Abstract
Effect of partially hydrolyzed guar gum (PHGG) level (1–5%), culture level (1.5–3.5%) and incubation time (4–8 h) on texture profile of yogurt was studied using response surface methodology. The fortification of partially hydrolyzed guar gum in yogurt decreased the firmness and gumminess while it increased the adhesiveness, cohesiveness and springiness of yogurt significantly at p < 0.01. The culture level did not affect the textural properties of yogurt significantly except gumminess whereas textural properties of yogurt were negatively correlated with incubation time. The coefficient of determination for hardness/hardness, adhesiveness, cohesiveness, springiness and gumminess were 0.9216, 0.9397, 0.8914, 0.8971 and 0.9156, respectively, which revealed that the models obtained were significant as coefficient of determination value was close to one. The optimum conditions obtained were PHGG level 3.37%, culture level 1.96% and incubation time 5.96 h which leads to preparation of yogurt with desired textural characteristics.
Keywords: Dairy products, Yogurt, Dietary fiber, Guar gum, Texture
Introduction
There has been growing interest in consumption of functional and nutraceutical dairy products prepared by incorporation of nutritional supplements. Dietary fiber fortification to ethnic and daily based foods is practiced. Plant materials which resist digestion and absorption in the human alimentary canal with or without fermentation are known as dietary fiber. These may include undigestible carbohydrate polymers and oligomers. Functionality of dietary fiber is due to its laxative action and control of glucose and cholesterol levels in blood (AACC 2001). Guar gum is obtained from seeds of Cyamopsis tetragonolobus plant by separating the endosperm from germ and husk followed by grinding the endosperm. Galactomannan is the major polysaccharide (~80%) present in guar gum. Galactomannan is composed of mannose and galactose. Mannose forms main linear backbone chain (β-1 → 4 linkage) whereas galactose units are attached (α-1 → 6) at every alternative mannose unit of backbone chain. This guar galactomannan portion is generally undigested in human intestinal tract when consumed. Hence, it is considered as functional dietary fiber. Water soluble nature of guar galactomannan makes it a soluble fiber. Guar galactomannan is designated as dietary fiber because of its role in physiological functions such as control of diabetes via blood glucose levels, control of heart disease via reduction in blood cholesterol levels and healthy digestive system via control absorption of nutrients and bowl movement. Guar gum when dispersed in solvent like water forms a very viscous solution. Due to its high viscosity in aqueous solution it is used as thickener and stabilizer in variety of foods such as processed fruits and vegetable products, dairy products, bakery products and confectionery products (Mudgil et al. 2014a). Native guar gum cannot be utilized as dietary fiber in food products because of its high viscosity property it negatively influences the processing and sensory attributes. To solve this problem, native guar gum is enzymatically hydrolyzed to prepare low viscosity partially hydrolyzed guar gum (PHGG). Finished product obtained after enzymatic hydrolysis is a water soluble gum with very low viscosity because enzymes used in hydrolysis process cuts the main backbone chain and reduces the molecular weight of the galactomannan. PHGG is similar to native guar gum with respect to molecular structure. Fiber fortified or enriched food products are prepared by food scientists to prevent the disease conditions resulting from intake of low-fiber foods or diet (Mudgil et al. 2012, 2014b). It is necessary to incorporate dietary fiber in the diet to meet the daily requirements of fiber intake. PHGG can be utilized for the development of fiber fortified food products because of its non-viscous, tasteless and odorless behavior (Yoon et al. 2008).
Yogurt is a globally consumed dairy product and has high nutritional and functional significance.
Food scientists have conducted various studies to assess the effect of nutritional improvement of yogurt via fortification of protein, fiber and fiber source in yogurt on quality of yogurt (Domagalla et al. 2005, 2006; Kaur et al. 2009; Ghasempour et al. 2012; Yang and Li 2010). Textural properties of yoghurt are significant indicators of the yoghurt quality (Lunardello et al. 2011). Functional properties of food products are undesirably influenced by addition of dietary fiber (Ellis 1985). Response surface methodology is used for standardization of process. It is based on statistics and mathematics based principles. It also generates regression equations. RSM is also utilized to study the influence of the variables on responses. Mathematical model describing the relation of variables and responses can also be generated using response surface methodology (Montgomery 2001). The current investigation was designed to analyze the influence of processing variables on the textural properties of yogurt.
