Abstract
The purpose of this study was to gain insights into the variations in quality characteristics of white-flesh peach fruits. Eighteen cultivars of north of China were investigated. The quality evaluation indicators, including color, physico-chemical and nutritional attributes were measured. Analysis of variance revealed that all the indicators showed significant differences among the cultivars, except edible rate. Principal Component Analysis (PCA) conducted to distinguish the indicators among cultivars, suggested that the most important factors affecting the peach quality could be reduced into nine principal components. Crude fiber content, glucose content, peel-b*, TA, moisture content, pulp-firmness, quinic content, shikimic content and edible rate could be regarded as the characteristic indicators for PC1, PC2, PC3,PC4, PC5, PC6, PC7, PC8 and PC9, respectively. Cluster analysis classified the different cultivars into five main groups on the basis of the measured quality evaluation indicators, and the results were in good accordance with PCA results.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2788-0) contains supplementary material, which is available to authorized users.
Keywords: White-flesh peach, Quality evaluation, Principle component analysis, Cluster analysis, Cultivars
Introduction
Peach (Prunus persica L. Batsch) originated from China,which had an annual production of 12,452,377 tons in 2014 (54.63% of the world’s production) (FAOSTAT). There are more than 700 peach cultivars growing in China. Based on the delicious flavor and attractive appearance, peach has been considered to be one of the most important and popular commodities consumed worldwide (Cano-Salazar et al. 2013). With the increase of the awareness of health, the nutritional value related to vitamin C, pectin and phenolic is also very important, which cannot be ignored in describing fruit quality (Liu et al. 2015). Generally, fruits with high external quality, such as shape, color and size, were more perceptible by consumers. The internal quality, such as texture, sugars, organic acids and nutritional compound contents, is correlated significantly to the taste and aroma of the fruits (Zeballos et al. 2015).
Recently, peach plant breeders foucs on not only increasing fruit size, color and broadening the range of maturity but also selecting peaches with high nutraceuticals and functional traits (Di Vaio et al. 2015). Therefore, sensory indicators are not the only means to evaluate the quality of peaches. The indicators should be suitable to evaluate the nutritional quality and useful to monitor the quality of peach fruits during ripening and storage (Versari et al. 2002). Color is one of the most important sensory quality attributes of fresh fruits. Color can influence consumer’s choice and preferences, characterize the maturity, as well as correlate with other quality attributes, such as sugar and acid content and flavor (Pathare et al. 2013). Soluble sugars and organic acids are important components of fruit taste and have an impact on the overall organoleptic quality of peaches. In most fresh peach fruits, the main soluble sugars are sucrose, fructose and glucose, while, the main organic acids are malic and citric acids. Sorbitol and quinic acid are sometimes also detected at low levels (Etienne et al. 2002). Evidences showed that peach fruits proved to be one of the most suitable sources of macroelements, especially potassium (K), which is known for reducing blood pressure and reduced the morbidity and mortality of cargiovascular disease (Dabbo et al. 2016). Peach fruits contain phenolics and vitamin C that both have been shown to display a high antioxidant potential and have crucial impacts on visual aspect and taste of fruit (Mokrani et al. 2016). Pectin plays an important role in fruit firmness during the softening. Moreover, it serves as structural elements and contributes greatly to the texture of fruit (Zhang et al. 2012). Other indicators such as firmness, weight, soluble solid content (SSC), titratable acid (TA) and moisture content were also reported to evaluate the quality of fresh peaches and distinguish peach cultivars (Valero et al. 2007; Crisosto and Crisosto 2005).
However, different quality factors which were closely related but were relatively independent increase the difficulty of cultivars characterization (Bi et al. 2015). It is necessary to search simple methods to analyze and characterize the cultivars based on the quality parameters. Some studies have focused on the methods of principal component analysis (PCA) and cluster analysis. PCA is one of the most popular multivariate techniques, which can reduce the dimensionality, compress the noise and correlates measurements in a simple informational sub-space of the data set (Goyeneche et al. 2014). Cluster analysis is often employed to classify the variation, which can show the pattern of relationship between individuals of a population (Singh et al. 2013).
The objective of this work was to characterize white-flesh peaches from different cultivars grown in northern China, and to select the cultivars that showed the high quality considering the sensory, physico-chemical, and nutritional profile. Further researches should be focused on the variability of peach components from different cultivars.
Materials and methods
Samples
Based on the recent increase in the production, eighteen white-flesh peaches (Prunus persica L. Batsch) were collected from the Germplasm Repository for peach in Pinggu District of Beijing between June and October, 2012 (Table S1). White-flesh peaches of similar size and maturity were collected. One hundred peaches were picked for each cultivar, and stored at 4 ± 0.5 °C for at most 5 days. The degree of ripening was evaluated based on the color. Briefly, the skin color of the fruit varied from light green to milk white. The flesh was slightly hard and exhibited red ground color, depending on the cultivars.
