Abstract
Objectives
This article reviews pharmacology, pharmacokinetic properties, clinical efficacy, and safety in metastatic breast cancer patients, as well as the predictive biomarkers for outcome of treatment with pemetrexed-based regimens.
Methods
PubMed, Embase, OVID, and the Cochrane Library databases were searched from the beginning of each database without any limitations to the date of publication. Search terms were ‘‘pemetrexed’’ or ‘‘LY231514’’ or “Alimta”, “metastatic breast cancer”, and “advanced breast cancer”.
Results
There were 15 studies (n = 1002) meeting our criteria for evaluation. Eight single-agent trials (n = 551) and seven using combinations with other agents (n = 451) were identified that evaluated pemetrexed for use in patients with metastatic breast cancer. Response rates to pemetrexed as a single agent varied from 8% to 31%, and with combination therapy have been reported to be between 15.8% and 55.7%. With routine supplementation of patients with folic acid, dexamethasone, and vitamin B12, the toxicity profile of these patients was mild, including dose-limiting neutropenia and thrombocytopenia, as well as lower grades of reversible hepatotoxicity and gastrointestinal toxicity. Expression of thymidylate synthase (TS) and other biomarkers are associated with the prognosis and sensitivity for pemetrexed in breast cancer.
Conclusion
Pemetrexed has shown remarkable activity with acceptable toxicities for treatment of metastatic breast cancer patients. Translational research on pemetrexed in breast cancer identified biomarkers as well as additional genes important to its clinical activity and toxicity. Further research is needed to clarify the role of pemetrexed in breast cancer treatment in order to guide oncologists.
Keywords: Metastatic breast cancer, Chemotherapy, Pemetrexed, Anthracycline, Taxane
Introduction
Breast cancer is the most common malignancy among women and is still a leading cause of mortality in women worldwide.1 Although impressive improvements have been made in adjuvant therapy with anthracyclines and taxanes, development of drug resistance to these agents in recurrent tumors is common, and a substantial proportion of breast cancer patients will eventually develop metastatic disease.2, 3, 4 In the management of metastatic breast cancer, current goals focus on prolonging survival and maintaining the quality of life by controlling symptoms and minimizing related toxicity.5 Currently, there is no single standard to guide oncologists in choosing additional chemotherapy for patients with metastatic breast cancer who are refractory to anthracyclines and taxanes.6 Drug therapy with agents such as capecitabine or ixabepilone is often used, but the response rates to these therapies are low.7, 8 More efficacious and safe chemotherapeutic agents, both for monotherapy and in combination with other agents, are needed to treat this pretreated population of patients with metastatic breast cancer.
Pemetrexed is a multi-targeted antifolate cytotoxic chemotherapy agent that has proven activity in several malignancies, including mesothelioma, lung, breast, colon, pancreatic, gastric, bladder, head and neck, and cervical cancers.9, 10 Pemetrexed has been approved for use in combination with cisplatin for first-line treatment of malignant pleural mesothelioma, as a single agent for advanced non-small-cell lung cancer (NSCLC), and for first-line treatment of nonsquamous NSCLC.11 It has a manageable toxicity profile that includes dose-limiting neutropenia and thrombocytopenia, as well as lower grades of reversible hepatotoxicity and gastrointestinal toxicity. Studies of pemetrexed have shown that the drug is effective in the treatment of previously treated metastatic breast cancer, and has an acceptable toxicity profile. This paper reviews the pharmacology, pharmacokinetics, clinical efficacy, safety, and role in therapy of pemetrexed in patients with metastatic breast cancer.
Material and methods
Search methods were conducted according to the Preferred Reporting of Systematic Reviews and Meta-Analysis (PRISMA) Statement guidelines.12 Medline, PubMed, Embase, OVID, and the Cochrane Library were searched from the beginning of each database without any limitations to the date of publication for relevant articles on human studies published in English. Search terms were ‘‘pemetrexed’’ or ‘‘LY231514’’or “Alimta”, “metastatic breast cancer”, and “advanced breast cancer”. The references of selected articles were also reviewed to identify additional publications. The study subjects should be patients with pathologically proven breast cancer who received pemetrexed containing regimens. Studies that did not provide at least the objective response rate or median survival or survival time were excluded.
Results
There were 15 studies (n = 1002) meeting our criteria for evaluation. Eight single-agent trials (n = 551) and seven that used combinations with other agents (n = 451) were identified that evaluated pemetrexed for use in patients with metastatic breast cancer who had been pretreated with anthracycline and taxane.
Pharmacology
The chemical name of pemetrexed is N-[4-[2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d] pyrimidin-5-yl]ethyl] benzoyl]-1-glutamic acid disodium salt (Fig. 1). It is a multitargeted antifolate agent that interferes with the synthesis of nucleic acids, resulting in a cytotoxic effect on neoplastic cells. Pemetrexed inhibits several enzymes in the de novo pathways of pyrimidine and purine biosynthesis which are required for the growth and survival of both normal cells and cancer cells, including thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyl transferase (GARFT).13, 14 These multiple mechanisms of action may explain the greater potency and broader spectrum of antitumor activity of pemetrexed in preclinical studies compared with other antimetabolites such as fluorouracil, methotrexate, or raltitrexed.13 Pemetrexed can inhibit colony formation of a variety of chemotherapy-resistant cancer cell lines.