Materials and methods
Materials
Guar galactomannan powder was procured from Hindustan Gums and Chemicals Ltd., Bhiwani, India. Guar gum sample procured from the industry was sifted via 200 mesh and stored in a refrigerator until used. All chemicals used in the study were of AR Grade (Central Drug House, India). Cellulase enzyme was procured from USB Corporation, USA. Locally available Milk and skim milk powder (SMP) were used in the study.
Manufacture of PHGG and analysis
PHGG was prepared via enzyme assisted hydrolysis of guar galactomannan powder of fine particle size. Cellulase from Aspergilus niger was used a hydrolyzing agent at pH 6 and temperature 50 °C for the preparing PHGG. Low viscosity aqueous solution collected in cellulase reaction was administered to filtration, freeze drying, grinding and sieving. Standard methods (AOAC, 1990) were used for the determination of parameters of proximate analysis except dietary fiber (Furda 1981).
Yogurt preparation
For production of set-type yogurt, milk solid-not-fat content was first standardized at 10% (w/v) with SMP and subjected to heating at 43 °C. Partially hydrolyzed guar gum was mixed in milk at concentration level of 1–5% with the help of laboratory homogenizer. Milk was then pasteurized at 90 °C for 10 min. After thermal treatment the milk was cooled to 43 °C. Inoculation of milk supplemented by PHGG was done with 1.5–3.5% culture. Milk was then incubated at 43 °C for 4–8 h. Finally, the yogurts were cooled to 10 °C. Yogurts were then stored in sealed container at 4 ± 1 °C before textural analysis was done.
Texture profile analysis of yogurt
Yogurts were analyzed for texture parameters. Texture Analyzer, TA-XT2i (Stable Micro Systems, Surrey, UK) was used to measure texture parameters like hardness, adhesiveness, cohesiveness, springiness and gumminess. Textural properties were analyzed by performing two sequential compression tests with a cylindric shaped probe with a diameter of 25 mm separated by a rest phase of 30 s. Samples were compressed up to 70% of their original length. Pre-test, during test and post-test speeds during textural analysis were 4, 1 and 1 mm per second, respectively. All the measurements were carried out in quintuplicate. Hardness, adhesiveness, cohesiveness, springiness and gumminess values were calculated from the obtained profiles using the software provided by Stable Microsystems.
Design of experiments
Present study involves the use of RSM to study the effect of process variables on textural characteristics of PHGG fortified yogurt. In present study, CCD (central composite design) of RSM was selected to analyze the influence of PHGG concentration (X1), starter culture concentration (X2) and incubation period (X3) on texture characteristics of yogurts such as hardness (Y1), adhesiveness (Y2), cohesiveness (Y3), springiness (Y4) and gumminess (Y5). A set of design was obtained from design expert consisting of 20 different combinations of variables. All the 20 combinations were carried out separately to obtain the response value of textural characteristics (Table 2). The range of variables selected for the analysis was PHGG concentration (1–5%), culture concentration (1.5–3.5%) and incubation period (4–8 h). Equation 1 represents the suggested model for the response variables.
| 1 |
where b0 is the value of responses at centre point of the experiments whereas bi, bii and bij represents linear, quadratic and cross-product coefficients, respectively.
Table 2.
