Abstract
Tumor necrosis factor-α (TNF-α) contributes to myocardial infarction (MI) injury. Polymorphism of TNF-α gene promoter region and secretion and release of TNF-α and its transformation by a series of signaling pathways are all changed at different points of pathophysiological process in MI. Researches also investigated TNF-α antagonists and their potential therapeutic role in the setting of MI and heart failure at both molecular and clinical level. This article briefly reviews TNF-α and its mechanism as a mediator in MI.
Keywords: Tumor necrosis factor-α, Myocardial infarction, Heart failure
During myocardial infarction (MI) injury, multiple factors at cell level are involved. Among them, the inflammatory immune response occurs in infarcted myocardium and neighboring tissues. This immune response manifests as an acute necrosis, hypertrophy, apoptosis of cardiomyocytes, and subsequent ventricular remodeling. The remodeling process sometimes could lead to congestive heart failure. Although both medication and surgical intervention can mitigate the clinical symptoms, the infarcted, dead myocardium remains an issue and could become substrate for chronic diseases. In recent years, the application of stem cells with pluripotent differentiation and proliferation for regenerative treatment of MI has been investigated. Researchers showed that tumor necrosis factor-α (TNF-α), as a key regulating factor in the inflammatory reaction, not only acted in combination with its ligand as a mediator in the inflammatory immune response but also worked independently in the setting of myocardial repair. In this article we briefly review the TNF-α origin, function, mechanism, gene polymorphism, and recent development in our understanding of this important mediator and its role during MI and the post MI tissue repair.
Source and biological function of TNF-α
TNF-α has two forms in the body: membrane-associated TNF-α (mTNF-α) and secreted TNF-α (sTNF-α) with molecular weights of 26 and 17 kD, respectively. sTNF-α is generally considered the active form of mTNF-α; this activation from mTNF-α to sTNF-α is facilitated by TNF-converting enzyme (TACE).
TNF-α is a ubiquitous cytokine. Many cells have the ability to produce and release it, including monocyte-macrophages, lymphocytes, smooth muscle cells, fibroblasts, endothelial cells, epithelial cells, and osteoblasts. TNF-α mRNA is also expressed in the lung, liver, spleen, thymus, and kidney under the physiological conditions. Inflammatory factors such as invasion of bacteria and viruses can rapidly induce the heart, pancreas, and other organs to synthesize and express TNF-α.1 Many studies2, 3, 4 of the heart have shown that a variety of injuries and inflammatory states, such as MI, myocardial ischemia, reperfusion, cardiac bypass surgery, and chronic heart failure (HF), can promote the production of TNF-α by cardiomyocytes.
Biological activity of TNF-α is realized through the combination of TNF-α and its receptor, TNF-α receptor (TNFR), which is expressed in the cell membranes. TNFR is divided into two types, namely TNFR1 and TNFR2, with molecular weights of 55 and 75 kD, respectively. TNFR1 is expressed across most of the surface of cell membrane, whereas TNFR2 is mainly expressed on the surface of endothelial cells and hematopoietic cells. Remarkably, the expression of TNFR2 gradually decreases with age.5 Furthermore, TNF-α and TNFR1 are confirmed to be expressed in both myocardial infarct zone and non-infarct zone upon MI, while TNFR2 is only expressed within the myocardial infarct zone.6 This suggests that the heart is both a target for and producer of TNF-α.
The activated TNF-α, sTNF-α, can combine with different receptors—TNFR1 or TNFR2—on the surface of a cell and then activate a variety of enzymes and regulate protein synthesis, resulting in a wide variety of biological effects. It can regulate the body's immune function and kill tumor cells directly; participate in the body's inflammatory response, tissue injury, shock, and other pathological process; and is also closely associated with the pathophysiology of several autoimmune diseases.7
Gene polymorphism of TNF-α and MI
Individual susceptibility plays an important role in the development of MI. Single nucleotide polymorphisms (SNPs) do not directly cause a healthy individual to develop MI, but may lead to individual susceptibility to a particular environmental factor or otherwise make an individual susceptible to MI. Studies have confirmed that a G/A polymorphism exists in the 308 promoter region (the 308th bp in the upstream region of transcription start site) of TNF-α gene, which may affect the transcription, expression, and biological activity of TNF-α. However, research on the relationship between SNPs in this region and the incidence of MI is contradictory. Recent research8 has shown that an individual's susceptibility to MI was closely related to genetic factors, which had trans-racial and intra-racial differences.
