Abstract
Vinegar stands as a highly appreciated fermented food product due to several functional properties and multiple applications. This work focuses on vinegar production from fruit wines derived from fruit concentrates, to attain a food product with nutritional added value. Four fruit vinegars (orange, mango, cherry and banana), were produced and characterized, with total acidities of 5.3 ± 0.3% for orange, 5.6 ± 0.2% for mango, 4.9 ± 0.4% for cherry and 5.4 ± 0.4% for banana. Acetification showed impact on aroma volatiles, mainly related to oxidative reactions. Minor volatiles associated with varietal aroma were identified, monoterpenic alcohols in orange vinegar, esters in banana vinegar, C13-norisoprenoids in cherry vinegar and lactones in mango vinegar, indicating fruit vinegars differentiated sensory quality. Total antioxidant activity analysis by FRAP, revealed fruit vinegars potential to preserve and deliver fruit functional properties. Antioxidant activity of fruit vinegars, expressed as equivalents of Fe2SO4, was of 11.0 ± 1.67 mmol L−1 for orange, 4.8 ± 0.5 mmol L−1 for mango, 18.6 ± 2.33 mmol L−1 for cherry and 3.7 ± 0.3 mmol L−1 for banana. Therefore, fruit vinegars presented antioxidant activity close to the reported for the corresponding fruit, and between 8 and 40 folds higher than the one found in commercial cider vinegar, demonstrating the high functional potential of these novel vinegar products.
Keywords: Vinegar, Fruit, Functional foods, Antioxidant activity, Chemical composition, Acetic fermentation
Introduction
Consumer concern and increasing knowledge about nutritional impact on human health lead to the advance of preventive medicine and nutraceuticals, a novel generation of food products with functional properties (Lobo et al. 2010). Vinegar is widely acknowledged by its functional features possessing antimicrobial properties, antioxidant activity, dietary, antidiabetic and antitumoral effect, as well as preventing cardiovascular diseases (Budak et al. 2014). Also highly acknowledged, fruit represent one of the main sources of nutrients with functional properties, being a source of phytochemicals with antioxidant activity, as well as flavors, colors and aromas. Antioxidants present in fruits have already been correlated with nutritional benefits, due to their ability to scavenge and inhibit free radicals formed during oxidative metabolism with harmful effects for human health (Gülçin 2012). Therefore, there is high interest on developing strategies for the delivery of such nutrients and vinegar poses as a strong candidate for the design of an enhanced functional food. Vinegar is not only an ingredient for food seasoning but also a main ingredient for formulation of beverages. Thus the market for vinegar related products is expected to grow, along with the demand for genuine, high quality fruit vinegar products (Chang et al. 2005).
New and improved products derived from vinegar are now starting to be developed and studied that fruit inclusion has a major role (Cejudo-Bastante et al. 2013). Fruit vinegar designation is also valid for products obtained by mixing juice with vinegar (Chang et al. 2005). However, considering consumer interest on quality and genuine food products, the establishment of vinegars produced solely from fruits is of upmost importance to guarantee a final product with fruit natural properties.
In a recent trend, some works have been reported on fruit vinegars characterization, focusing on specific features of the product including major volatile analysis of persimmon and strawberry vinegars (Ubeda et al. 2011) and antioxidant features of rabbiteye blueberry vinegar pomace (Su and Silva 2006). In an effort to a more global approach, this work presents an overall analysis of process dynamics, chemical composition and antioxidant activity of four novel orange, mango, cherry and banana vinegars, produced from whole fruit concentrates.
Materials and methods
Chemicals
The following chemicals were used for the standards: citric acid monohydrate (99.5%) (Merck), absolute ethanol (99.5%) (Panreac), l(−)-Malic Acid (99%) (Acros Organics), acetic acid glacial (99.7%). For GC-FID the following standards were used: acetaldehyde (≥99.5%), methyl acetate (≥99.9%), 1-propanol (≥99.9%), 2-methyl-1-propanol (≥99.8%), 2-methyl-1-butanol (≥98%), 3-methyl-1-butanol (≥99.8%), 2,3-butanediol, levo (≥99.0%), 2,3-butanediol, meso (≥99.0%) from (Fluka) and ethyl acetate (99.8%), methanol (≥99.8%), diethyl succinate (99.0%) from (Sigma-Aldrich). For GC–MS: 1-octanol (≥99.5%), furfuryl alcohol (≥98%), 1-dodecanol (≥98.5%), isobutyl acetate (≥98.5%), 2-phenylethyl acetate (≥99.0%), fenchol (≥99.0%), borneol (>95.0%), trans-furan linalool oxide and cis-furan linalool oxide (≥97.0%), isobutyric acid (≥99.5%), butyric acid (≥99.5%), hexanoic acid (≥98.0%), decanoic acid (≥98.0%), benzaldehyde (≥99.0%), acetoin (≥97.0%) from Fluka, 3-ethoxy-1-propanol (97%), benzyl alcohol (≥99.0%), ethyl butyrate (99.0%), 3-methylbutyl acetate (≥99.0%), ethyl hexanoate (≥99.9%), Z-3-hexenyl acetate (≥98%), ethyl octanoate (≥99.0%), ethyl 3-hydroxybutyrate (99.0%), ethyl decanoate (≥99.0%), benzyl acetate (≥99.0%), linalool (97%), terpinen-4-ol (≥99.0%), β-citronellol (95%), nerol (97%), geraniol (98%), eugenol (99%), 4-vinylguaiacol (≥98%), 4-vinylphenol (12%), acetovanillone (98%), zingerone (≥96%), 3-methylbutyric acid (99%), 2-methylbutyric acid (98%), octanoic acid (≥99.5%), isovaleric acid (99%) methoxyfuraneol (≥97%), furaneol (≥98%), γ-decalactone (≥98%), 2-methyltetrahydrothiophen-3-one (≥97%), 2-(methylthio)ethanol (99%), methionol (98%), 6-methyl-5-hepten-2-one (99%) from Sigma-Aldrich, isopulegol (>85.0%) from TCI, myrcenol (≥90.0%) from Ventós and α-terpineol (≥98.0%) from Merck. For the FRAP assay the following reagents were used: 2,4,6-tris(2-pyridyl)-s-triazine (≥98%), Iron(III) chloride (>97%) and sodium acetate (≥99%), all from Sigma-Aldrich.