Experimental design for partially hydrolyzed guar gum fortified yoghurt with respective variables (X) and response value (Y)
| Run | X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | Y5 |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.00 | 2.50 | 6.00 | 50.3 | 36.1 | 0.58 | 0.88 | 29.2 |
| 2 | 1.81 | 3.09 | 4.81 | 54.7 | 37.2 | 0.53 | 0.82 | 29.0 |
| 3 | 3.00 | 2.50 | 8.00 | 52.0 | 36.9 | 0.57 | 0.86 | 29.6 |
| 4 | 3.00 | 1.50 | 6.00 | 41.4 | 36.4 | 0.57 | 0.85 | 23.6 |
| 5 | 4.19 | 1.91 | 7.19 | 46.1 | 41.1 | 0.58 | 0.87 | 26.7 |
| 6 | 5.00 | 2.50 | 6.00 | 38.7 | 43.6 | 0.62 | 0.94 | 24.0 |
| 7 | 1.81 | 1.91 | 7.19 | 52.0 | 36.2 | 0.53 | 0.80 | 27.6 |
| 8 | 3.00 | 2.50 | 4.00 | 52.3 | 37.3 | 0.58 | 0.87 | 30.3 |
| 9 | 4.19 | 3.09 | 7.19 | 41.9 | 41.7 | 0.56 | 0.84 | 23.5 |
| 10 | 3.00 | 2.50 | 6.00 | 49.7 | 37.4 | 0.58 | 0.87 | 28.8 |
| 11 | 3.00 | 2.50 | 6.00 | 51.8 | 35.9 | 0.59 | 0.93 | 30.6 |
| 12 | 1.00 | 2.50 | 6.00 | 53.7 | 35.4 | 0.52 | 0.78 | 27.9 |
| 13 | 3.00 | 3.50 | 6.00 | 39.6 | 38.0 | 0.56 | 0.83 | 22.2 |
| 14 | 1.81 | 3.09 | 7.19 | 54.1 | 37.5 | 0.51 | 0.79 | 27.6 |
| 15 | 1.81 | 1.91 | 4.81 | 52.6 | 36.7 | 0.54 | 0.83 | 28.4 |
| 16 | 4.19 | 3.09 | 4.81 | 43.9 | 42.3 | 0.59 | 0.93 | 25.9 |
| 17 | 3.00 | 2.50 | 6.00 | 52.2 | 35.5 | 0.57 | 0.90 | 29.8 |
| 18 | 4.19 | 1.91 | 4.81 | 47.3 | 41.8 | 0.59 | 0.91 | 27.9 |
| 19 | 3.00 | 2.50 | 6.00 | 51.6 | 36.6 | 0.60 | 0.91 | 31.0 |
| 20 | 3.00 | 2.50 | 6.00 | 49.3 | 36.2 | 0.57 | 0.89 | 28.1 |
X1 (PHGG level), X2 (culture level), X3 (incubation time) for dependent response Y1 (hardness), Y2 (adhesiveness), Y3 (cohesiveness), Y4 (springiness) and Y5 (gumminess)
Analysis of data
The data obtained after performing designed experiments was subjected to analysis for standardization of variables in relation to response values of hardness, adhesiveness, cohesiveness, springiness and gumminess of PHGG fortified yogurts. Stat-Ease software (Design-Expert 8.0.4.1 2010) was used for statistical analysis such ANOVA and regression analysis.
Regression equations were obtained via 2nd order polynomial model fitting to data obtained after carrying out the experiments. Components of regression models were analyzed for their statistical significance. Parameters selected for analyzing the statistical significance of models include coefficient of determination and lack of fit test (Weng et al. 2001). The effects of variables at linear, quadratic and interactive level with respect to responses were also analyzed.
Optimization
Algebraic standardization technique was used to obtain optimal process variable. The desirability function was selected for the optimization of responses. For algebraic standardization, all process variables were put up in the selected range whereas responses such as hardness, cohesiveness, cohesiveness and springiness of yogurts were put up at maximum level, although adhesiveness and gumminess of yogurts were put up at minimum level.
In contemplation of reaching to optimum value, goal functions are joined into a comprehensive blended function, D(x), which is also known as desirability function (Myers et al. 2009).
| 2 |
where, d1, d2,…,dn are the dependent variables and ‘n’ is the total number of dependent variables in the measure.
Algebraic standardization figures a point where “desirability function” enhances to maximal.
Results and discussion
Analysis of PHGG
Proximate analysis parameters of PHGG are summarized in Table 1. Results obtained in the investigations suggested that the partially hydrolyzed guar gum consists of good quantity of dietary fiber especially soluble dietary fiber. Partially hydrolyzed guar gum comprised of 80.43% of soluble fibre and 2.70% of insoluble fibre (Table 1). Identical results have been published by researchers in the literature (Yoon et al. 2008).