Antonicelli et al9 found that in Italians, people with the TNF-α-AA+GA polymorphism were at a higher risk of ST-segment elevation MI than those not expressing the polymorphism. Chang et al8 found that there appeared to be gender differences in susceptibility to acute MI, which was induced by the polymorphism of TNF-α-308A/G. Males with TNF-α-308AA+GA were more susceptible to MI compared to males without the polymorphism. Interestingly, this difference in susceptibility between those with and without the polymorphism was not seen among women. Chang et al considered that this might be related to the 308th bp in the upstream region of transcription start site of TNF-α, where an adenine nucleotide replaced a guanine nucleotide and in doing so, enhanced the translation of TNF-α mRNA. Consequently, the secretion of TNF-α into plasma was increased and apoptosis initiated because of the presence of abnormal activation of apoptosis pathways of cardiomyocytes, thus accelerating the formation and rupture of unstable plaques, which further increased the risk of cardiovascular events. While this explanation is compelling, the specific molecular mechanism remains to be elucidated. Chu et al10 conducted a clinical case-control study and meta-analysis on the Chinese Han population to investigate the relationship between the polymorphism of TNF-α-308A/G and susceptibility to MI, which included a total of 10 items (including gender, age, smoking, and diabetes) with 1975 enrollees. They found in both their case-control study and meta-analysis that there was no association between the TNF-α 308A/G polymorphism and the risk of MI.
In addition to the -308 polymorphism, several other polymorphisms exist in the gene for TNF-α, including an A/G polymorphism in the -238 promoter region and C/T polymorphisms in the -806 and -857 sites. Recent research suggests that these polymorphisms may be associated with susceptibility to MI. Through stratified analysis on gender and region, Bennet et al11 found that compared to carriers of the TNF-α-238G allele, females with the TNF-α-238A allele had a lower risk for MI. Hou12 also had similar findings: based on a multivariate analysis on polymorphisms of TNF-α in 804 Han Chinese patients with coronary heart disease (504 with a history of MI) and 905 healthy controls, it was found that compared with the carriers of the TNF-α-238G allele, nonsmokers with the TNF-α-238A allele had a lower risk for coronary heart disease and lower susceptibility to MI. Thus it could be speculated that transformation from adenine to guanine at the -238 promoter regions of TNF-α gene might lead to the reduction of mRNA transcription activity and thus cause the decrease in generation of TNF-α. Kaluza et al13 confirmed this hypothesis by using luciferase reporter assay. They found that transcriptional activity in people with the TNF-α-238A allele was significantly reduced, and compared with the control group, monocytes carrying the TNF-α-238A allele in the peripheral blood, when stimulated, produced dramatically less TNF-α. It is worth noting that in individuals who smoke, polymorphism of TNF-α-806-C/T significantly increased their susceptibility to MI.12 However, since this polymorphism is rare, the extent that it affects TNF-α expression and function is not yet clear and needs to be further studied and its role in increasing susceptibility to MI confirmed. Bennet et al11 found that in healthy smokers, compared with individuals carrying the TNF-α-857C allele, individuals with the TNF-α-857T allele had significantly increased levels of sTNF-α. Bennet et al believed that smoking increased the activity of NF-κВ in vivo, thus inducing the expression of TNF-α and this induction was greater in individuals carrying the TNF-α-857T/T allele. Interestingly, despite the increased levels of TNF-α, these individuals were not more susceptible to MI. This lack of correlation may be reflective of a threshold phenomenon, in that a certain level of TNF-α may be reached before any increased susceptibility to MI is seen.
It is well known that TNF-α can influence the lipid metabolism, increase insulin resistance of obese individuals, and play an important role in the pathophysiology of MI. The complement cascade can also promote the progress of atherosclerotic plaques through participation in the inflammatory reaction of local blood vessels, and thus may play a leading role in the regulation of local inflammatory response to MI. Therefore, is there a potential synergy between polymorphisms in the TNF-α gene and the complement regulatory gene? Szalai et al14 studied 318 patients with severe coronary heart disease and 248 healthy controls and found that individuals with alleles TNF-α-308A or C4A∗Q0 had increased susceptibility to MI. Furthermore, individuals carrying both the TNF-α-308A and C4A∗Q0 alleles were at an even greater risk of MI. They argued that when both the genes were expressed, their products interacted, resulting in co-stimulatory activity which led to an inflammatory reaction. This reaction disrupts the inflammatory balance and may lead to the rupture of unstable plaques, thus facilitating the transformation from stable coronary heart disease to MI.
Overall, the influence of polymorphisms in TNF-α on the risk of MI remains controversial. Influences of different subjects, samples, and other environmental factors on the results cannot yet be ruled out. Thus, despite some evidence that polymorphisms in TNF-α increase susceptibility to MI, more research is needed to confirm the role of TNF-α and its variants in the pathogenesis of MI.