Fig. 1.
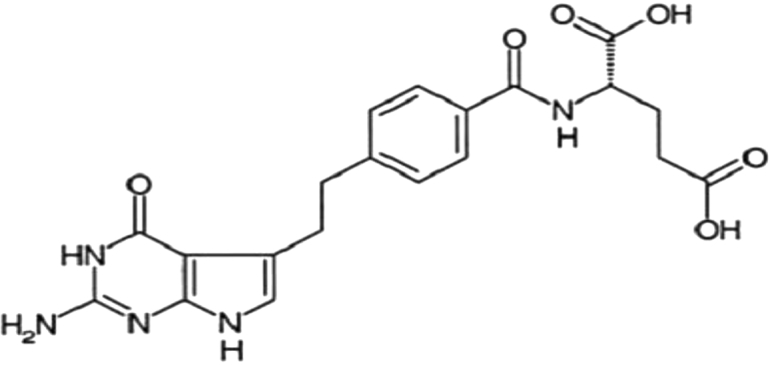
Structure of pemetrexed.
Pharmacokinetic profile
Pemetrexed is administered by an intravenous route only, and it is rapidly eliminated (half-life of 3.5 h and a total systemic clearance of 91.8 ml/min), mainly via the kidneys, with 70–90% of the administered drug recoverable in the urine within 24 h. Only a limited amount of the drug is metabolized by the liver.15 Eighty percent of pemetrexed is bound to plasma proteins, where it gets rapidly distributed and reaches peak plasmatic levels within 30 min. Its clearance correlates with renal function and may be safely used with vitamin supplementation in patients with a creatinine clearance of ≥45 ml/min.16 The pharmacokinetics of a pemetrexed dose does not interfere significantly with the metabolism of other drugs by cytochrome P450 isozymes; therefore, it is feasible to safely administer it in combination with many other cytotoxic or targeted agents.17
Pemetrexed exhibits a moderate toxicity profile at a dose of 500 mg/m2 by 10-min infusion once every 21 days with myelosuppression being the dose-limiting toxicity.18 Folic acid added to the diet in preclinical studies reduced toxicities while maintaining antitumor activity. Based on this observation and clinical toxicities, folic acid and vitamin B12 dietary supplementation has been recently introduced into all ongoing trials.
Clinical efficacy
Single-agent studies with pemetrexed in metastatic breast cancer
Single-agent pemetrexed has shown promising activity and a favorable toxicity profile in patients with locally advanced or metastatic breast cancer.19 Those patients were untreated or minimally pretreated with one or two prior chemotherapies, or heavily pretreated with three to five prior chemotherapies. Depending on the degree of the previous cumulative treatment, response rates ranged from 31% in the cohort of previously untreated patients20 to 8% in the most heavily pretreated cohort tested21 (Table 1).
Table 1.
Results of single-agent trials with pemetrexed.
| Author (year) | No. of evaluable patients | Median age (years) | Dose (mg/m2) | Chemotherapy cycle (range) | Objective response rate (%) | Duration of response (months) | Overall survival (months) | Toxicities |
|---|---|---|---|---|---|---|---|---|
| Gomez 200620 | 76 (61) | 46 | 500, q3w | 2.9 (1–3) | 19 PR (31) | NR | NR | G4 neutropenia (8.2%) |
| 34 SD (56) | G3 aminotransferase (52.4%) | |||||||
| O'Shaughnessy200521 | 80 (75) | 53 | 500, q3w | 3 (1–31) | 3 CR (4) | 5.8 | 8.2 | G3/4 neutropenia (38.8%) |
| 3 PR (4) | G3 lymphopenia (35.0%) | |||||||
| 27 SD (36) | G3/4 aminotransferase (27.6%) | |||||||
| Miles200122 | 38 (36) | 52 | 600, q3w | 5 (1–9) | 1 CR (3) | 8 | 13 | G3/4 neutropenia (47.0%) |
| 9 PR (25) | G3/4 thrombocytopenia (15.7%) | |||||||
| G3/4 rash (18.2%) | ||||||||
| Matin200323 | 77 (72) | 55 | 600, q3w | NR | 3 CR (4) | 5.5 | NR | G3/4 neutropenia (56.0%) |
| 12 PR (16) | G3/4 thrombocytopenia (19.0%) | |||||||
| G3/4 rash (10.0%) | ||||||||
| Spielmann200124 | 72 (31) | 55 | 600, q3w | NR | 1 CR (3) | 5.4 | 12.8 | G3/4 neutropenia (58.0%) |
| 7 PR (23) | G3/4 thrombocytopenia (16.0%) | |||||||
| 13 SD (42) | G3/4 nausea (10.0%) | |||||||
| Llombart-Cussac200625 | 79 (78) | 56 | 500, q3w | 4 (1–23) | 7 PR (9) | 3.1 | 10.5 | G3/4 neutropenia (36.4%) |
| 35 SD (45) | G3/4 lymphopenia (53.3%) | |||||||
| G3/4 aminotransferase (7.7%) | ||||||||
| Robert201128 | 37 (35) | 61.4 | 600, q2w | NR | 1 CR (3) | 4.1 | 18.9 | G3/4 neutropenia (37.2%) |
| 8 PR (23) | ||||||||
| Llombart-Cussac200743 | 47 | 56 | 600, q3w | 6 (1–29) | 8 PR (17) | 4.2 | NR | G3/4 neutropenia (19.2%) |
| G3 leukopenia (6.4%) | ||||||||
| 45 | 61 | 900, q3w | 5 (1–18) | 2 CR (4.4) | 4.1 | 21.4 | G3/4 neutropenia (13.3%) | |
| 5 PR (11.1) | G3 leukopenia (8.8%) | |||||||
| G3 thrombocytopenia (4.4%) |
CR: complete response; PR: partial response; SD, stable disease; NR: not reported.