Table 1.
Analysis of partially hydrolyzed guar gum
| Parametersa | |
|---|---|
| Moisture (%) | 8.02 ± 0.30 |
| Ash (%) | 2.56 ± 0.12 |
| Protein (%) | 1.95 ± 0.32 |
| Fat (%) | 1.09 ± 0.37 |
| SDF (%) | 80.43 ± 0.96 |
| IDF (%) | 2.70 ± 0.42 |
| TDF (%) | 83.13 ± 1.34 |
aThe values are mean ± SD of determinations made in triplicates
Diagnostic models checking
Experimental data obtained from the experiments were studied using response surface analysis.
ANOVA was used to describe the statistical importance of the models. Insignificant F-value of lack-of-fit test for all models was observed that depict the model’s competence in prediction of response values (Table 2). Analysis of R2 values of the models are another validation method of the models. R2 values for hardness, adhesiveness, cohesiveness, springiness and gumminess were 0.92, 0.93, 0.89, 0.89 and 0.91, respectively. R2 value close to one indicates accuracy of prediction. In present study, model F-value, lack-of-fit test F-value, c.v. and R2 values denotes the acceptability of the models (Table 3).
Table 3.
Regression analysis of second order polynomial models for various responses
| Predictor | β (coded factors) | ||||
|---|---|---|---|---|---|
| Hardness | Adhesiveness | Cohesiveness | Springiness | Gumminess | |
| Intercept | 50.72 | 36.24 | 0.58 | 0.90 | 29.55 |
| X1 | −4.35*** | 2.42*** | 0.028*** | 0.042*** | −1.11*** |
| X2 | −0.47 | 0.41 | −0.004 | −0.004 | −0.51* |
| X3 | −0.36 | −0.16 | −0.006* | −0.015** | −0.51* |
| X1 X2 | −1.47* | −0.087 | 0.001 | 0.001 | −0.72* |
| X1 X3 | −0.25 | −0.14 | −0.001** | −0.008 | −0.17 |
| X2 X3 | −0.10 | 0.11 | −0.003 | −0.006 | −0.22 |
| X21 | 0.99* | 1.41*** | −0.007* | −0.014** | −1.07*** |
| X22 | −3.00*** | 0.59** | −0.009** | −0.021*** | −2.15*** |
| X23 | 1.12* | 0.56** | −0.005 | −0.012* | 0.35 |
| ANOVA | |||||
| Model (F-value) | 13.06 | 17.31 | 9.12 | 9.69 | 12.06 |
| Lack-of-fit (F-value) | 4.24 | 2.49 | 1.42 | 0.88 | 0.71 |
| R2 (%) | 92.16 | 93.97 | 89.14 | 89.71 | 91.56 |
| c.v. (%) | 4.01 | 2.28 | 2.27 | 2.42 | 3.59 |
* Significant at < 0.1, ** significant at p < 0.05, *** significant at p < 0.01; X1, PHGG level; X2, culture level; Xp3, incubation time
Response surface plotting
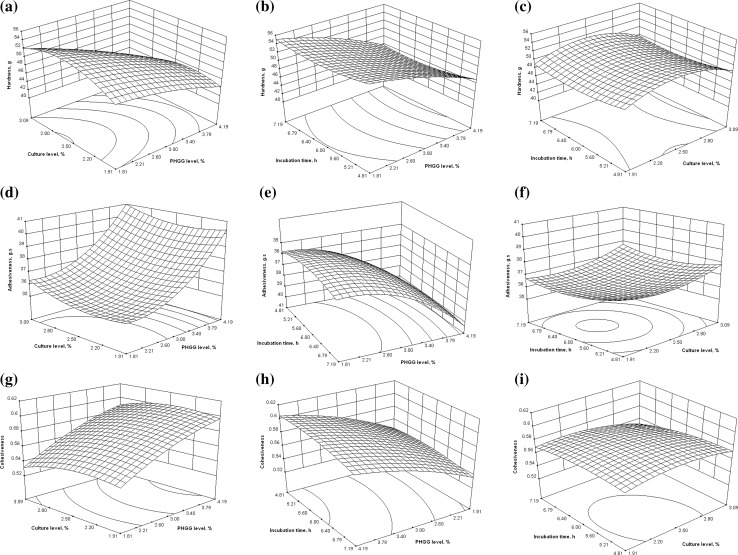
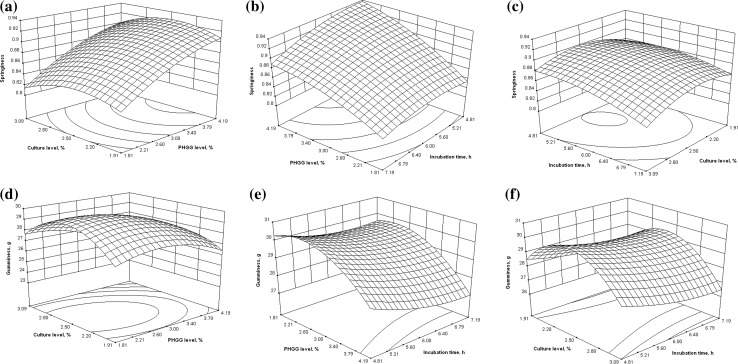
The influence of process variable i.e. PHGG concentration, culture concentration and incubation period on the responses i.e. adhesiveness, hardness, cohesiveness, gumminess and springiness of yogurt were studied using response surfaces. The graphs from experimental data are represented in Figs. 1 and 2.
Fig. 1.
Effect of processing variables on hardness, adhesiveness and cohesiveness of yogurt
Fig. 2.
Effect of processing variables on springiness and gumminess of yogurt
Effect on hardness