Fruit vinegar production
For the production of fruit vinegars, fruit wines were produced from fruit concentrates in the previously optimized conditions (Coelho et al. 2015). Fruit wines were then centrifuged at 10,000g during 15 min to remove yeast and suspended solids. After ethanol quantification, fruit wines were diluted with sterile water to the desired initial alcoholic strength and inoculated with a natural isolate of acetic bacteria (confirmed as Acetobacter sp.), previously grown in YE medium [1% (m/v) yeast extract and 6% (v/v) ethanol] and collected by centrifugation at 4000 min−1 during 15 min, re-suspended in the diluted fruit wine in a pre-inoculum/diluted fruit wine volumetric ratio of 1:2. Then 100 mL acetic fermentations were conducted in triplicate in Erlenmeyer flasks fitted with cotton stoppers allowing gas exchange, at 30 °C with 200 min−1 orbital agitation. Acetification was monitored by periodical sampling and measurement of total acidity by colorimetric titration with 0.1 mol L−1 NaOH, using 1% phenolphthalein as indicator. Samples were also collected to follow ethanol-acetic acid conversion.
Fruit vinegars characterization
Ethanol and organic acids
Ethanol and organic acids were measured by high performance liquid chromatography using a Varian Metacarb 87H column and H2SO4 5 mmol L−1 mobile phase at 0.7 mL min−1. Organic acids were measured using a Jasco 870-UV detector at 210 nm and ethanol was measured using a Jasco RI-1530 detector. Calibration curves from pure standards were used for quantification.
Antioxidant activity
Antioxidant activity was quantified using Ferric Reducing Antioxidant Power (FRAP) assay. 10 μL of each sample was mixed, in a 96 well microplate, with 290 μL of FRAP reagent. FRAP reagent used in the assay was prepared by mixing a 10 mmol L−1 2,4,6-tris (1-pyridyl)-5-triazine (TPTZ) solution (made with 40 mmol L−1 HCl) with a 20 mmol L−1 FeCl3 solution and 300 mmol L−1 acetate buffer (pH 3.6) in a 1:1:10 volumetric proportion. Samples were incubated at 37 °C during 15 min followed by absorbance measurement at 593 nm. Antioxidant activity was expressed as Fe2SO4 equivalents, using the proper calibration curve.
Major volatile compounds
Major volatile compounds were quantified using a Chrompack CP-9000 gas chromatograph with a split/splitless injector, a flame ionization detector (FID) and a capillary column, coated with CP-Wax 57CB (50 m × 0.25 mm; 0.2 μm film thickness, Chrompack), by direct injection of the samples with 4-nonanol as internal standard. Injector and detector temperatures were 250 °C. Oven temperature was initially held at 60 °C, for 5 min, then programmed to rise from 60 to 220 °C, at 3 °C min−1, and maintained at 220 °C for 10 min. Carrier gas was helium 4× (Praxair) at a flow rate of 1 mL min−1 (125 kPa at the head of the column). 1 μL of sample was injected in split mode (15 mL min−1) for analysis. Quantification was performed using software Star-Chromatography Workstation version 6.41 (Varian) supported by response factors and retention times determined with pure standards. Independent fermentation triplicates were analyzed for determination of experimental deviations.
Minor volatile compounds
Minor volatiles were analyzed by GC–MS after extraction of 8 mL of fruit vinegar with 400 μL of dichloromethane, with 3-octanol as internal standard. A gas chromatograph Varian 3800 with a 1079 injector and an ion-trap mass spectrometer Varian Saturn 2000 was used. 1 μL injections were made in splitless mode (30 s) in a Sapiens-Wax MS column (30 m × 0.15 mm; 0.15 μm film thickness, Teknokroma). Carrier gas was helium 4× (Praxair) at a constant 1.3 mL min−1 flow. Detector was set to electronic impact mode with an ionization energy of 70 eV, a mass acquisition range from 35 to 260 m/z and 610 ms acquisition interval. Oven temperature was initially set to 60 °C for 2 min and then raised from 60 to 234 °C at a rate of 3 °C min−1, raised from 234 to 250 °C at 10 °C min−1 and finally maintained at 250 °C for 10 min. Injector temperature was 250 °C with 30 mL min−1 split flow. Compounds were identified using MS Workstation version 6.9 (Varian) software, by comparing mass spectra and retention indices with those of pure standards. Minor compounds were quantified as 3-octanol equivalents. Independent fermentation triplicates were analyzed for determination of experimental deviations.