TNF-α and its receptors as well as MI and complications
TNF-α is not expressed in normal cardiomyocytes, but after MI, myocardial tissue with ischemia and anoxia activates cardiomyocytes and myocardial local mononuclear macrophages, which causes the myocardium in the infarcted zone and infarction border zone to produce large amounts of TNF-α.15 Concurrently, the expression of TNFR1 and 2 also increases significantly,16 with the degree of elevation positively correlated with the infarction size, the damage to heart function, and the risk of this disease.17 This suggests that the expression of TNF-α and its receptors is closely related to the pathogenesis of MI.
After MI, TNF-α is combined with TNFR1/2 to play a pleiotropic role: When combined with TNFR1, the complex is mainly involved in the inflammatory reaction and ventricular remodeling after MI, leading to cardiomyocyte apoptosis and cardiotoxicity; whereas when combined with TNFR2, the complex inhibits the inflammatory reaction and ventricular remodeling after MI, thus reducing the apoptosis of cardiomyocytes and protecting the heart.17 TNF-α thus has a dual function in myocardium after MI that acts with time and dose dependence; in the short term, low doses of TNF-α could protect the myocardium; in the long term, however, high-dose secretion of TNF-α has toxic effects on cardiomyocytes.18 In addition, TNF-α can also affect the cardiomyocyte metabolism and weaken the balance between inflammatory and anti-inflammatory factors in MI zone.
TNF-α and MI
TNF-α, interacting with TNFRs after MI, has broad biological activities. When TNF-α combines with TNFR1, the secretion of apoptosis-related proteins (e.g., FADD and TRADD) and inflammatory factors increases, which promotes the progress of ventricular remodeling.5, 19 Arslan et al20 found that TNF-α in combination with TNFR1 induced the secretion of apoptosis-related proteins with RIP1 (receptor interacting protein 1) dependence, which could be blocked by activation of TAK1 (TGF β-activated kinase-1). They also found that TNF-α combined with TNFR1 could activate the NF-κΒ pathway, intensify endothelial cells to express VCAM-1 and ICAM-1, and increase the infiltration of neutrophils into the infarction area, and even cause delayed generation of toxic substances such as superoxide and perforin and seriously influence myocardial contraction and recovery from MI.21
While NF-κB is activated by the combination of TNF-α and TNFR2, expression of inflammatory cytokines IL-6 and IL-1β is downregulated to reduce the injury resulting from the inflammatory reaction. Additionally, expression of angiogenic growth factors VEGF and bFGF is increased to accelerate neovascularization, thus improving the prognosis of MI. Furthermore, downregulated TNFR2 can cause the enhancement of TNFR1.5, 19 Westbrook et al22 found that the combination of TNF-α and TNFR1/2 could cause DNA damage in various cells including T lymphocytes, but NF-κВ inhibitors and IL-10 could significantly reduce the DNA damage. Therefore, it was speculated that the mechanism of DNA damage might be an imbalance of the redox reaction due to reactive oxygen species (ROS) produced by the interaction of TNF-α and TNFRs. However, whether this mechanism is actually occurring in cardiomyocytes during MI is yet to be clarified.
Combination of adiponectin, expressed by adipocytes, and its ligand AdipoR1, which is on the surface of cardiomyocytes, could increase the intake of glucose, enhance lipid metabolism, increase sensitivity of cardiomyocytes to insulin and anti-atherosclerosis; weaken the inflammatory reaction and apoptosis of cardiomyocytes and reduce oxidative stress; and maintain and improve heart function. After MI, however, the increased TNF-α combined with TNFR1 upregulates the secretion of ATF3 (activating transcription factor 3), an adiponectin expression inhibitor which reduces the secretion of adiponectin, while TNF-α combined with TNFR2 downregulates the expression of ATF3 to increase the secretion of adiponectin. Thus, the overall influence of ATF3 on adiponectin is dependent on the balance between the combination of TNF-α with TNFR1 and TNFR2.23, 24
It is notable that TNF-α can play a protective role independently of its receptors. Rathi et al25 found that after MI, low concentrations of TNF-α in vivo inhibited KCL-induced migration of Ca2+ to cardiomyocytes, thus improving myocardial systolic function. Lacerda et al26 had shown that low doses of TNF-α could inhibit the function of mitochondrial state 3 respiration after anoxia-reoxygenation injury of cardiomyocytes, reduce the release of proton leak-dependent uncoupling protein and transmembrane potential, weaken the glutamate-dependent breath, and increase the recovery of mitochondrial respiratory rate, thus improving mitochondrial function. This protective mechanism is realized with the regulation of the ROS as well as the sphingomyelin pathway. In later studies, Lacerda et al27 also found that a certain amount of TNF-α in vivo could also inhibit the secretion of leptin to reduce the injury caused by myocardial ischemia/reperfusion in diabetic mice.