Hardness is the most important parameter for evaluation of yogurt texture. It is regarded as the force required to attain a certain deformation and is considered as a measure of firmness of the yogurt. Response surface plots of hardness of yogurt as a function of PHGG level, culture level and incubation time are shown in Fig. 1. Hardness (g) of PHGG fortified yogurts ranged from 38.7 to 54.7 depending on the varying levels of PHGG level, culture level and incubation time. Difference in hardness of yogurt with varying PHGG level and culture level are shown in Fig. 1(a). On PHGG supplementation, hardness of yogurt remains almost same and then decreased with increase in PHGG concentration. Domagalla et al. (2006) observed similar effects when yogurt samples were enriched with oat-maltodextrin. Balasubramanyam and Kulkarni (1991) also reported the increase in yogurt softness with addition of mango pulp. PHGG supplementation up to 2–2.5% did not affect the hardness of the yogurt and further increase in PHGG level decreased the hardness of yogurt. An increase in hardness of yogurt was observed by increase in culture level. At about 2–2.5% of culture level showed highest level of hardness. Beyond 2.5% culture level, there were observed a decrease in yogurt firmness. The influence of varying incubation time and PHGG level on hardness of yogurt is shown in Fig. 1(b). The influence of PHGG level on yogurt was found sharper than incubation time. PHGG incorporation up to 2.5% did not affect the hardness of yogurt while further rise in PHGG level decreased the hardness of yogurt. The hardness of PHGG fortified yogurt was not significantly affected by varying incubation time. The ideal range for yogurt with firm texture was observed between 5.5 and 6.0 h. Figure 1(c) shows the influence of incubation time and level of culture on hardness of PHGG fortified yogurt. Both incubation time and culture level showed opposite effects on the hardness of yogurt. The surface graph showed that with increase in incubation time hardness of yogurt showed slight decrease and then slight increase. Maximum hardness of yogurt was observed at 5 h of incubation time. With increase in the culture level, hardness of yogurt first increased and then decreased. The optimum region for maximum hardness was near 2.5% culture level and 5.0 h of incubation time. The no decrease in hardness of yogurt up to 2.5% supplementation of PHGG may be due to its water absorption which could give strength to the yogurt and stabilize the aqueous and serum phase. Beyond 2.5% concentration of PHGG decrease in hardness values of yogurt may be justified with the viscosity effect of PHGG. PHGG, at higher concentration, might show higher viscosity and thus may have a film forming action which restricts the interaction of culture medium and milk components and desired texture of yogurt could not be attained at higher levels of PHGG supplementation. Hence at higher levels, PHGG decreased the hardness of yogurt.