Three recent single-group, phase II clinical trials have demonstrated single pemetrexed activity and a manageable safety profile in patients with refractory metastatic breast cancer. In one study conducted by Gomez and colleagues,20 61 advanced breast cancer patients were given pemetrexed (500 mg/m2) on a 21-day cycle. The objective response rate was 31% (all partial responses) and the stable disease rate was 56%. In another study by Miles and colleagues,22 38 patients with metastatic breast cancer were given pemetrexed (600 mg/m2) as first-line therapy. The objective response rate was 28%, and median duration of the response was eight months and the median overall survival was 13 months. Martin and colleagues23 have shown similar results with an objective response rate of 20.9%, a stable disease rate was 43%, and the median duration of response and overall survival were 5.5 and 10.7 months in 72 patients.
Spielmann and colleagues24 investigated using pemetrexed (600 mg/m2) in 72 metastatic breast cancer patients who had been heavily pretreated. The overall response rate was 26%; the median duration of the response was 5.4 months and median survival time was 12.8 months. Their study suggests promising therapeutic activity in metastatic breast cancer patients previously treated with both anthracyclines and taxanes. O'Shaughnessy and colleagues21 showed the same activity of pemetrexed in 80 heavily pretreated patients, and improvements in patient-reported symptoms ranged from 16.2% for the intensity of pain to 32.1% for nausea. Contrarily, Llombart-Cussac and colleagues25 reported that the response to pemetrexed salvage treatment was low in this kind of patient, but was generally well tolerated by patients who had been previously treated for breast cancer.
Pemetrexed pharmacokinetic studies in NSCLC patients have suggested that TS inhibition is short-lived, especially with concurrent vitamin B12 and folic acid supplementation, suggesting that an every 2-week schedule may be optimal.26, 27 Therefore, some studies evaluate the activity of pemetrexed on a biweekly schedule, as first-line treatment for advanced or metastatic breast cancer. Robert et al28 used pemetrexed 600 mg/m2 every 2 weeks as first-line chemotherapy in 35 evaluable patients, the overall response rate was 26%, and the clinical benefit rate was 40%. Median progression-free survival and overall survival were 4.1 months and 18.9 months, respectively.
Combination studies with pemetrexed in metastatic breast cancer
In various pemetrexed-based combination therapies for breast cancer, response rates were 15.8–55.7% when used with cyclophosphamide, carboplatin, or gemcitabine (Table 2).
Table 2.
Results of combination trials with pemetrexed.
| Author (year) | No. of evaluable patients | Median age (years) | Dose (mg/m2) | Chemotherapy cycle (range) | Objective response rate (%) | Duration of response (months) | Overall survival (months) | Toxicities |
|---|---|---|---|---|---|---|---|---|
| Dittrich 201230 | 42 | 59 | PEM-600, d1 | 5.5 (1–10) | 8PR (19.1) | NR | NR | G3/4 neutropenia (21.5%) |
| CTX-600, d1, q3w | 18SD (42.9) | G3/4 leukopenia (16.6%) | ||||||
| 61 | 56 | PEM-1800, d1 | 6 (1–10) | 20PR (32.8) | 6.6 | NR | G3/4 neutropenia (27.9%) | |
| CTX-600, d1, q3w | 26SD (42.6) | G3/4 leukopenia (26.2%) | ||||||
| G3 lymphopenia (35.0%) | ||||||||
| Garin200831 | 50 | 55.5 | PEM-600, d1 | 8 (1–13) | 27PR (54) | 10.3 | NR | G3/4 neutropenia (86.0%) |
| CBP-AUC = 5.0, d1, q3w | 15SD (30) | G3 thrombocytopenia (10.0%) | ||||||
| G3 anemia (18.0%) | ||||||||
| Amadori201332 | 65 (64) | 52 | PEM-600, d1 | 6.3 | 17PR (26.6) | 7.7 | NR | G3/4 neutropenia (36.9%) |
| CBP-AUC = 5.0, d1, q3w | 23SD (35.9) | G3/4 thrombocytopenia (24.0%) | ||||||
| 66 (61) | 51.5 | GEM-1200 | 6.2 | 2CR (3.3) | 7.5 | NR | G3/4 neutropenia (60.6%) | |
| VB-30, d1, 8, q3w | 16PR (26.3) | |||||||
| Deng201333 | 19 | NR | PEM-500, d1 | 2 | 3PR (15.8) | NR | 10.3 | G3/4 neutropenia (52.7%) |
| L#-35, d1, q3w | 11SD (57.9) | G3/4 thrombocytopenia (21.3%) | ||||||
| Ma CX200634 | 59 | 51 | PEM-500, d8 | 5 (1–22) | 14PR (24) | 3.7 | 10.3 | *G3/4 neutropenia (83.0%) |