Results and discussion
Production of fruit vinegars
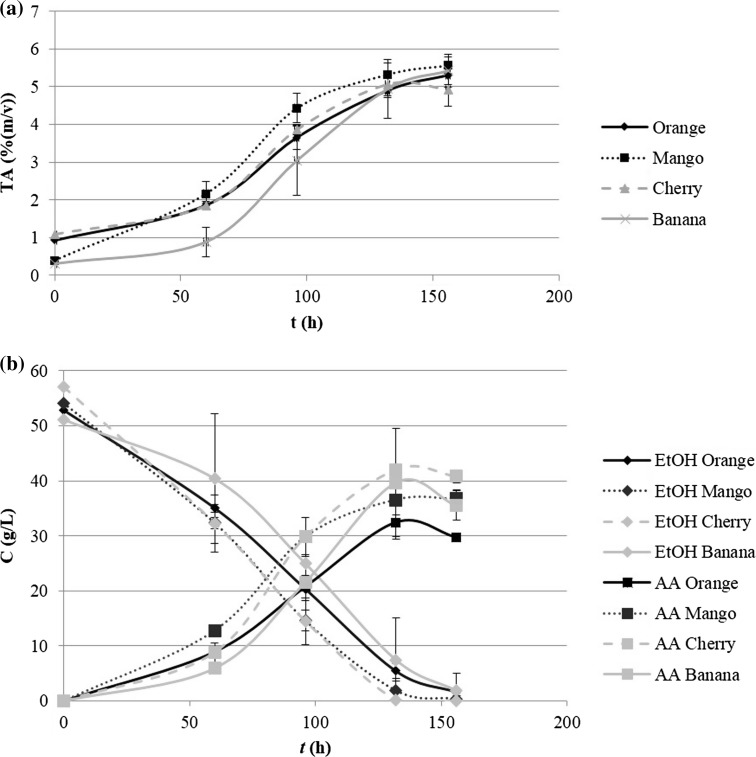
Alcoholic fermentation allowed the production of fruit wines with alcoholic strengths (v/v) of 8.6 ± 0.22%, 11 ± 0.70%, 7.9 ± 0.72%, 11.5 ± 2.08% for orange, mango, cherry and banana respectively, coherent with the previously described (Coelho et al. 2015) and within concentrations feasible for vinegar production. Total acidity profiles, represented in Fig. 1), allowed acetification profiling. As seen, it was possible to produce vinegar from all fruit wines, with total acidities between 5 and 6% (m/v), as required. Initial total acidity was similar for orange and cherry, higher than the one observed for banana and mango, due to fruits natural composition. Orange, cherry and banana acetifications presented a 50 h lag phase whereas mango initiated acetification immediately. All acetifications reached stationary phase between 130 and 150 h of fermentation. Ethanol/acetic acid conversion profiles are presented in Fig. 1). In most cases, a slight decrease of acetic acid concentration at the end of acetification was observed, which can be linked to acetic acid over oxidation due to substrate depletion (Gullo and Giudici 2008). Acetic acid reached maximum concentration between 40 and 50 g L−1. Such values don’t fit the total acidity measured, being complemented by additional organic acids. Citric acid was found in fruit vinegars at 17.5 ± 0.26 g L−1 for orange, 4.9 ± 0.11 g L−1 for mango, 2.4 ± 0.16 g L−1 for cherry and 2.6 ± 0.06 g L−1 for banana vinegar. Malic acid was only found in cherry and banana vinegars in the concentrations of 18.4 ± 0.22 and 2.0 ± 0.05 g L−1 respectively. Thus, fruit vinegars possessed lower content of acetic acid when compared to traditional wine vinegars for the same total acidity, due to the presence of fruit characteristic organic acids. Final ethanol concentration was residual but ethanol-acetic acid conversion efficiency was rather low, at percentages of the theoretical yield of 45 ± 1% in orange, 52 ± 2% in mango, 55 ± 2% in cherry and 55 ± 4% in banana acetifications. This low efficiency can be expected when taking into account the system used for acetification, the method and long fermentation times, leading to ethanol losses by evaporation. Acetic acid yield and productivity can be further improved by alternative methods/setups for acetification, which is out of the scope of the current work.
Fig. 1.
Acetic acid production profiling throughout acetification time (t) measured as a total acidity (TA) and b ethanol-acetic acid conversion. Errors represent standard deviation of fermentation triplicates.
Created using Microsoft Office Professional Plus 2013
Characterization of volatile composition
Major volatile compounds
Nine major volatile compounds were identified by GC-FID in the fruit vinegars, as presented in Table 1. Overall major volatile compound content in fruit vinegars was considerably lower when compared to the reported for the corresponding fruit wines. Apart from the dilution for acetification, major volatile compound losses can also be related to evaporation due to the long acetification time and/or to non-specific oxidations performed by acetic bacteria. Fruit vinegars showed lower acetaldehyde content than fruit wines and in some cases this compound was absent from the analyzed samples. Acetaldehyde is an intermediary of ethanol oxidation to acetic acid which tends to accumulate in low oxygen conditions (Ribéreau-Gayon et al. 2006). Thus, considering ethanol-acetic acid conversion there was no accumulation of this major volatile and its concentration decreased. This decrease was also observed for higher alcohols 3-methyl-1-butanol, 2-methyl-1-butanol and methanol, previously correlated with unspecific oxidation by acetic acid bacteria (Ubeda et al. 2011). Regarding esters, a lower concentration of ethyl acetate was observed in fruit vinegars. Ethyl ester hydrolysis phenomena has been previously correlated with active ethanol consumption during acetic acid bacteria metabolism (Callejón et al. 2009). Methyl acetate concentration increased for orange and mango acetifications, which can be expected taking into account the correlation between methyl ester formation and methanol content in acidic conditions (Morales et al. 2002). Diethyl succinate concentration was higher, which can be a direct result of the strain or acetification conditions used (Callejón et al. 2008). Furthermore, some compounds previously quantified in the fruit wines were not found in the fruit vinegars. Such is the case of 2,3-butanediol in its levo and meso forms, which is believed to have been converted to acetoin during the acetification, identified in the minor compound analysis and coherent with the oxidation–reduction balance reported in previous works (Ribéreau-Gayon et al. 2006).