Effect on adhesiveness
Adhesiveness is regarded as the force required to remove the adhered material in the mouth while eating. It is considered as a measure of stickiness of yogurt and is inversely related to eating quality of yogurt. Adhesiveness (g s) of PHGG fortified yogurts ranged from 35.4 to 43.6 depending on the varying levels of PHGG, culture and time of incubation. Effect of varying levels of culture and PHGG on the adhesiveness of yogurt is shown in Fig. 1(d). Adhesiveness of the yogurt increased sharply upon addition of yogurt with PHGG up to 3%, beyond that adhesiveness increased sharply at low as well as high culture levels. Domagala et al. (2005) also researched the effect of incorporation of oat-maltodextrin on textural and rheological characteristics of yogurt. They reported that incorporation of oat-maltodextrin increased the adhesiveness of yogurt. Adhesiveness of yogurt is not significantly influenced by culture level. However, adhesiveness of yogurt decreased up to 2.5% culture level. Beyond this, adhesiveness of yogurt showed a rise with further increase in levels of culture at lower as well as higher PHGG levels. The effect of PHGG level and incubation time on adhesiveness of yogurt is shown in Fig. 1(e). With change in incubation time, adhesiveness of yogurt did not show any change. Adhesiveness of yogurt did not show any significant change up to 2.6% PHGG level. After this level, adhesiveness of yogurt increased sharply with increase in PHGG level. Adhesiveness of yogurt was observed minimum at incubation time of 6 h and PHGG levels of 2–2.6%. Figure 1f shows the effect of culture levels and incubation time on adhesiveness of PHGG fortified yogurt. Adhesiveness showed first decreasing and then increasing trend with increase in incubation time as well as culture level. Minimum adhesiveness was noticed in middle range of culture level and incubation time.
Effect on cohesiveness
Cohesiveness is the level to which a material can be deformed before it is ruptured and is measure of the strength of internal bonds. It is related to consumer acceptability of yogurt and is an important parameter for analyzing the yogurt texture. The effect of PHGG and water levels on the cohesiveness of yogurt is shown in Fig. 1(g). Cohesiveness of the yogurt showed an increase with increasing levels of PHGG level at lower and higher levels of culture. Similar results were observed when yogurt was supplemented with oat-maltodextrin (Domagala et al. 2005). Cohesiveness of yogurt showed similar relationship with culture level at lower PHGG level as well as at higher PHGG level. Cohesiveness of PHGG yogurt showed a little rise and then fall with increase in culture level. Combined effect showed cohesiveness of yogurt increased with increase in both the variables. The relationship between PHGG level, incubation time and cohesiveness is represented in Fig. 1(h). The increased concentration of PHGG increased the cohesiveness of yogurt. Incubation time showed no significant effect on the cohesiveness of yogurt. However, maximum cohesiveness of PHGG fortified can be seen at minimum incubation time. Figure 1(i) showed the relationship between incubation time, culture level and cohesiveness of yogurt. Cohesiveness of yogurt was observed maximum middle range of culture level and lower levels of incubation temperature. The increase in cohesiveness with PHGG supplementation might be due to viscosity imparted by PHGG which could provide strength to the yogurt structure. Cohesiveness of yogurt may increase with concentration of PHGG which may increase the viscosity of the system.
Effect on springiness
Springiness is the rate at which the sample returns to its native dimensions after the deforming force is removed. Springiness of the yogurt was found to be more influenced by PHGG level. The springiness of yogurt was not significantly affected by the varied culture levels. Springiness of the yogurt increased with the increase in PHGG level as shown in Fig. 2(a). Springiness of yogurt was observed maximum at PHGG levels 3.5–4.2% and culture level 2.2–2.5%. Figure 2(b) shows the effect of PHGG level and mixing time on springiness of yogurt. The effect of PHGG levels on the springiness of yogurt was more prominent than the effect of incubation time. Springiness of yogurt was observed maximum at PHGG levels 3.7–4.2% and incubation time of 5–5.5 h. The relationship among springiness, incubation time and culture level is shown in Fig. 2(c). Springiness of yogurt decreased with increase in incubation time. Springiness of yogurt showed an increase initially but then decreased slightly upon further increase in culture level. Maximum value of springiness was observed in the middle range of culture level and at minimum incubation time. The increase in chewiness of the yogurt upon PHGG supplementation could be due to the viscosity effect of PHGG which might have further strengthened the structure of yogurt. Hence, the increased springiness of yogurt was observed.