| GEM-1250, d1, 8, q3w | 9SD (15) | febrile neutropenia (14.0%) | ||||||
| G3/4 thrombocytopenia (27.0%) | ||||||||
| Pippen201035 | 21 | 50.7 | PEM-500, d1 | 4 (1–8) | 5PR (23.8) | 4.01 | 16.2 | G3/4 neutropenia (71.0%) |
| GEM-1000, d1, 8, q3w | 10SD (47.6) | febrile neutropenia (10.0%) | ||||||
| G3/4 leukopenia (24.0%) | ||||||||
| 52 | 53.5 | PEM-500, d1 | 5 (1–38) | 2CR (3.85) | 3.19 | 13.4 | G3/4 neutropenia (33.0%) | |
| GEM-1500, d1, 8, q2w | 8PR (15.38) | febrile neutropenia (6.0%) | ||||||
| 26SD (50) | G3/4 leukopenia (14.0%) | |||||||
| Dent201036 | 16 (14) | 54 | PEM-500, d1 | 5 (1–13) | 7SD (50) | 3.2 | 5.9 | G3/4 febrile neutropenia (19.0%) |
| GEM-1500, d1, q2w | ||||||||
PEM: pemetrexed; CTX: cyclophosphamide; CBP: carboplatin; AUC: area under the concentration/time curve; L#: Lobaplatin; GEM: gemcitabine; VB: vinorelbine; OS: overall survival; CR: complete response; PR: partial response; SD: stable disease; NR: not reported; G: grade; *: without vitamin B12 and folic acid supplementation.
Pemetrexed-cyclophosphamide
One study investigated the maximum tolerated dose (MTD) of pemetrexed and cyclophosphamide combination therapy for 57 patients with locally advanced or metastatic breast cancer.29 The dose escalation started with pemetrexed 400 mg/m2 and cyclophosphamide 400 mg/m2, and reached tumor growth delay after 13 dose escalation steps of either pemetrexed or cyclophosphamide with doses of 2400 mg/m2 of pemetrexed and 600 mg/m2 of cyclophosphamide. Among the 50 patients evaluable for efficacy, 13 (26%) patients had a partial response and 17 (34%) patients had stable disease from the lowest dose level. They also observed the antitumor activity measured by the response rate of two different doses of pemetrexed (600 or 1800 mg/m2) in combination with cyclophosphamide (600 mg/m2)30; the group receiving 600 mg/m2 was discontinued as the response rate (19.1%) was lower than targeted. In the 1800 mg/m2 arm, the partial response and stable disease rates were 32.8% and 42.6%, respectively, and the median progression-free survival was 6.3 months. The initial schedule selected for further investigation in phase II trials was pemetrexed at 600 mg/m2. During the subsequent phase II development the dose of pemetrexed was adjusted to 500 mg/m2 due to bone marrow and gastrointestinal toxicities. The adjusted dose of pemetrexed was well tolerated throughout the late-phase drug development program.
Pemetrexed-platinum-based compounds
A phase II study by Garin and colleagues investigating pemetrexed (600 mg/m2) combined with carboplatin (area under the curve of 5) administered every three weeks in 50 patients with locally advanced (30%) or metastatic (70%) breast cancer.31 Only one fourth of the patients had received prior adjuvant chemotherapy. The partial response rate was 54.0% and the median time to disease progression was 10.3 months.
A randomized phase II non-comparative study by Amadori et al32 investigated pemetrexed-carboplatin and gemcitabine–vinorelbine combination therapies in patients pretreated with anthracycline and taxanes. Both combinations showed moderate efficacy and were well tolerated. Deng and colleagues33 observed that the combination of pemetrexed (500 mg/m2) and lobaplatin (35 mg/m2) was modestly active in 19 heavily pretreated metastatic breast cancer patients. The response rate was 15.8% and the median survival time was 10.3 months.
Pemetrexed-gemcitabine
A phase II study34 defined the efficacy and toxicity of pemetrexed, 500 mg/m2 (intravenous; day 8), in combination with gemcitabine, 1250 mg/m2 (intravenous; day 1 and 8), in 59 patients with metastatic breast cancer. The overall response rate was 24%, median survival time was 10.3months, and the 1-year survival rate was 49%.
Another phase II trial35 evaluated pemetrexed 500 mg/m2 and gemcitabine 1000 mg/m2 or 1500 mg/m2 given on a 21-day or a 14-day schedule in patients with advanced breast cancer previously treated with taxanes. The response rates were 23.8% and 19.2% and the median survival times were 16.2 months and 13.4 months, respectively.