Table 1.
Volatile composition, with correspondent mean concentration (C) of banana, orange, mango and cherry vinegars and fruit wines*, along with the corresponding sensory descriptors and their reported perception thresholds (PT).
* Adapted from Coelho et al. (2015)
| Banana | Orange | Mango | Cherry | Descriptors | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C/(μg L−1) | C/(μg L−1) | C/(μg L−1) | C/(μg L−1) | PT/(μg L−1) | ||||||
| Wine* | Vinegar | Wine* | Vinegar | Wine* | Vinegar | Wine* | Vinegar | |||
| Minor volatiles | ||||||||||
| Alcohols | ||||||||||
| 3-Ethoxy-1-propanol | 403.8 ± 145.8 | 66 ± 42 | 217.6 ± 56.5 | 103 ± 13.8 | 294.3 ± 75.4 | 84 ± 44 | 68 ± 5.0 | 35 ± 46 | 50,000b [1] | |
| 1-Octanol | – | – | 1052.5 ± 117.6 | – | – | – | – | 10,000 [1]b | Coconut, nuts, oily [2] | |
| Furfuryl alcohol | – | – | – | – | 161.4 ± 43.9 | – | – | – | 15,000 [3]a | Hay, moldy [3] |
| Benzyl alcohol | – | – | 56.1 ± 17.1 | – | 17.4 ± 3.7 | – | 382.0 ± 31.5 | 546 ± 353 | 200,000a [4] | Almonds, bitter [2] |
| 1-Dodecanol | – | – | – | 47 ± 19 | ||||||
| Esters | ||||||||||
| Isobutyl acetate | – | – | – | – | 342.1 ± 46.3 | – | 59.2 ± 1.4 | – | 1605 [5]a | Banana, fruity, sweet [2] |
| Ethyl butyrate | – | 299 ± 42.9 | 121.4 ± 0.6 | – | 364.5 ± 115.3 | – | 52.3 ± 9.7 | – | 20b [1], [6] | Fruity [7], papaya, sweet, butter, apple [2] |
| 3-Methylbutyl acetate | 3762.4 ± 1460.0 | – | 293.0 ± 61.8 | – | 2674.0 ± 879.1 | – | 1034.4 ± 16.1 | – | 30 [6], [1]b | Banana, apple, solvent [2] |
| Ethyl hexanoate | 911.8 ± 400.0 | – | 154.4 ± 45.6 | – | 413.6 ± 99.4 | – | 122.4 ± 8.0 | – | 14 [8]a | Apple, fruity, aniseed, sweet [4] |
| Z-3-hexenyl acetate | – | – | – | – | – | – | 14.5 ± 0.3 | – | – | – |
| Ethyl octanoate | 465.5 ± 160.3 | – | 127.4 ± 35.9 | – | 95.0 ± 13.2 | – | 42.6 ± 7.4 | – | 5 [8]a | Apple, sweet, fruity [2] |
| Ethyl 3-hydroxybutyrate | 45.3 ± 15.2 | 16 ± 7.0 | 9.2 ± 0.2 | – | 319.1 ± 50.0 | 158 ± 57.8 | 4.1 ± 0.6 | – | 20,000a [4] | |
| Ethyl decanoate | 273.4 ± 134.3 | – | 38.9 ± 11.0 | – | 29.4 ± 14.2 | – | 16.6 ± 2.0 | – | 200 [8]a | Fatty acid, fruity, apple, solvent [2] |
| Benzyl acetate | – | – | – | – | – | – | 35.7 ± 3.0 | 14 ± 4.3 | ||
| 2-Phenylethyl acetate | 659.4 ± 203.5 | – | 296.3 ± 81.3 | – | – | 99 ± 7.4 | 98.0 ± 9.3 | – | 250b [6] | Roses, honey, apple, sweet [2] |
| Monoterpenic alcohols | ||||||||||
| Linalool | – | – | 6725.1 ± 1560 | 2541 ± 298.7 | 44.3 ± 16.4 | – | 38.6 ± 5.6 | – | 25.2a [8] | Aniseed, terpene [2], lemon [9] |
| Isopulegol I | – | – | 183.5 ± 39.7 | 23 ± 1.5 | – | – | – | – | ||
| Fenchol | – | – | – | – | 41.7 ± 6.0 | – | – | – | 50 [10]c | Muddy [11] |
| Terpinen-4-ol | – | 12,403.5 ± 3146.1 | 7785 ± 324.2 | 91.3 ± 20.9 | – | 14.4 ± 5.8 | – | |||
| Myrcenol | – | – | – | – | 35.0 ± 2.8 | – | – | – | ||
| Borneol | – | – | – | – | 53.5 ± 15.0 | – | – | – | ||
| α-Terpineol | – | 3682.7 ± 983.9 | 2720 ± 97.47 | 1036.0 ± 275.3 | – | 11.8 ± 3.7 | – | 250a [8] | Pine, terpene [4] | |
| β-Citronellol | – | 306.6 ± 80.7 | 196 ± 7.77 | – | 9.3 ± 1.6 | – | 100b [1] | Citronella [12] | ||
| Nerol | – | 279.9 ± 32.2 | 128 ± 16.5 | – | 7.5 ± 2.1 | – | 400–500c [13] | Lime, floral-hyacinth, roses [4] | ||
| Geraniol | 241.1 ± 63.9 | 13.5 ± 1.3 | ||||||||
| Monoterpenic oxides and diols | ||||||||||
| trans-furan linalool oxide | 92.9 ± 10.7 | |||||||||
| cis-furan linalool oxide | 93.3 ± 20.0 | 70.0 ± 22.4 | ||||||||
| 8-Hydroxy-6,7-dihydrolinalool | 168.1 ± 54.5 | |||||||||
| E-8-hydroxylinalool | 131.7 ± 30.7 | 261.4 ± 99.2 | 11.4 ± 1.1 | |||||||
