 5-year survival rate in patients with angina based on the experience of 5107 patients.
Feedback system is the essential part of any well-organized system. The continuous uninterrupted feedback results can be used as learning matrix. Neural network relearning from updated matrix helps to make decisions using new experience of exceptional cases, thus improving decision-making.
aLYNX concept shows possibility to use an artificial intelligence algorithm or a neural network model in decision-making system so as to avoid possible mistakes and to remind the doctors to review tactics once more in selected cases. Such system should include registry with significant factors, decisions and results; machine learning process based on the registry data; using the machine learning results as the adviser.
We do hope that soulless machine will never make decisions by itself in consideration of the ethical issues and the real needs of patients, but correct learning dataset including significant factors, tactics and late results makes it possible to build a model to avoid potential mistakes.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Kolh P., Windecker S., Alfonso F. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardiothorac Surg. 2014;46:517–592. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 2.Garg S., Serruys P.W., Silber S. The prognostic utility of the SYNTAX score on 1-year outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All Comers Trial. JACC Cardiovasc Interv. 2011;4:432–441. doi: 10.1016/j.jcin.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Lu Q., Tian G., Zhang Y., Lu M., Lin X., Ma A. Low HDL-C predicts risk and PCI outcomes in the Han Chinese population. Atherosclerosis. 2013;226:193–197. doi: 10.1016/j.atherosclerosis.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Selivanov S.G., Guzairov M.B. [Systemotechnics of Innovative Production Preparation in Industry]. М: Mashinostroenie; 2012. Sistemotechnica innovatsionnoy podgotovki proizvodstva v mashinostroenii. [Google Scholar]
- 5.Poole D., Mackworth A., Goebel R. Oxford University Press; New York: 1998. Computational Intelligence: A Logical Approach. [Google Scholar]
- 6.Buzaev I.V., Programma E.V.M. 2004. Osteon Software Bol'nitsa. Registration Number 2004610140. Official nyj byulleten Programmy dlya EhVM Bazy dannyh Topologii integral'nyh mikroskhem. [Google Scholar]
- 7.Nikolaeva I.E., Plechev V.V., Nagaev I.A. 2013. Programma EVM. On-line sistema dispansernogo nabludeniya dlya respublicanskogo kardiologicheskogo dispansera. Registration Number 2013619040. Oficial'nyj byulleten' «Programmy dlya EhVM Bazy dannyh Topologii integral'nyh mikroskhem». [Google Scholar]
- 8.Fülöp J. Hungarian Academy of Sciences; Budapest: 2005. Introduction to Decision Making Methods. Working Paper 05–6, Laboratory of Operations Research and Decision Systems, Computer and Automation Institute. [Google Scholar]
- 9.Keeney R.L., Raiffa H. Wiley; New York: 1976. Decision with Multiple Objectives: Performances and Value Trade-offs. [Google Scholar]
- 10.Bose U., Davey A.M., Olson D.L. Multi-attribute utility methods in group decision making: past applications and potential for inclusion in GDSS. Omega. 1997;25:691–706. [Google Scholar]
- 11.Csáki P., Rapcsák T., Turchányi P., Vermes M. 1993. Research and Development for Group Decision Aid in Hungary by WINGDSS, A Microsoft Windows Based Group Decision Support System; p. 19. Working Paper of the Laboratory of Operations Research and Decision Systems (LORDS) WP 93-9. [Google Scholar]
- 12.Dyer R.F., Forman E.H. Group decision support with the analytic hierarchy process. Decis support Syst. 1992;8:99–124. [Google Scholar]
- 13.Macharis C., Springael J., De Brucker K., Verbeke A. PROMETHEE and AHP: the design of operational synergies in multicriteria analysis: strengthening PROMETHEE with ideas of AHP. Eur J Oper Res. 2004;153:307–317. [Google Scholar]
- 14.Leyva-Lopez J.C., Fernandez-Gonzalez E. A new method for group decision support based on ELECTRE III methodology. Eur J Oper Res. 2003;148:14–27. [Google Scholar]