In addition, Dent et al36 reported an open label phase II study of biweekly treatments with the same combination in patients with metastatic breast cancer. Sixteen patients received pemetrexed (500 mg/m2) on day-1 of chemotherapy treatment immediately prior to gemcitabine (1500 mg/m2). Median progression-free survival was 3.2 months and median overall survival was 5.9 months. But this study did not meet the criteria for proceeding to the second stage of accrual as the rate of early disease progression was more than 40% and the response rate was 0. The different levels of activity may be a reflection of different baseline characteristics of patients or the schedule of administration; if gemcitabine had been preceded by pemetrexed the treatment may have been more active.
Tolerability
Adverse events of pemetrexed treatment include dose-limiting myelosuppression, skin rash, mucosal toxicities, elevation in transaminases and asthenia, as well as lower grades of reversible hepatotoxicity and gastrointestinal toxicity. However, since routine supplementation with folic acid, dexamethasone, and vitamin B12 in patients treated with pemetrexed has been instituted, the toxicities are modest. In the above studies clinicians found that the toxicities were generally less frequent in those who received the supplementation (Table 1, Table 2). Therefore, this agent is of particular interest in the palliative treatment setting and could be utilized in high risk patients, especially the elderly or poor performance status patients.18, 37, 38, 39 Moreover, pemetrexed with folic acid, dexamethasone and vitamin B12 can reduce the level of plasma homocysteine, and thus pemetrexed-associated toxicities, allowing dose escalation of pemetrexed without compromising its antitumor activity.40, 41
A phase I study42 evaluated the effect of folate or vitamin supplementation on the toxicity, tolerability, and pharmacokinetics of pemetrexed in patients with locally advanced or metastatic breast cancer. In this study, the pemetrexed doses tolerated with vitamin supplementation were significantly higher than those tolerated in earlier studies without supplementation, and toxicities were independent of the type of vitamin supplementation or prior myelosuppressive treatment. The recommended dose of pemetrexed is 1050 mg/m2 in lightly pretreated patients and 800 mg/m2 in heavily pretreated patients, irrespective of the type of vitamin supplementation. Therefore, the maximum tolerated dose of pemetrexed as a single agent is probably higher than the dose actually utilized in clinical practice.
Llombart-Cussac et al43 observed response rates of 17% and 15.6% when pemetrexed was given at 600 and 900 mg/m2 to metastatic breast cancer patients in a first-line setting on day-1 of a 21-day cycle. They showed that lower doses of pemetrexed appear to have the same activity as when the higher, 900 mg/m2 dose, was given. The dose of 600 mg/m2 has been the recommended as the pemetrexed dose for phase II studies44 and has shown activity in previous phase II studies in patients with metastatic breast cancer.22, 23, 24
Predictive biomarkers for pemetrexed
Recent studies have reported that thymidylate synthase (TS) expression in tumor tissues is significantly associated with the prognosis in patients with several types of malignant tumors; such as mesothelioma, gastric cancer, and colorectal cancer45, 46, 47, 48, 49. A clinical study evaluating 5-FU based therapy in patients with breast cancer indicated that lower pretreatment levels of TS protein were predictive of the response to chemotherapy.50 Gomez and colleagues20 also explored potential correlations between treatment outcome (antitumor activity) and molecular target expression in patients with untreated breast cancer. Patients with “low” baseline TS expression levels (≤71) were more likely to respond to pemetrexed than patients with “high’’ baseline TS (>71). Hence, lower pretreatment TS expression levels may be associated with enhanced clinical activity of pemetrexed. In addition, the observed decreases in DHFR and GARFT expression after pemetrexed treatment highlight the potential clinical importance of these targets.
Other molecules involved with folate metabolism, transport, and mechanism of action may similarly affect pemetrexed efficacy and toxicity. Llombart-Cussac et al43 assessed 49 patients for the expression levels of 12 pemetrexed-related genes. They found the response rates and median time to tumor progression for high versus low thymidine phosphorylase (TP) expression were 27.6% versus 6.3% and 5.4 months versus 1.9 months, and the folylpolyglutamate synthetase (FPGS) high-expression subgroup had greater response rate and median time to progression than low-expression subgroup which were 37.5% vs. 10.0% and 8.6 vs. 3.0 months. Only γ-Glutamyl hydrolase (GGH) expression correlated with the occurrence of grade 3/4 toxicities; 78.6% patients with high GGH expression experienced a grade 3/4 toxicity, whereas for low GGH expression was 27.3%.
Pippen and colleagues35 found a trend toward an increased response rate in estrogen receptor-negative (ER–) patients compared with estrogen receptor-positive (ER+) patients on the 14-day pemetrexed treatment schedule, consistent with previous studies.51 In their study, two patients with ER–/PR–/human epidermal growth factor receptor 2-negative (HER2–) tumors achieved CR when treated with the 14-day schedule. In addition, only 75–80% of human epidermal growth factor receptor 2-positive (HER2+) patients on 14-day and 21-day schedule had previously received trastuzumab, and a higher rate of trastuzumab use among the HER2+ patients might have resulted in higher response rates and/or more durable responses in that subset of patients. However, it is noteworthy that HER2+ patients in Pippen's study responded to treatment at a rate comparable to that of the HER2– patients. Schneeweiss et al52 reported to the contrary that the clinical effect of pemetrexed-based chemotherapy in early breast cancer was irrelevant to the hormone receptor status.