| Z-8-dihydroxylinalool | – | 139 ± 11.1 | – | 14.3 ± 2.1 | – | |||||
| C13-norisoprenoids | ||||||||||
| 3-Hydroxy-β-damascone | – | – | – | 50.8 ± 4.2 | 44 ± 8.5 | |||||
| 3-Hydroxy-7,8-dihydro-α-ionone | 12.2 ± 1.8 | |||||||||
| 3-Oxo-α-ionol | 30.1 ± 16.7 | – | 506.5 ± 156.1 | 181 ± 25.0 | 40.8 ± 12.2 | – | 664.7 ± 75.9 | 690 ± 155 | ||
| 3-Oxo-7,8-dihydro-α-ionol | 63.1 ± 35.9 | 280.7 ± 84.3 | 154.7 ± 39.6 | 45.0 ± 6.0 | ||||||
| Volatile phenols | ||||||||||
| Eugenol | 8204.6 ± 3027.4 | 1417 ± 189.5 | 236.2 ± 76.1 | 118 ± 5.43 | – | 224.6 ± 39.0 | 271 ± 52.7 | 6 [8]; 15 [3]a | Clove-like [7] [3] | |
| 4-Vinylguaiacol | 188.3 ± 48.7 | 18 ± 9.7 | 2890.2 ± 976.7 | 1631 ± 90.63 | 329.8 ± 90.1 | – | 6.1 ± 0.4 | – | 130 [3]; 1100 [8]a | Phenolic, bitter [2]; pharmaceutic-spicy [11] |
| 4-Vinylphenol | – | – | 636.8 ± 298.3 | 370 ± 26.1 | 23.1 ± 5.0 | – | 6.3 ± 0.9 | – | 180a [3] | Stramonium [3]; pharmaceutic [11] |
| Acetovanillone | 85.9 ± 38.1 | 49.7 ± 21.8 | – | 18.2 ± 2.6 | 1000 [4]a | |||||
| Zingerone | 37.0 ± 15.9 | – | 282.3 ± 90.9 | – | – | – | 12.1 ± 4.4 | 11 ± 3.4 | ||
| Volatile fatty acids | ||||||||||
| Propanoic acid | – | 510 ± 240 | 32.6 ± 9.6 | – | 40.8 ± 15.2 | 260 ± 221 | – | 467 ± 225 | 8100 [14]b | Vinegar [15] |
| Butanoic acid | 408.3 ± 153.6 | 14 ± 5.4 | 77.9 ± 26.8 | 77 ± 7.8 | 687.9 ± 186.9 | 476 ± 192 | 7.0 ± 2.4 | 28 ± 16 | 173 [8]a | Butter, cheesy, sweat [2] |
| 2-Methylpropanoic acid | 539.5 ± 188.6 | 898 ± 222 | – | – | 97.8 ± 18.8 | 559 ± 192 | 21.0 ± 2.6 | 443 ± 166 | 2300 [8]a | Sweaty, bitter, vinegar [2] |
| Isovaleric acid | 5345 ± 1022 | 4349 ± 460.7 | 8552 ± 3350 | 5863 ± 2438 | 33.4 [8]; 8 [16]a | Cheesy, sweaty, old hops [2] | ||||
| 3-Methyl + 2-methylbutyric acids | 727.8 ± 242.2 | 39.2 ± 9.7 | 241.8 ± 63.1 | 30.3 ± 3.3 | ||||||
| Hexanoic acid | 1047.0 ± 389.9 | 323 ± 80 | 971.5 ± 259.4 | 856 ± 46.7 | 731.6 ± 147.5 | 378 ± 63.1 | 193.8 ± 21.7 | – | 420 [8]a | Fatty acid, oily, sweaty [2]; green [12] |
| Octanoic acid | 2090.8 ± 797.0 | 129 ± 15.8 | 1047.1 ± 324.6 | 1660 ± 171.2 | 918.3 ± 219.2 | 344 ± 66.0 | 543.5 ± 56.5 | 338 ± 67.3 | 500 [8]a | Fatty acid, oily, sweaty [2] |
| Decanoic acid | 545.1 ± 174.4 | – | 21.9 ± 5.6 | 97.1 ± 9.7 | ||||||
| Lactones | ||||||||||
| Methoxyfuraneol | 48.1 ± 10.1 | |||||||||
| Furaneol | – | – | 1180.1 ± 281.5 | 383.93 ± 132.67 | – | 37 [16]a | Caramel [7] | |||
| γ-Decalactone | 22.9 ± 3.8 | 1000 [1]b | ||||||||
| Sulfur compounds | ||||||||||
| 2-Methyltetrahydrothiophen-3-one | 19.8 ± 2.3 | |||||||||
| 2-(Methylthio)ethanol | – | – | – | – | 50.5 ± 8.2 | – | – | – | ||
| Methionol | 65.0 ± 20.6 | – | – | – | 510.3 ± 115.9 | 55 ± 81 | 4.1 ± 0.7 | – | 1000 [8]a | Cooked potato-like [7] |
| Carbonyl compounds | ||||||||||
| Acetoin | 11,752 ± 3468.6 | 7062 ± 1619 | 5985 ± 3229 | 3828 ± 1192 | 152,600 [14]; 30,000 [1]b | Fruity, moldy, woody [2] | ||||
| Benzaldehyde | 15 ± 6.4 | – | – | 364.0 ± 48.3 | 28 ± 10 | 5000 [1]b | Almond [14] | |||
| 6-Methyl-5-hepten-2-one | 364.0 ± 48.3 | |||||||||
| Major volatiles | ||||||||||
| Acetaldehyde | 5260 ± 710 | – | 13,300 ± 1380 | 3435 ± 209.7 | 20,800 ± 2510 | – | 7430 ± 1180 | – | 25 [7]c | Fresh, green [7] |
| Methyl acetate | 3380 ± 270 | – | 6840 ± 1050 | 7039 ± 3251 | 6890 ± 1350 | 16,539 ± 1833 | – | – | ||
| Ethyl acetate | 66,100 ± 38,000 | – | 13,300 ± 480 | 6906 ± 2152 | 39,500 ± 2560 | 8522 ± 3155 | 18,400 ± 1040 | – | 7500 [6]b | Solvent, fruity [2] |
| Methanol | 42,400 ± 11,700 | 23,000 ± 4146 | 213,000 ± 40,200 | 41,911 ± 2058 | 109,000 ± 31,400 | 46,013 ± 13,519 | 16,800 ± 6070 | 25,564 ± 4147 | ||