TNF-α and myocardial ischemia/reperfusion injury after MI
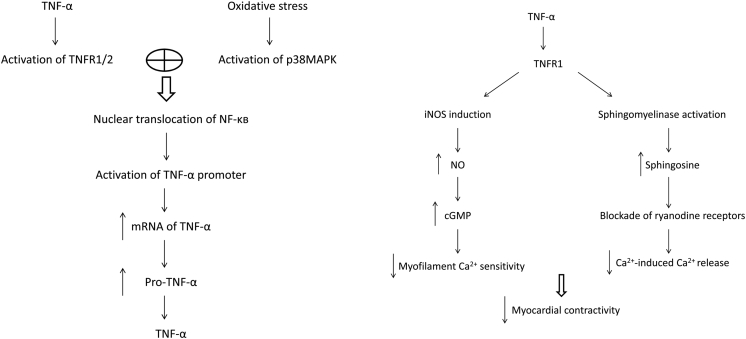
During reperfusion therapy after MI, ischemia/reperfusion injury or no-reflow occurs frequently, which is closely related with TNF-α and often accompanied by arrhythmia, myocardial stunning, left ventricular systolic dysfunction, microvascular injury, and progressive myocardial necrosis. The pathological mechanisms include excessive accumulation of Ca2+ in cardiomyocytes, production of large amounts of oxy radicals, and activation of a variety of oxidoreductases.28, 29 TNF-α is a key regulator in the process through several ways,2, 30, 31 such as combining with TNFR1 to induce the synthesis of NO, reducing the sensitivity of myofilament to Ca2+, or activating sphingomyelinase to weaken the release of Ca2+ induced by Ca2+, thus leading to the occurrence of arrhythmia and a decrease in ventricular systolic function. TNF-α can also activate the NF-κB pathway by TNFR1, which results in the vicious circle of TNF-α and pro-inflammatory cytokines, which further aggravates the injury. This activity can be blocked by TNFR2 to a certain extent. Some of these pathways are shown in Fig. 1.
Fig. 1.
Proposed outline of the pathway of tumor necrosis factor α (TNF-α) synthesis and effect of TNF-α on myocardial contractility. TNF-R: TNF-α receptor; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor kappaB; mRNA: messenger RNA; pro-TNF-α: TNF-α propeptide; cGMP: cyclic GMP; iNOS: inducible nitric oxide synthase; NO: nitric oxide.
TNF-α and arrhythmia after MI
TNF-α plays the central role in inflammatory response and immunoregulation as the acute phase reactive proteins in vivo, and MI itself also involves in the inflammatory reaction. TNF-α also participates in the ventricular remodeling and repairs process after MI. Ventricular arrhythmia, however, is considered as one of the common complications after MI, so does TNF-α also play a role in it?
Xiao et al32 found that in mice which had experimentally induced MI, the occurrence of ventricular arrhythmia (including ventricular premature beat, ventricular tachycardia, sinus bradycardia, and arrhythmia) correlated with an increased level of TNF-α protein and mRNA within the infarcted area. Moreover, Xiao et al observed mice hearts in vitro and found that TNF-α could significantly increase Ca2+ concentration in cardiomyocytes, which could be blocked by TNF-α inhibitors. It is well known that the pathogenesis of arrhythmia is correlated with an imbalance of ion flow in cardiomyocytes, and TNF-α is able to regulate this ion flow. TNF-α can adjust the internal flow of Ca2+ in cardiomyocytes by the PLA2/AA pathway, thus affecting the contraction of cardiomyocytes. TNF-α can also restrain the delayed rectifier potassium current in the PKA way. Thus, TNF-α can increase the Ca2+ concentration of cardiomyocytes after MI; until the delay after depolarization of cardiomyocytes reached the threshold, arrhythmia occurred. Results similar to Xiao et al were seen by Shimoda et al.33
Based on Xiao's experiment, Chen et al15 further found that at 10 min after MI, the level of TNF-α protein and mRNA began to rise in the ischemic infarcted zone and infarction border zone of myocardium in mice. This increase peaked at 20–30 min and then the level declined gradually. Time for appearance of ventricular fibrillation is basically identical with TNF-α concentration curve. During this period, dispersion of monophasic action potential duration (MAPD) in infarction border zone increased, but no such change was found in the infarction border zone or normal region. TNF-α inhibitors significantly decreased the frequencies of ventricular fibrillation and dispersion of MAPD in infarction border zone, suggesting that TNF-α could increase the risk of ventricular fibrillation after MI by increasing the dispersion of MAPD in infarction border zone.