Discussion
The studies discussed in this review demonstrate that pemetrexed is well tolerated as a single agent and can be an important contribution to combination chemotherapy regimens. Pemetrexed can provide an adequate regimen for metastatic breast cancer therapy. Dose-dense, single-agent pemetrexed did not appear to improve efficacy compared to the standard every 3-week schedule, but its reduced side-effect profile may make it a fitting agent for use in combination with other chemotherapeutics or with targeted agents. The combination of pemetrexed with cyclophosphamide or platinum-based compounds represents a regimen of reasonable efficacy and acceptable tolerability for metastatic breast cancer patients who have been pretreated with an anthracycline plus a taxane. Pemetrexed and gemcitabine is clinically active and a 14-day schedule appears to result in fewer serious toxicities. Further assessment of this combination in a randomized trial of various breast cancer patient populations is warranted.
However, it is difficult to compare results of different studies because of the relatively small number of patients and potential differences in the patient populations. Conducting future studies comparing the combination of pemetrexed and other chemotherapeutic agents with pemetrexed alone in a larger patient population would seem to be a reasonable approach. Therefore, decision-making regarding treatment selection must take into account multiple patient and tumor factors and the therapeutic indices of the available treatments should be considered in the context of the individual patient.
With routine supplementation of patients with folic acid, dexamethasone, and vitamin B12, the toxicity profile of these patients was mild, including dose-limiting neutropenia and thrombocytopenia, as well as lower grades of reversible hepatotoxicity and gastrointestinal toxicity. Furthermore, the relative role of folic acid compared to vitamin B12 should be scrutinized, whether there is an effect of these vitamins on the antitumor effect, and whether vitamin supplementation may allow for a clinically meaningful dose escalation beyond 600 mg/m2. There is a clear need for an optimal dose of pemetrexed for metastatic breast cancer that prolongs survival and is well tolerated.
Expression of thymidylate synthase (TS) and other biomarkers are associated with the prognosis and sensitivity for pemetrexed in breast cancer. The impact of pemetrexed on the expression of its main target enzymes needs to be further explored in order to better predict treatment outcomes and side effects to this agent. Future translational research studies will include investigation of biomarkers of pemetrexed treatment as well as additional genes critical to the folate pathway and breast cancer. Such studies may ultimately allow us to create individualized treatment regimens that offer an improved therapeutic profile by identifying patients most likely to benefit from pemetrexed. In addition, further research is needed to understand the economic implications of these regimens, including the broader societal effects and the value to the patients.
Conflicts of interest
We report no conflicts of interest.
Acknowledgments
This study was supported by the Anticancer Key Technologies R & D Program of Tianjin (NO. 12ZCDZSY16200).
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Sheri A., Johnston S. New developments and future directions in systemic therapy. Clin Oncol (R Coll Radiol) 2013;25:117–126. doi: 10.1016/j.clon.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kataja V., Castiglione M. Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(suppl 2):ii11–13. doi: 10.1093/annonc/mdn072. [DOI] [PubMed] [Google Scholar]
- 4.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Colozza M., de Azambuja E., Personeni N., Lebrun F., Piccart M.J., Cardoso F. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12:253–270. doi: 10.1634/theoncologist.12-3-253. [DOI] [PubMed] [Google Scholar]
- 6.Moreno-Aspitia A., Perez E.A. Anthracycline- and/or taxane-resistant breast cancer: results of a literature review to determine the clinical challenges and current treatment trends. Clin Ther. 2009;31:1619–1640. doi: 10.1016/j.clinthera.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Miller K.D., Chap L.I., Holmes F.A. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 8.Oostendorp L.J., Stalmeier P.F., Donders A.R., van der Graaf W.T., Ottevanger P.B. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. Lancet Oncol. 2011;12:1053–1061. doi: 10.1016/S1470-2045(11)70045-6. [DOI] [PubMed] [Google Scholar]
- 9.Shih C., Chen V.J., Gossett L.S. LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–1123. [PubMed] [Google Scholar]