| 1-Propanol | 193,000 ± 50,000 | – | 116,000 ± 3670 | 2865 ± 374.5 | 87,400 ± 9050 | – | 236,000 ± 29,300 | – | ||
| 2-Methyl-1-propanol | 63,700 ± 19,100 | 5657 ± 3874 | 15,300 ± 1410 | 2641 ± 302.4 | 45,000 ± 1340 | 3240 ± 450.1 | 24,500 ± 1650 | – | 550 [7]c | Malty [7] |
| 2-Methyl-1-butanol | 24,900 ± 7260 | 4035 ± 924.3 | 18,700 ± 810 | 3443 ± 473.5 | 43,100 ± 2290 | 6557 ± 2171 | 14,900 ± 1580 | – | 1200 [7]c | Malty [7] |
| 3-Methyl-1-butanol | 100,000 ± 26,300 | 5876 ± 4682 | 69,700 ± 1270 | 3372 ± 675.6 | 164,000 ± 11,500 | 3937 ± 1330 | 120,000 ± 1160 | – | 220 [7]c | Malty [7] |
| 2,3-Butanediol, levo | 365,000 ± 79,500 | – | 208,000 ± 26,000 | – | 245,000 ± 81,800 | – | 305,000 ± 63,400 | – | ||
| 2,3-Butanediol, meso | 119,000 ± 27,300 | – | 65,000 ± 11,700 | – | 93,400 ± 31,800 | – | 87,600 ± 20,700 | – | ||
| Diethyl succinate | 1420 ± 420 | 16,407 ± 2027 | – | 9074 ± 1461 | – | 9097 ± 1226 | – | 11,918 ± 1156 | ||
Errors represent standard deviation of fermentation triplicates
[1] Moreno et al. (2005), [2] Meilgaard (1975), [3] Boidron et al. (1988), [4] Gómez-Míguez et al. (2007), [5] Ferreira et al. (2002), [6] Guth (1997), [7] Czerny et al. (2008), [8] Ferreira et al. (2000), [9] Escudero et al. (2004), [10] La Guerche et al. (2006), [11] Boutou and Chatonnet (2007), [12] Ribéreau-Gayon et al. (2006), [13] Ribéreau-Gayon et al. (1975), [14] Étievant (1991), [15] Siebert et al. (2005), [16] Kotseridis and Baumes (2000)
aThreshold in model solution, bThreshold in hydroalcoholic solution, cThreshold in water, – Not detected
Minor volatile compounds
Fruit vinegars presented distinctive compositions of minor volatile compounds, (Table 1). Volatile fatty acids were found in all four fruit vinegars, which can be expected when taking into account the ability of Acetobacter to oxidize other organic compounds beyond ethanol (Sengun and Karabiyikli 2011). Isovaleric acid was the main volatile fatty acid found in fruit vinegars. Its formation was expected from the metabolism of 3-methyl-1-butanol by acetic acid bacteria (Ubeda et al. 2011). Acetoin was also found in all fruit vinegars at high proportions, which was expected when taking into account the oxidative balance of 2,3-butanediol previously discussed. Highlighting distinguishing features in minor volatile compounds, orange vinegar presented distinctive content of monoterpenic alcohols with descriptors coherent with orange aroma. For instance linalool, α-terpineol and β-citronellol were found above perception thresholds, which correlated with orange and citric descriptors. Despite lacking for minor volatiles typically associated with cherry or red fruits sensory descriptors, cherry vinegar presented distinctive composition of C13-norisoprenoids and benzaldehyde which have been previously correlated with cherry aroma (Coelho et al. 2015). Banana vinegar presented higher content of esters, with ethyl butyrate above the perception threshold, associated with fruity aroma descriptors. Regarding mango, furaneol, a lactone associated with mango sensory descriptors, was found relating with varietal aroma (Kulkarni et al. 2013). Overall minor volatile concentration and diversity in fruit vinegars was lower than in the corresponding fruit wines. Esters, alcohols and monoterpenic alcohols content and diversity were overall lower, and minor volatile fatty acids content was higher, potentially caused by previously discussed phenomena related to Acetobacter metabolism.