TNF-α and progress of HF after MI
After MI, there is significant rise in TNF-α in vivo, which affects the occurrence, development, and prognosis of HF, including promotion of left ventricular dilation and remodeling, which result in left ventricular systolic dysfunction and regulation of cardiomyocyte hypertrophy. For individuals with HF due to MI, the higher the concentration of TNF-α in vivo, the weaker their predicted cardiac function, and the higher the mortality of the individual.34
Researches showed that TNF-α could be involved in the process of HF through a series of signaling pathways,35, 36 such as inducing the uncoupling of beta-adrenergic receptors, increasing the generation of ROS, and prompting the synthesis of inducible nitric oxide synthase (iNOS). In addition, TNF-α might also increase the secretion of other pro-inflammatory cytokines (e.g., IL-6, IL-1), which in turn reinforce the myocardial injury potential of TNF-α. Moreover, TNF-α could downregulate the synthesis of cardiac α-actin and α-myosin heavy chain which is related to a decline in ventricular systolic function in HF.37 In addition to the decline in ventricular systolic function, for individuals with HF after MI, continuous high expression of TNF-α in vivo could also change the structure of the heart, such as promoting hypertrophy of cardiomyocytes and increasing the apoptosis of cardiomyocytes and myocardial fibrosis.
Excessive expression of TNF-α in cardiomyocytes after MI or infiltration of chronic high doses of TNF-α in vivo often causes adaptive hypertrophy of cardiomyocytes,38, 39 thus leading to left ventricular hypertrophy and dilation.40 TNF-α could increase the transcription of hypertrophic genes by activation of p38MAPK and NF-κB pathways.41 The changes in protein expression induced by TNF-α are ROS dependent.42 Therefore, although TNF-α antagonists can be used to balance the excessive expression of TNF-α in vivo, TNF-α then can effectively weaken the hypertrophy and remodeling of cardiomyocytes after HF.43
Some studies have found that in HF the apoptosis of cardiomyocytes increases significantly40; oxidative stress enhances and acts on the constituent proteins of mitochondrial permeability transition pore (MPTP)—adenosine transitional protein and voltage-dependent anion channel (VDAC) to open the pore.44 TNF-α could increase the release of mitochondrial cytochrome c to the cytoplasm by improving the synthesis and secretion of arachidonic acid,45 to weaken the mitochondrial transmembrane potential and induce apoptosis of mature ventricular myocytes and even the incidence of pacing-induced HF.36, 46 TNF-α directly regulates mitochondrial function, especially the increased formation of ROS which increases the release of cytochrome c, which initiates apoptotic signals. In addition, the increasing Ca2+ may stimulate the apoptosis of cardiomyocytes, which may be related to the activation of calpain induced by increased TNF-α.47, 48 It is important to note that the combination of TNF-α and TNFR1 could cause the continuous activation of NF-κВ and the special position of iNOS in the mechanism of cardiomyocyte apoptosis cannot be ignored31; however, the role of TNFR2 in the protection of myocardial function also should not be ignored.
Related studies confirmed that upon HF, synthesis of interstitial infiltration, extracellular matrix components, and connexin was all increased to some extent.40 By increasing the synthesis of ROS and reducing the secretion of tissue inhibitor of MP (TIMP), TNF-α upregulated the expression of matrix metalloproteinase (MMP)49 and broke the balance between MMP and TIMP, thus changing the content of myocardial collagen fibers, which eventually aggravated ventricular remodeling and even caused ventricular rupture.31
In spite of this understanding, basic and clinical research aiming at treating HF through antagonism of TNF-α has not been successful. Berthonneche et al50 found that in mice with HF after MI there was no effect when they were given monomeric recombinant human soluble TNF-α receptor type II (soluble TNFR2) treatment. Eugene et al treated HF patients with infliximab (a TNF-α inhibitor), and the results showed that low doses had no benefit in individuals with HF, while high doses of infliximab, on the contrary, led to the deterioration of HF.51 Therefore, although research suggests a link between TNF-α and the pathogenesis of HF, the potential exploitation of this pathway for treatment purposes needs further studies.