Analytical determinations
Color
Color values on the skin (peel-color) and flesh (pulp-color) was measured by a reflectance chromameter (D25LT, Huterlab, USA), the three CIE coordinates: L* (lightness), a* (greenness–redness) and b* (blueness–yellowness) (CIE 1976) were determined for each cultivar. Therefore, L*, a*, and b* were applied to describe the color of the skin and the flesh of white-flesh peach.
Organic acid and soluble sugar content
Organic acids were extracted with Milli-Q water (92 mL) and ethanol (8 mL) for 30 min, following the recommendations of Flores et al. (2012) with some modifications. After the extraction, the homogenate was centrifuged and the supernatant was filtered through a 0.45 μm cellulose ester filter before measurement. Contents of soluble sugar (glucose, fructos and sucrose) were determined according to the description by Chinnici et al. (2005) with some modification. Ten grams of samples was ground and extracted by 100 mL Millio-Q water for 3 h, the supernatant filtered through a 0.45 μm cellulose ester filter before measurement. Both organic acids and soluble sugars were separated by using high-performed anion exchange chromatograph (HPAEC) with Dionex system (ICS-3000 Bio-L system, USA).
Content of phenolic compounds
The content of phenolic compounds was determined according to the Folin- Ciocalteu method (2009) with some modification. Five grams of fruit material was ground with 0.5 N HCL in methanol/Milli-Q water (80% v/v). The supernatant was recovered and measured by using a spectrophotometer (UV-1800, Shimadzu, Japan) at 725 nm. The content of phenolic content was expressed in milligrams of catechol equivalents (GAE) per 100 g of fresh weight (FW).
Pectin content
The content of pectin was measured based on the method described by Ibarz et al. (2006). Briefly, 0.5 g of samples and 0.5 mL of distilled water were added followed by the addition of 5 mL the sulphuric/tetraborate solution and placed in a water–ice bath. A 0.1 mL of m-hydroxydiphenyl solution were added. A spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) was used to determine the pectin content (mg g−1) at 520 nm.
Firmness
A texture Analyzer (A-XT2i; SMS; England) with a 6-mm tip was used to evaluate the firmness of peaches (peel-firmness) and peaches without peel (pulp-firmness). The test was carried out on four sides of the peach fruit. The peel-firmness and the pulp-firmness were expressed as the maximum force (N) during the extrusion process and the mean value of force values (N) during the 6–10 s in the extrusion process, respectively.
Mineral contents
According to the method described by Leterme et al. (2006), samples (1 g) were calcined at 450 °C for 6 h. The ash was then weighted and put in solution in 5 mL HNO3/HClO4 (2/1 v/v). The solution was filtered and recovered in a flask, added with pure HNO3, heated and diluted. The minerals, including potassium (K), calcium (Ca), sodium (Na), magnesium (Mg), phosphorus (P), were then analyzed by an atomic absorption spectrophotometer (Perkin-Elmer Analyst 100, Waltham, MA, USA) equipped with Perkin-Elmer intensitron lamps.
Other quality evaluation indicators
Moisture content, titrable acidity content (TA), ascorbic acid content and crude fiber content were determined according to standard AOAC Official Method 934.01 (1999), standard AOAC Official Method 942.15 (1942), AOAC Official Method 985.26 (1985) and AOAC Official Method 992.16 (1992), respectively.
The weight of samples were determined by a digital balance (CPA124S, Sartorius, Germany), and the edible rate was calculated as the ratio between the part suitable for eating and the whole fruit (g 100 g−1). Soluble solids content (SSC) was measured by a refractometer (MASTER-α, ATAGO, Japan) and expressed as °Brix. pH values were measured with a pH-meter (PHS 3C, Leici, China).
Statistical analysis
All the experiments were conducted in triplicate and reported with the mean results and standard deviation. Analysis of variance (ANOVA) was carried out to determine any significant differences in measurement and considered the significant at p < 0.05. Principal component analysis (PCA) was applied to integrate the individual characteristic indicators of white-flesh peaches and reflect relationship between characteristic indicators and the cultivars. Cluster analysis (CA) allowed us to group the different cultivars based on their similarities. ANOVA, PCA and CA were performed by suing SPSS 12.0.1 (SPSS for Windows, Rel. 12.0.1. 2004. Chicago: SPSS Inc).
Result and discussion
Descriptive statistics of white-flesh peaches
Color
Characteristics of color indicators of white-flesh peaches from 18 cultivars were shown in Table 1. There were significant differences among cultivars in terms of L* value, a* value and b* value of peach peel and pulp (p ≤ 0.01). The range of coefficient of variation (CV) varied from 7.89 to 119.30% between the white-flesh peaches, signifying that the degree of variation of the color indicators was different. The biggest CV was observed in pulp-a* (119.30%), and the smallest CV was found in pulp-L* (7.89%).