Effect on gumminess
Gumminess is another important parameter for textural analysis of yogurt. Level of acceptance of gumminess in yogurt depends on the consumer acceptability. It may vary from person to person. Figure 2(d) shows the effect of culture and PHGG level on gumminess of yogurt. Gumminess of yogurt increased upon increase in concentrations of culture and PHGG. Gumminess of yogurt decreased slightly at higher levels of culture and PHGG. Gumminess of yogurt was observed maximum at the middle region in surface graph for both the variables (i.e. culture level and PHGG level). Gumminess of yogurt changes significantly with change in PHGG levels as compared to change in incubation time (Fig. 2e). Gumminess of yogurt remained almost constant up to 2.5% supplementation of PHGG. Further supplementation of PHGG beyond 3.5% decreased the gumminess of yogurt. Incubation time did not significantly affect the gumminess of yogurt. Gumminess decreased as the incubation time increased for yogurt preparation. Figure 2(f) represents the surface graph showing effect of incubation time and culture level on gumminess of yogurt. Gumminess of yogurt was not so much affected by incubation time. Surface graph showed a curve behavior for gumminess with respect to changes in culture level. Gumminess of the yogurt was observed maximum in the middle region of culture level and was observed almost same at all levels of incubation time. The results obtained in the present study revealed that yogurt fortified with PHGG at 3–3.5% level produce yogurt with firm texture, maximum springiness and cohesiveness and minimum gumminess and adhesiveness. Yogurt prepared with optimized level of variables showed improvement in the textural properties. The results obtained in the present research suggest the use of partially hydrolyzed guar gum as a source for enriching the yogurt with dietary fiber and also in improving the textural properties of yogurt.
Optimization of variables and model verifications
Design-Expert (8.0.4.1 2010) program was used for algebraic standardization of process variables via designating assertive restraints on each independent and dependent variable. Standardized value for each variable was obtained and is represented in Table 4. Affirmative tests were carried out for authentication of models at standardized values of autonomous variables (PHGG concentration 3.37% (~3.4%), culture concentration 1.96% (~2%) and incubation period 5.96 h (~6 h). The substantial experimental obtained were marginally low as compared to forecast values predicted by model except adhesiveness of yogurt. The substantial experimental values achieved at standardized values of responses were 47.1 (hardness), 37.5 (adhesiveness), 0.58 (cohesiveness), 0.87 (springiness) and 27.3 (gumminess) for yogurt sample. Hence, affirmative experiment ratifies the results obtained after carrying out the experiment and likewise regression models.
Table 4.
Numerical optimization of processing variables
| Parameters | Goal | Lower Limit | Upper Limit | Optimum value |
|---|---|---|---|---|
| PHGG level (%) | In range | 1.0 | 5.0 | 3.37 |
| Culture level (%) | In range | 1.5 | 3.5 | 1.96 |
| Incubation time (h) | In range | 4.0 | 8.0 | 5.96 |
| Reponses | ||||
| Hardness | Maximize | 38.7 | 54.7 | 47.7 |
| Adhesiveness | Minimize | 35.4 | 43.6 | 37.3 |
| Cohesiveness | Maximize | 0.51 | 0.62 | 0.59 |
| Springiness | Maximize | 0.78 | 0.94 | 0.90 |
| Gumminess | Minimize | 22.2 | 31.0 | 28.0 |
Conclusion
Textural profile analysis (TPA) is considered as a helpful technique for evaluating the firmness (hardness), adhesiveness, cohesiveness, gumminess and springiness in dairy products such as yogurt. Response surface methodology (RSM) along with TPA was effectively applied for optimization of process variables for preparation of PHGG fortified yogurt. Desired values of hardness, adhesiveness, cohesiveness, springiness and gumminess of yogurt supplemented with PHGG were obtained after conducting regression analysis. Results suggested that all the three variables showed a greater effect on adhesiveness, cohesiveness and springiness than hardness and gumminess of yogurt. Optimum values of culture level, PHGG level and incubation time were 2%, 3.4% 6 h, respectively for the preparation of PHGG fortified yogurt with desired textural characteristics.