Stem cell therapy when TNF-α adjusting MI
Stem cells and hematopoietic cells have strong regeneration and differentiation abilities and can differentiate into other cells including cardiomyocytes. Therefore, in recent years, studies on stem cells and hematopoietic progenitor cells to repair myocardial injury have become common. In addition, the inflammatory reaction is involved in the pathological changes of almost all cardiovascular diseases (e.g., MI, HF, and myocarditis); therefore, it is necessary to understand the relationship between stem cells, hematopoietic progenitor cells, and TNF-α in the treatment of myocardial injury.
There is evidence that while TNF-α is markedly increased in the plasma of individuals with congestive HF, the levels of CD34+ stem cells in peripheral blood, endothelial progenitor cells (EPCs) in circulating blood, and hematopoietic precursor cells in bone marrow are decreased.52, 53 Moreover, TNF-α can directly inhibit infiltration of hematopoietic precursor cells CD34+ induced by stem cell factor (SCF).54
Interestingly, the above injury function of TNF-α is realized by the interaction with its receptor TNFR1; however, when combined with TNFR2, the complex is conducive to maintain the function of the stem cell. For example, Chen et al55 found that cardiomyocytes expressing TNF-α combined with TNFR2 on the surface of the embryonic stem cells led to more migration and differentiation of embryonic stem cells into cardiomyocytes. Studies also found that mesenchymal stem cells expressing TNFR2 could reduce inflammatory reactions and improve cardiac function in individuals with MI.56 In addition, TNF-α may also promote the secretion of GM-CSF to influence the differentiation of stem cells.57 In conclusion, the ligand types of TNF-α play an important role in the regulation of stem cell function, and the synthesis and regulation depend on the amount and proportion of TNFR1 and TNFR2 expressed.
Conclusions
Since TNF-α was first described in 1975, many studies about the correlation of TNF-α and MI had been published. Through decades of effort, some mechanisms of TNF-α in the progress of MI have been identified, such as the polymorphisms in gene expression, inflammatory reaction, metabolic regulation, and the change of the ion flow channel. Despite the understanding we have gained over the past several decades, there are still many gaps in it: for example, specific regulation of the polymorphism of coding TNF-α gene promoter region to the expression of TNF-α and its interaction with various environmental factors to affect MI progress. In addition, when to use the TNF-α antagonists in the treatment of HF and how much the amount is the most suitable all remain to be elucidated in the further experiments. Considering the roles of TNF-α in MI and its subsequent progress, further investigation on the mechanism of TNF-α will promote better treatment of MI and its complications.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81100220).
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Tang X., Marciano D.L., Leeman S.E., Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc Natl Acad Sci U S A. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng M., Yan H., Chen Y. Suppression of NF-kappaB reduces myocardial no-reflow. PLoS One. 2012;7:e47306. doi: 10.1371/journal.pone.0047306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eremenko A.A., Chernova E.V., Vinnitskiĭ L.I. Effect of clarithromycin on the systemic inflammatory response syndrome severity in patients after myocardial revascularization surgery. Anesteziol Reanimatol. 2012:67–71. [PubMed] [Google Scholar]
- 4.Adamy C., Le Corvoisier P., Candiani G. Tumor necrosis factor alpha and glutathione interplay in chronic heart failure. Arch Mal Coeur Vaiss. 2005;98:906–912. [PubMed] [Google Scholar]
- 5.Kishore R., Tkebuchava T., Sasi S.P. Tumor necrosis factor-alpha signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv Exp Med Biol. 2011;691:433–448. doi: 10.1007/978-1-4419-6612-4_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deten A., Zimmer H.G. Heart function and cytokine expression is similar in mice and rats after myocardial infarction but differences occur in TNFalpha expression. Pflugers Arch. 2002;445:289–296. doi: 10.1007/s00424-002-0930-x. [DOI] [PubMed] [Google Scholar]