Table 1.
Variation of color of flesh-white peach from different cultivars
| Indicator | Range | Mean | SD | CV (%) | ANOVA |
|---|---|---|---|---|---|
| Peel-L* | 34.01–52.97 | 44.79 | 4.76 | 10.64 | ** |
| Peel-a* | 8.36–22.9 | 14.30 | 3.49 | 24.42 | ** |
| Peel-b* | 7.76–15.66 | 12.44 | 2.07 | 16.64 | ** |
| Pulp-L* | 52.78–68.87 | 61.17 | 4.80 | 7.89 | ** |
| Pulp-a* | −3.98–9.96 | 3.16 | 3.77 | 119.30 | ** |
| Pulp-b* | 10.26–16.46 | 13.50 | 1.93 | 14.28 | ** |
SD and CV mean standard deviation and coefficient of variation, respectively
** p ≤ 0.01
Fruit color is one of the most important traits for the commercial value of white-flresh peach fruits, and it can be affected by the color phenotype and environment conditions. With respect to the fruit peel, the L* value ranged from 52.97 (Jingyan) to 34.01 (2000-6-9). “Guibao” showed a significantly greater fruit peel a* value (22.90) than other cultivars indicated that “Guibao” had the most intense red color. The red coloration of fruit skin is mainly due to the accumulation of anthocyanins. “Jingyu” had the highest b* value (15.66) and “Wan 9” was the lowest one (7.76). For fruit pulp, “Guibao” had higher L* values than the other cultivars, “Wan 9” showed the most intense red color (9.96) and “Jingyu” the lowest (−3.98), significant differences in b* values were also observed, the highest b* value was 16.46 (2000-6-9), and the lowest value was 10.26 (Wan 9).
Analysis of physical indicators
The CV of physical indicators varied from 1.82 to 35.51% between all the cultivars (Table 2). The lowest CV was observed in edible rate (1.82%), which also showed no significant differences among the 18 cultivars. The correlation coefficient of r = 0.626 (p < 0.01) between the edible rate and fruit weight was observed (Table S2). Fruit weight is an important factor in quality evaluation (Marini and Sowers 1994), which can influence the yield and consumer acceptance, and even the processing industry (Sansavini and Corelli-Grappadelli 1996). The weights of white-flesh peaches varied from 183.59 to 320.67 g, which were subject to the normal distribution. All fruits had high moisture content, ranging from 83.29 g 100 g−1 (Jingyan) to 91.89 g 100 g−1 (Dajiubao). The pH and TA (%) ranged from 5.49 (Jingyan) to 4.09 (Dajiubao) and 0.16 (Qingfeng) to 0.49 (Dajiubao), respectively. SSC and SSC/TA were significantly influenced by different cultivars (p ≤ 0.01), with mean values ranging from 7.93 (Yanhong) to 11.88 °Brix (Jingmi) and from 21.06 (2000-6-9) to 67.01 (Yanghong), respectively. The SSC/TA is the expression of taste equilibrium, fruits with low values are perceived as sour, while high values are equally unpleasant (Montevecchi et al. 2012). Firmness is one of the most important indicators of ripening and can be used to predict the shelf life (Iglesias and Echeverria 2009). Among the white-flesh peach fruits analyzed, peel-firmness and pulp-firmness also showed significant differences among cultivars (Table 2). The peel-firmness and pulp-firmness of white-flesh peach fruits ranged from 27.97 to 53.19 N and from 11.35 and 30.88 N, respectively. There was a linear relationship between the pulp-firmness and peel-firmness (y = 0.7141x − 9.3124, r = 0.93) (Table S2). The differences in physical indicators of white-fresh peach fruits can result in the differences in taste and flavor and even the commercial value.
Table 2.
Variation of physical indicators of flesh-white peach from different cultivars
| Indicator | Range | Mean | SD | CV (%) | ANOVA |
|---|---|---|---|---|---|
| Weight (g) | 183.59–320.67 | 258.90 | 36.54 | 14.11 | ** |
| Edible rate (g 100 g−1) | 91.88–98.93 | 95.82 | 1.74 | 1.82 | n.s. |
| Moisture content (g 100 g−1) | 83.29–91.89 | 87.56 | 2.17 | 2.48 | ** |
| pH | 4.08–5.49 | 4.64 | 0.41 | 8.87 | ** |
| TA (malic acid %) | 0.16–0.49 | 0.29 | 0.09 | 30.46 | ** |
| SSC (°Brix) | 7.93–11.88 | 10.67 | 0.92 | 8.65 | ** |
| SSC/TA | 21.06–67.01 | 40.06 | 12.67 | 31.64 | ** |
| Peel-firmness (N) | 27.97–53.19 | 38.62 | 7.93 | 20.54 | ** |
| Pulp-firmness (N) | 11.35–30.88 | 17.93 | 6.10 | 34.03 | ** |
SD and CV mean standard deviation and coefficient of variation, respectively
Peel-firmness and pulp-firmness mean firmness of peach with peel and firmness of peach without peel, respectively
SSC and TA mean soluble solid content and titratable acid content, respectively
n.s. not significant
** p ≤ 0.01
Analysis of chemical and nutritional indicators
Statistical characteristics of chemical and nutritional indicators of white-flesh peaches from 18 cultivars are shown in Table 3. The CV varied from 13.08% (Mg) to 72.79% (citric acid), signifying that the degree of variation of the chemical and nutritional indicators was different.