References
- AACC The definition of dietary fiber. Cereal Food World. 2001;46:112–126. [Google Scholar]
- Design Expert version 8.0.4.1 (2010) Stat-Ease, Inc., MN, USA
- Domagalla J, Sady M, Grega T, Bonczar G. The influence of storage time on rheological properties and texture of yoghurts with the addition of oat-maltodextrin as a fat substitute. Int J Food Prop. 2005;8:439–448. doi: 10.1080/10942910500267489. [DOI] [Google Scholar]
- Domagalla J, Sady M, Grega T, Bonczar G. Rheological properties and texture of yoghurts when oat-maltodextrin is used as a fat substitute. Int J Food Prop. 2006;9:1–11. doi: 10.1080/10942910600588776. [DOI] [Google Scholar]
- Ellis PR. Fiber and food products. In: Leeds AR, Avenell A, editors. Dietary fiber perspectives: reviews and bibliography. London: John Libbey Company Limited; 1985. pp. 83–105. [Google Scholar]
- Furda I. Simultaneous analysis of soluble, insoluble dietary fibre. In: James WPT, Theander O, editors. The analysis of dietary fibre in food. New York: Marcel Dekker Inc.; 1981. pp. 163–172. [Google Scholar]
- Ghasempour Z, Alizadeh M, Bari MR. Optimization of probiotic yoghurt production containing Zedo gum. Int J Dairy Tech. 2012;65:118–125. doi: 10.1111/j.1471-0307.2011.00740.x. [DOI] [Google Scholar]
- Kaur H, Mishra HN, Kumar P. Textural properties of mango soy fortified probiotic yoghurt: optimization of inoculums level of yoghurt and probiotic culture. Int J Food Sci Tech. 2009;44:415–424. doi: 10.1111/j.1365-2621.2008.01789.x. [DOI] [Google Scholar]
- Lunardello KA, Yamashita F, Benassi MDT, Barros-de-renesis CMV. The physicochemical characteristics of nonfat set yoghurt containing some hydrocolloids. Int J Dairy Tech. 2011;65:260–267. doi: 10.1111/j.1471-0307.2011.00762.x. [DOI] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. Singapore: Wiley; 2001. [Google Scholar]
- Mudgil D, Barak S, Khatkar BS. Soluble fibre and cookie quality. Agro Food Ind Hi Tech. 2012;23:15–17. [Google Scholar]
- Mudgil D, Barak S, Khatkar BS. Guar gum: processing, properties and food applications—A Review. J Food Sci Technol. 2014;51:409–418. doi: 10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgil D, Barak S, Khatkar BS. Optimization of enzymatic hydrolysis of guar gum using response surface methodology. J Food Sci Technol. 2014;51:1600–1605. doi: 10.1007/s13197-012-0678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC, Anderson-Cook CM. Response surface methodology: process and product optimization using designed experiments. New York: Wiley; 2009. [Google Scholar]
- Weng W, Liu W, Lin W. Studies on the optimum models of the dairy product Kou Woan Lao using response surface methodology. Asian Austral J Anim Sci. 2001;14:1470–1476. doi: 10.5713/ajas.2001.1470. [DOI] [Google Scholar]
- Yang M, Li L. Physicochemical, textural and sensory characteristics of probiotic soy yoghurt prepared from germinated soybean. Food Tech Biotech. 2010;48:490–496. [Google Scholar]
- Yoon SJ, Chu DC, Juneja LR. Chemical and physical properties, safety and application of partially hydrolyzed guar gum as a dietary fiber. J Clin Biochem Nutr. 2008;42:1–7. doi: 10.3164/jcbn.2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]