- 7.Barnabe C., Martin B.J., Ghali W.A. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 8.Chang W.T., Wang Y.C., Chen C.C. The -308G/A of tumor necrosis factor (TNF)-alpha and 825C/T of guanidine nucleotide binding protein 3 (GNB3) are associated with the onset of acute myocardial infarction and obesity in Taiwan. Int J Mol Sci. 2012;13:1846–1857. doi: 10.3390/ijms13021846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonicelli R., Olivieri F., Cavallone L. Tumor necrosis factor-alpha gene -308G>A polymorphism is associated with ST-elevation myocardial infarction and with high plasma levels of biochemical ischemia markers. Coron Artery Dis. 2005;16:489–493. doi: 10.1097/00019501-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chu H., Yang J., Mi S. Tumor necrosis factor-alpha G-308 A polymorphism and risk of coronary heart disease and myocardial infarction: a case-control study and meta-analysis. J Cardiovasc Dis Res. 2012;3:84–90. doi: 10.4103/0975-3583.95359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennet A.M., van Maarle M.C., Hallqvist J. Association of TNF-alpha serum levels and TNFA promoter polymorphisms with risk of myocardial infarction. Atherosclerosis. 2006;187:408–414. doi: 10.1016/j.atherosclerosis.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Hou L., Huang J., Lu X. Polymorphisms of tumor necrosis factor alpha gene and coronary heart disease in a Chinese Han population: interaction with cigarette smoking. Thromb Res. 2009;123:822–826. doi: 10.1016/j.thromres.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Kaluza W., Reuss E., Grossmann S. Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J Invest Dermatol. 2000;114:1180–1183. doi: 10.1046/j.1523-1747.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 14.Szalai C., Füst G., Duba J. Association of polymorphisms and allelic combinations in the tumour necrosis factor-alpha-complement MHC region with coronary artery disease. J Med Genet. 2002;39:46–51. doi: 10.1136/jmg.39.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Zhang Q., Liao Y.H. Effect of tumor necrosis factor-alpha on neutralization of ventricular fibrillation in rats with acute myocardial infarction. Mediat Inflamm. 2011;2011:565238. doi: 10.1155/2011/565238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielczarek-Palacz A., Sikora J., Kondera-Anasz Z., Smycz M. Changes in concentrations of tumor necrosis factor TNF and its soluble receptors type 1 (sTNF-r1) and type 2 (sTNF-R2) in serum of patients with ST-segment elevation myocardial infarction. Wiad Lek. 2011;64:71–74. [PubMed] [Google Scholar]
- 17.Nilsson L., Szymanowski A., Swahn E., Jonasson L. Soluble TNF receptors are associated with infarct size and ventricular dysfunction in ST-elevation myocardial infarction. PLoS One. 2013;8:e55477. doi: 10.1371/journal.pone.0055477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fajardo L.F., Kwan H.H., Kowalski J., Prionas S.D., Allison A.C. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- 19.Monden Y., Kubota T., Inoue T. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H743H753. doi: 10.1152/ajpheart.00166.2007. [DOI] [PubMed] [Google Scholar]
- 20.Arslan S.C., Scheidereit C. The prevalence of TNFalpha-induced necrosis over apoptosis is determined by TAK1-RIP1 interplay. PLoS One. 2011;6:e26069. doi: 10.1371/journal.pone.0026069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y.C., Kim C.W., Kim Y.M. Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur J Pharmacol. 2009;614:91–97. doi: 10.1016/j.ejphar.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Westbrook A.M., Wei B., Hacke K. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis. 2012;27:77–86. doi: 10.1093/mutage/ger063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito Y., Fujioka D., Kawabata K. Statin reverses reduction of adiponectin receptor expression in infarcted heart and in TNF-alpha-treated cardiomyocytes in association with improved glucose uptake. Am J Physiol Heart Circ Physiol. 2007;293:H3490–H3497. doi: 10.1152/ajpheart.00310.2007. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Zhao J., Zhang Y. Differential regulation of TNF receptor 1 and receptor 2 in adiponectin expression following myocardial ischemia. Int J Cardiol. 2013;168:2201–2206. doi: 10.1016/j.ijcard.2013.01.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathi S.S., Xu Y.J., Dhalla N.S. Mechanism of cardioprotective action of TNF-alpha in the isolated rat heart. Exp Clin Cardiol. 2002;7:146–150. [PMC free article] [PubMed] [Google Scholar]
- 26.Lacerda L., McCarthy J., Mungly S.F. TNFalpha protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol. 2010;105:751–762. doi: 10.1007/s00395-010-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacerda L., Opie L.H., Lecour S. Influence of tumour necrosis factor alpha on the outcome of ischaemic postconditioning in the presence of obesity and diabetes. Exp Diabetes Res. 2012;2012:502654. doi: 10.1155/2012/502654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolli R., Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 29.Dhalla N.S., Golfman L., Takeda S., Takeda N., Nagano M. Evidence for the role of oxidative stress in acute ischemic heart disease: a brief review. Can J Cardiol. 1999;15:587–593. [PubMed] [Google Scholar]
- 30.Saini H.K., Xu Y.J., Zhang M. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp Clin Cardiol. 2005;10:213–222. [PMC free article] [PubMed] [Google Scholar]