- 10.Adjei A.A. Pemetrexed (ALIMTA), a novel multitargeted antineoplastic agent. Clin Cancer Res. 2004;10:4276s–4280s. doi: 10.1158/1078-0432.CCR-040010. [DOI] [PubMed] [Google Scholar]
- 11.Tomasini P., Greillier L., Khobta N., Barlesi F. The place of pemetrexed in the management of non-small-cell lung cancer patients. Expert Rev Anticancer Ther. 2013;13:257–266. doi: 10.1586/era.12.171. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–130. [PMC free article] [PubMed] [Google Scholar]
- 13.Shih C., Habeck L.L., Mendelsohn L.G., Chen V.J., Schultz R.M. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine-based antifolate LY231514 (MTA) Adv Enzyme Regul. 1998;38:135–152. doi: 10.1016/s0065-2571(97)00017-4. [DOI] [PubMed] [Google Scholar]
- 14.Racanelli A.C., Rothbart S.B., Heyer C.L., Moran R.G. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–5474. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinaldi D.A. Overview of phase I trials of multitargeted antifolate (MTA, LY231514) Semin Oncol. 1999;26:82–88. [PubMed] [Google Scholar]
- 16.Mita A.C., Sweeney C.J., Baker S.D. Phase I and pharmacokinetic study of pemetrexed administered every 3 weeks to advanced cancer patients with normal and impaired renal function. J Clin Oncol. 2006;24:552–562. doi: 10.1200/JCO.2004.00.9720. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen J.B. Pharmacokinetic evaluation of pemetrexed. Expert Opin Drug Metab Toxicol. 2011;7:919–928. doi: 10.1517/17425255.2011.587411. [DOI] [PubMed] [Google Scholar]
- 18.Hanauske A.R., Chen V., Paoletti P., Niyikiza C. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist. 2001;6:363–373. doi: 10.1634/theoncologist.6-4-363. [DOI] [PubMed] [Google Scholar]
- 19.Dittrich C. Use of pemetrexed in breast cancer. Semin Oncol. 2006;33:S24–S28. doi: 10.1053/j.seminoncol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Gomez H.L., Santillana S.L., Vallejos C.S. A phase II trial of pemetrexed in advanced breast cancer: clinical response and association with molecular target expression. Clin Cancer Res. 2006;12:832–838. doi: 10.1158/1078-0432.CCR-05-0295. [DOI] [PubMed] [Google Scholar]
- 21.O'Shaughnessy J.A., Clark R.S., Blum J.L. Phase II study of pemetrexed in patients pretreated with an anthracycline, a taxane, and capecitabine for advanced breast cancer. Clin Breast Cancer. 2005;6:143–149. doi: 10.3816/CBC.2005.n.016. [DOI] [PubMed] [Google Scholar]
- 22.Miles D.W., Smith I.E., Coleman R.E., Calvert A.H., Lind M.J. A phase II study of pemetrexed disodium (LY231514) in patients with locally recurrent or metastatic breast cancer. Eur J Cancer. 2001;37:1366–1371. doi: 10.1016/s0959-8049(01)00117-4. [DOI] [PubMed] [Google Scholar]
- 23.Martin M., Spielmann M., Namer M. Phase II study of pemetrexed in breast cancer patients pretreated with anthracyclines. Ann Oncol. 2003;14:1246–1252. doi: 10.1093/annonc/mdg339. [DOI] [PubMed] [Google Scholar]
- 24.Spielmann M., Martin M., Namer M., duBois A., Unger C., Dodwell D.J. Activity of pemetrexed (ALIMTA, multitargeted antifolate, LY231514) in metastatic breast cancer patients previously treated with an anthracycline and a taxane: an interim analysis. Clin Breast Cancer. 2001;2:47–51. doi: 10.3816/CBC.2001.n.010. [DOI] [PubMed] [Google Scholar]
- 25.Llombart-Cussac A., Theodoulou M., Rowland K. Pemetrexed in patients with locally advanced or metastatic breast cancer who had received previous anthracycline and taxane treatment: phase II study. Clin Breast Cancer. 2006;7:380–385. doi: 10.3816/CBC.2006.n.054. [DOI] [PubMed] [Google Scholar]
- 26.Dudek A.Z., Larson T., McCleod M.J. Phase 1/2 dose escalating study of twice-monthly pemetrexed and gemcitabine in patients with advanced cancer and non-small cell lung cancer. J Thorac Oncol. 2008;3:394–399. doi: 10.1097/JTO.0b013e318169cdc4. [DOI] [PubMed] [Google Scholar]
- 27.Li K.M., Rivory L.P., Clarke S.J. Pemetrexed pharmacokinetics and pharmacodynamics in a phase I/II study of doublet chemotherapy with vinorelbine: implications for further optimisation of pemetrexed schedules. Br J Cancer. 2007;97:1071–1076. doi: 10.1038/sj.bjc.6603995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert N.J., Conkling P.R., O'Rourke M.A. Results of a phase II study of pemetrexed as first-line chemotherapy in patients with advanced or metastatic breast cancer. Breast Cancer Res Treat. 2011;126:101–108. doi: 10.1007/s10549-010-1286-0. [DOI] [PubMed] [Google Scholar]
- 29.Dittrich C., Petruzelka L., Vodvarka P. A phase I study of pemetrexed (ALIMTA) and cyclophosphamide in patients with locally advanced or metastatic breast cancer. Clin Cancer Res. 2006;12:7071–7078. doi: 10.1158/1078-0432.CCR-05-2829. [DOI] [PubMed] [Google Scholar]