Antioxidant activity
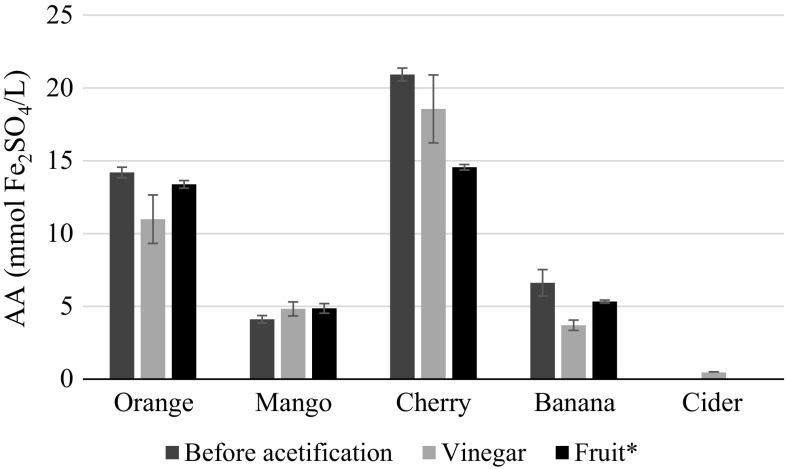
FRAP analysis, presented in Fig. 2 allowed an insight on fruit vinegars antioxidant activity and their comparison with commercial cider vinegar.
Fig. 2.
Antioxidant activity (AA), measured by FRAP, before acetic fermentation and in fruit vinegars, compared to commercial cider vinegar and the one reported for the corresponding fruits. Errors represent standard deviation of fermentation triplicates.
*Adapted from Fu et al. (2011). Created using Microsoft Office Professional Plus 2013
Cherry and orange vinegars demonstrated higher antioxidant activity, consistent with the previously reported for the corresponding fruits (Fu et al. 2011) and fruit wines (Coelho et al. 2015). Moreover, it can be seen that antioxidant activity values found in fruit vinegars were similar to the naturally occurring in the corresponding fruits, which can be due to the utilization of fruit concentrates. Overall, antioxidant activity in the reported fruit vinegars was between 8 and 40 folds higher than traditional cider vinegar. The utilization of alternative fruits in their concentrated form, allowed the production of antioxidant enhanced vinegars, maintaining functionality and adding value to vinegar products.
Conclusion
Four fruit vinegars were produced and characterized from industrial whole fruit concentrates. Acetification of fruit wines was feasible in the studied conditions and fruit vinegars acidity was within the required parameters. Volatile compounds analysis allowed an insight of Acetobacter metabolism on aroma composition. Despite the transformations observed, fruit vinegars presented minor volatiles coherent with varietal aroma. Antioxidant activity was found in all four fruit vinegars at values close to the ones reported for the given fruits, and remarkably higher than the one found in cider vinegar. The already demonstrated importance of antioxidant rich foods in human nutrition, which inhibit the harmful activity of free radicals naturally generated during oxidative metabolism, reinforces the value of vinegar production from antioxidant rich raw materials. Therefore, the combination of fruit antioxidant content with vinegar’s nutritional benefits strengthens the viability of fruit vinegar production from fruit concentrates for the preservation, delivery and enhancement of functional features.
Acknowledgements
Authors would like to acknowledge the financial funding of: FruitVinegarDRINK QREN Project (Ref. 23209), Project “BioInd—Biotechnology and Bioengineering for improved Industrial and Agro-Food processes, REF. NORTE-07-0124-FEDER-000028” Co-funded by the Programa Operacional Regional do Norte (ON.2 – O Novo Norte), QREN, FEDER and the FCT Strategic Project Pest OE/EQB/LA0023/2013. Authors would also like to acknowledge the participation of Mendes Gonçalves S.A. and Frulact S.A. staff, for the active input, which led to the work basis and rationale.