- 31.Hamid T., Gu Y., Ortines R.V. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119:1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao H., Chen Z., Liao Y. Positive correlation of tumor necrosis factor-alpha early expression in myocardium and ventricular arrhythmias in rats with acute myocardial infarction. Arch Med Res. 2008;39:285–291. doi: 10.1016/j.arcmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda Y., Satoh M., Nakamura M., Akatsu T., Hiramori K. Activated tumour necrosis factor-alpha shedding process is associated with in-hospital complication in patients with acute myocardial infarction. Clin Sci (Lond) 2005;108:339–347. doi: 10.1042/CS20040229. [DOI] [PubMed] [Google Scholar]
- 34.Dunlay S.M., Weston S.A., Redfield M.M., Killian J.M., Roger V.L. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625–631. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulick T., Chung M.K., Pieper S.J., Lange L.G., Schreiner G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe G.W., Marin-Garcia J., Konig A. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–H1820. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 37.Patten M., Krämer E., Bünemann J. Endotoxin and cytokines alter contractile protein expression in cardiac myocytes in vivo. Pflugers Arch. 2001;442:920–927. doi: 10.1007/s004240100612. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi Y., Otsu K., Nishida K. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2002;34:233–240. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama T., Nakano M., Bednarczyk J.L. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation. 1997;95:1247–1252. doi: 10.1161/01.cir.95.5.1247. [DOI] [PubMed] [Google Scholar]
- 40.Funakoshi H., Zacharia L.C., Tang Z. A1 adenosine receptor upregulation accompanies decreasing myocardial adenosine levels in mice with left ventricular dysfunction. Circulation. 2007;115:2307–2315. doi: 10.1161/CIRCULATIONAHA.107.694596. [DOI] [PubMed] [Google Scholar]
- 41.Barnes P.J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K., Fushimi K., Kouchi H. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 43.Jobe L.J., Meléndez G.C., Levick S.P. TNF-alpha inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariappan N., Soorappan R.N., Haque M., Sriramula S., Francis J. TNF-alpha-induced mitochondrial oxidative stress and cardiac dysfunction: restoration by superoxide dismutase mimetic Tempol. Am J Physiol Heart Circ Physiol. 2007;293:H2726–H2737. doi: 10.1152/ajpheart.00376.2007. [DOI] [PubMed] [Google Scholar]
- 45.Scorrano L., Penzo D., Petronilli V., Pagano F., Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J., Liu M., Kennedy R.H., Liu S.J. TNF-alpha-induced impairment of mitochondrial integrity and apoptosis mediated by caspase-8 in adult ventricular myocytes. Cytokine. 2006;34:96–105. doi: 10.1016/j.cyto.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Bajaj G., Sharma R.K. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558–1564. doi: 10.1016/j.bbrc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 48.Galvez A.S., Diwan A., Odley A.M. Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 49.Awad A.E., Kandalam V., Chakrabarti S. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kgamma-dependent manner. Am J Physiol Cell Physiol. 2010;298:C679–C692. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- 50.Berthonneche C., Sulpice T., Boucher F. New insights into the pathological role of TNF-alpha in early cardiac dysfunction and subsequent heart failure after infarction in rats. Am J Physiol Heart Circ Physiol. 2004;287:H340–H350. doi: 10.1152/ajpheart.01210.2003. [DOI] [PubMed] [Google Scholar]
- 51.Chung E.S., Packer M., Lo K.H., Fasanmade A.A, Willerson J.T., Anti-TNF Therapy Against Congestive Heart Failure Investigators Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 52.Iversen P.O., Woldbaek P.R., Tønnessen T., Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R166–R172. doi: 10.1152/ajpregu.2002.282.1.R166. [DOI] [PubMed] [Google Scholar]
- 53.Valgimigli M., Rigolin G.M., Fucili A. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 54.Rusten L.S., Smeland E.B., Jacobsen F.W. Tumor necrosis factor-alpha inhibits stem cell factor-induced proliferation of human bone marrow progenitor cells in vitro. Role of p55 and p75 tumor necrosis factor receptors. J Clin Invest. 1994;94:165–172. doi: 10.1172/JCI117303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Ke Q., Yang Y. Cardiomyocytes overexpressing TNF-alpha attract migration of embryonic stem cells via activation of p38 and c-Jun amino-terminal kinase. FASEB J. 2003;17:2231–2239. doi: 10.1096/fj.03-0030com. [DOI] [PubMed] [Google Scholar]
- 56.Bao C., Guo J., Lin G., Hu M., Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J. 2008;42:56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- 57.Arcuri F., Toti P., Buchwalder L. Mechanisms of leukocyte accumulation and activation in chorioamnionitis: interleukin 1 beta and tumor necrosis factor alpha enhance colony stimulating factor 2 expression in term decidua. Reprod Sci. 2009;16:453–461. doi: 10.1177/1933719108328609. [DOI] [PMC free article] [PubMed] [Google Scholar]