- 30.Dittrich C., Solska E., Manikhas A. A phase II multicenter study of two different dosages of pemetrexed given in combination with cyclophosphamide as first-line treatment in patients with locally advanced or metastatic breast cancer. Cancer Invest. 2012;30:309–316. doi: 10.3109/07357907.2012.658938. [DOI] [PubMed] [Google Scholar]
- 31.Garin A., Manikhas A., Biakhov M. A phase II study of pemetrexed and carboplatin in patients with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2008;110:309–315. doi: 10.1007/s10549-007-9722-5. [DOI] [PubMed] [Google Scholar]
- 32.Amadori D., Carrasco E., Roesel S. A randomized phase II non-comparative study of pemetrexedcarboplatin and gemcitabinevinorelbine in anthracycline- and taxane-pretreated advanced breast cancer patients. Int J Oncol. 2013;42:1778–1785. doi: 10.3892/ijo.2013.1869. [DOI] [PubMed] [Google Scholar]
- 33.Deng Q.Q., Huang X.E., Ye L.H., Lu Y.Y., Liang Y., Xiang J. Phase II trial of Loubo(R) (Lobaplatin) and pemetrexed for patients with metastatic breast cancer not responding to anthracycline or taxanes. Asian Pac J Cancer Prev. 2013;14:413–417. doi: 10.7314/apjcp.2013.14.1.413. [DOI] [PubMed] [Google Scholar]
- 34.Ma C.X., Steen P., Rowland K.M. A phase II trial of a combination of pemetrexed and gemcitabine in patients with metastatic breast cancer: an NCCTG study. Ann Oncol. 2006;17:226–231. doi: 10.1093/annonc/mdj054. [DOI] [PubMed] [Google Scholar]
- 35.Pippen J., Elias A.D., Neubauer M. A phase II trial of pemetrexed and gemcitabine in patients with metastatic breast cancer who have received prior taxane therapy. Clin Breast Cancer. 2010;10:148–153. doi: 10.3816/CBC.2010.n.020. [DOI] [PubMed] [Google Scholar]
- 36.Dent S.F., Gertler S., Verma S. A phase II study of biweekly pemetrexed and gemcitabine in patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2010;65:557–561. doi: 10.1007/s00280-009-1064-z. [DOI] [PubMed] [Google Scholar]
- 37.Fossella F.V., Gatzemeier U. Phase I trials of pemetrexed. Semin Oncol. 2002;29:8–16. doi: 10.1053/sonc.2002.30764. [DOI] [PubMed] [Google Scholar]
- 38.O'Shaughnessy J.A., Gennari A., Conte P. Pemetrexed: a promising new treatment for breast cancer. Semin Oncol. 2002;29:36–41. doi: 10.1053/sonc.2002.30766. [DOI] [PubMed] [Google Scholar]
- 39.Sudoh J., Gemma A. Pemetrexed. Gan To Kagaku Ryoho. 2008;35:1033–1038. [PubMed] [Google Scholar]
- 40.Niyikiza C., Hanauske A.R., Rusthoven J.J. Pemetrexed safety and dosing strategy. Semin Oncol. 2002;29:24–29. doi: 10.1053/sonc.2002.37465. [DOI] [PubMed] [Google Scholar]
- 41.Vogelzang N.J., Rusthoven J.J., Symanowski J. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 42.Takimoto C.H., Hammond-Thelin L.A., Latz J.E. Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res. 2007;13:2675–2683. doi: 10.1158/1078-0432.CCR-06-2393. [DOI] [PubMed] [Google Scholar]
- 43.Llombart-Cussac A., Martin M., Harbeck N. A randomized, double-blind, phase II study of two doses of pemetrexed as first-line chemotherapy for advanced breast cancer. Clin Cancer Res. 2007;13:3652–3659. doi: 10.1158/1078-0432.CCR-06-2377. [DOI] [PubMed] [Google Scholar]
- 44.Hanauske A.R., Dittrich C., Otero J. Overview of phase I/II pemetrexed studies. Oncology (Williston Park) 2004;18:18–25. [PubMed] [Google Scholar]
- 45.Righi L., Papotti M.G., Ceppi P. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010;28:1534–1539. doi: 10.1200/JCO.2009.25.9275. [DOI] [PubMed] [Google Scholar]
- 46.Lenz H.J., Leichman C.G., Danenberg K.D. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–182. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 47.Yamachika T., Nakanishi H., Inada K. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer. 1998;82:70–77. [PubMed] [Google Scholar]
- 48.Karlberg M., Ohrling K., Edler D., Hallstrom M., Ullen H., Ragnhammar P. Prognostic and predictive value of thymidylate synthase expression in primary colorectal cancer. Anticancer Res. 2010;30:645–651. [PubMed] [Google Scholar]
- 49.Qiu L.X., Tang Q.Y., Bai J.L. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384–2389. doi: 10.1002/ijc.23822. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura R., Nagao K., Miyayama H. Thymidylate synthase levels as a therapeutic and prognostic predictor in breast cancer. Anticancer Res. 1999;19:5621–5626. [PubMed] [Google Scholar]
- 51.Carey L.A., Dees E.C., Sawyer L. The triple negative paradox:primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 52.Schneeweiss A., Marme F., Ruiz A. A randomized phase II trial of doxorubicin plus pemetrexed followed by docetaxel versus doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant treatment of early breast cancer. Ann Oncol. 2011;22:609–617. doi: 10.1093/annonc/mdq400. [DOI] [PubMed] [Google Scholar]