References
- Boidron JN, Chatonnet P, Pons M. Effect of wood on aroma compounds of wine. Connaiss Vigne Vin. 1988;22:275–294. [Google Scholar]
- Boutou S, Chatonnet P. Rapid headspace solid-phase microextraction/gas chromatography/mass spectrometric assay for the quantitative determination of some of the main odorants causing off-flavours in wine. J Chromatogr A. 2007;1141:1–9. doi: 10.1016/j.chroma.2006.11.106. [DOI] [PubMed] [Google Scholar]
- Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. J Food Sci. 2014;79:757–764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- Callejón RM, Tesfaye W, Torija MJ, Mas A, Troncoso AM, Morales ML. HPLC determination of amino acids with AQC derivatization in vinegars along submerged and surface acetifications and its relation to the microbiota. Eur Food Res Technol. 2008;227:93–102. doi: 10.1007/s00217-007-0697-6. [DOI] [Google Scholar]
- Callejón RM, Tesfaye W, Torija MJ, Mas A, Troncoso AM, Morales ML. Volatile compounds in red wine vinegars obtained by submerged and surface acetification in different woods. Food Chem. 2009;113:1252–1259. doi: 10.1016/j.foodchem.2008.08.027. [DOI] [Google Scholar]
- Cejudo-Bastante MJ, Durán E, Castro R, Rodríguez-Dodero MC, Natera R, García-Barroso C. Study of the volatile composition and sensory characteristics of new Sherry vinegar-derived products by maceration with fruits. LWT Food Sci Technol. 2013;50:469–479. doi: 10.1016/j.lwt.2012.08.022. [DOI] [Google Scholar]
- Chang R-C, Lee H-C, Ou AS-M. Investigation of the physicochemical properties of concentrated fruit vinegar. J Food Drug Anal. 2005;13:356–384. [Google Scholar]
- Coelho E, Vilanova M, Genisheva Z, Oliveira JM, Teixeira JA, Domingues L. Systematic approach for the development of fruit wines from industrially processed fruit concentrates, including optimization of fermentation parameters, chemical characterization and sensory evaluation. LWT Food Sci Technol. 2015;62:1043–1052. doi: 10.1016/j.lwt.2015.02.020. [DOI] [Google Scholar]
- Czerny M, Christlbauer M, Christlbauer M, Fischer A, Granvogl M, Hammer M, Hartl C, Hernandez NM, Schieberle P. Re-investigation on odor thresholds of key food aroma compounds and development of an aroma language based on odor qualities of defined aqueous odorant solutions. Eur Food Res Technol. 2008;228:265–273. doi: 10.1007/s00217-008-0931-x. [DOI] [Google Scholar]
- Escudero A, Gogorza B, Melús MA, Ortín N, Cacho J, Ferreira V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J Agric Food Chem. 2004;47:3303–3308. doi: 10.1021/jf9813790. [DOI] [PubMed] [Google Scholar]
- Étievant P. Volatile compounds in foods and beverages. In: Maarse H, editor. Wine. New York: Marcel Dekker; 1991. pp. 483–546. [Google Scholar]
- Ferreira V, López R, Cacho J. Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric. 2000;80:1659–1667. doi: 10.1002/1097-0010(20000901)80:11<1659::AID-JSFA693>3.0.CO;2-6. [DOI] [Google Scholar]
- Ferreira V, Ortin N, Escudero A, López R, Cacho J. Chemical characterization of the aroma of Grenade rosé wines: aroma extract dilution analysis, quantitative determination and sensory reconstitution studies. J Agric Food Chem. 2002;50:4048–4054. doi: 10.1021/jf0115645. [DOI] [PubMed] [Google Scholar]
- Fu L, Xu B-T, Xu X-R, Gan R-Y, Zhang Y, Xia E-Q, Li H-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Gómez-Míguez MJ, Cacho JF, Ferreira V, Vicario IM, Heredia FJ. Volatile components of Zalema white wines. Food Chem. 2007;100:1464–1473. doi: 10.1016/j.foodchem.2005.11.045. [DOI] [Google Scholar]
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Gullo M, Giudici P. Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol. 2008;125:46–53. doi: 10.1016/j.ijfoodmicro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- Guth J. Quantitation and sensory studies of character impact odorants of different white wine varieties. J Agric Food Chem. 1997;45:3027–3032. doi: 10.1021/jf970280a. [DOI] [Google Scholar]
- Kotseridis Y, Baumes R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J Agric Food Chem. 2000;48:400–406. doi: 10.1021/jf990565i. [DOI] [PubMed] [Google Scholar]
- Kulkarni R, Chidley H, Deshpande A, Schmidt A, Pujari K, Giri A, Gershenzon J, Gupta V. An oxidoreductase from ‘Alphonso’ mango catalyzing biosynthesis of furaneol and reduction of reactive carbonyls. SpringerPlus. 2013;2:494. doi: 10.1186/2193-1801-2-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Guerche S, Dauphin B, Pons M, Blancard D, Darriet P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J Agric Food Chem. 2006;54:9193–9200. doi: 10.1021/jf0615294. [DOI] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilgaard M. Flavor chemistry of beer part II: flavor and threshold of 239 aroma volatiles. Tech Q Master Brew Assoc Am. 1975;12:151–168. [Google Scholar]
- Morales ML, Tesfaye W, Garcia-Parrilla MC, Casas JA, Troncoso AM. Evolution of the aroma profile of sherry wine vinegars during an experimental aging in wood. J Agric Food Chem. 2002;50:3173–3178. doi: 10.1021/jf011313w. [DOI] [PubMed] [Google Scholar]
- Moreno J, Zea L, Moyano L, Medina M. Aroma compounds as marker of the changes in sherry wines subjected to biological ageing. Food Control. 2005;16:333–338. doi: 10.1016/j.foodcont.2004.03.013. [DOI] [Google Scholar]
- Ribéreau-Gayon J, Peynaud E, Ribéreau-Gayon P, Sudraud P. Traité d’Oenologie: Sciences et Techniques du Vin. Paris: Dunod; 1975. [Google Scholar]
- Ribéreau-Gayon P, Glories Y, Maujean A, Dubordieu D. Handbook of enology: the chemistry of wine stabilization and treatments. New York: Wiley; 2006. [Google Scholar]
- Sengun IY, Karabiyikli S. Importance of acetic acid bacteria in food industry. Food Control. 2011;22:647–656. doi: 10.1016/j.foodcont.2010.11.008. [DOI] [Google Scholar]
- Siebert T, Smyth HE, Capone DL, Neuwöhner C, Pardon KH, Skouroumounis GK, Herderich MJ, Sefton MA, Pollnitz AP. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal Bioanal Chem. 2005;381:937–947. doi: 10.1007/s00216-004-2992-4. [DOI] [PubMed] [Google Scholar]
- Su M, Silva J. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006;97:447–451. doi: 10.1016/j.foodchem.2005.05.023. [DOI] [Google Scholar]
- Ubeda C, Callejón RM, Hidalgo C, Torija MJ, Mas A, Troncoso AM, Morales ML. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography–mass spectrometry method. Food Res Int. 2011;44:259–268. doi: 10.1016/j.foodres.2010.10.025. [DOI] [Google Scholar]