Table 3.
Variation of chemical and nutritional indicators of flesh-white peach from different cultivars
| Indicator | Range | Mean | SD | CV (%) | ANOVA |
|---|---|---|---|---|---|
| Pectin (mg g−1) | 1.55–6.10 | 3.33 | 1.18 | 35.51 | ** |
| Total phenolics (mg GAE 100 g−1) | 14.27–51.51 | 28.15 | 10.66 | 37.85 | ** |
| Ascorbic acid (mg 100 g−1) | 17.47–57.38 | 26.03 | 10.64 | 40.86 | ** |
| Crude fiber (g 100 g−1) | 0.34–1.6 | 0.66 | 0.37 | 55.85 | ** |
| P (mg kg−1) | 160.00–345.50 | 222.00 | 44.87 | 20.32 | ** |
| Ca (mg kg−1) | 24.50–74.20 | 40.93 | 13.26 | 32.40 | ** |
| Na (mg kg−1) | 1.87–26.00 | 10.19 | 6.13 | 60.18 | ** |
| K (mg kg−1) | 1320.00–2360.00 | 1883.89 | 338.09 | 17.95 | ** |
| Mg (mg kg−1) | 62.15–97.70 | 74.92 | 9.80 | 13.08 | ** |
| Glucose (mg g−1) | 1.87–8.33 | 5.64 | 1.92 | 34.12 | ** |
| Fructose (mg g−1) | 1.35–12.16 | 8.48 | 3.11 | 36.69 | ** |
| Sucrose (mg g−1) | 35.00–106.91 | 84.71 | 15.25 | 18.00 | ** |
| Quinic acid (mg g−1) | 1.11–5.28 | 2.02 | 0.90 | 44.67 | ** |
| Shikimic acid (mg g−1) | 0.01–0.55 | 0.23 | 0.156 | 68.71 | * |
| Malic acid (mg g−1) | 1.53–8.54 | 3.94 | 1.51 | 38.26 | ** |
| Citric acid (mg g−1) | 0.1–2.67 | 1.03 | 0.75 | 72.79 | ** |
SD and CV mean standard deviation and coefficient of variation, respectively
** p ≤ 0.01; * p ≤ 0.05
Pectin is covalently bound to the cell wall (Posé et al. 2013), which is associated with maturity of fruits (Zhang et al. 2010). Additionally, pectin as the dietary fiber has been shown to lower blood cholesterol levels and reduce the risk for coronary heart disease (Zhang et al. 2015). Pectin content showed significant variation between the investigated cultivars. The highest pectin content was recorded in “Jingyu” (6.10 mg g−1), while the lowest content was observed in “Hanlumi” (1.55 mg g−1). With respect to phenolic and ascorbic acid, the content values varied from 14.27 to 51.51 mg GAE 100 g−1 (Cuiyu” and “Dajiubao”, respectively) and 0.17 mg 100 g−1 (Zaoyu) to 0.57 mg 100 g−1 (Qingfeng), respectively. The significant negative correlation was observed between ascorbic acid content and pulp-a* value (r = −0.677 **), while the significant positive correlation was found between ascorbic acid content and TA value (r = 0.683**). Phenolic compounds represent the main antioxidant phytochemicals present in peaches. Meanwhile, ascorbic acid also contributes to their antioxidant capacity. Therefore, the two indicators are important to evaluating the quality of peach fruits. All fruits were low in crude fiber; “Zaoyu” had the highest crude fiber content (1.60 g 100 g−1) and “Dadong” had the lowest value (0.34 g 100 g−1).
The variation of mineral compositions of white-flesh peaches from different cultivars are shown in Table 3. Among the cultivars analyzed, the concentration of minerals (measured in mg kg−1 fresh weight) was found to range between 160.00–345.50 for P, 24.50–74.20 for Ca, 1.87–26.00 for Na, 1320.00–2360.00 for K and 62.15–97.70 for Mg, which were significantly different among the cultivars. Obviously, peaches were rich in K and had low content of Na, and suitable for people with hypokalemian and iron-deficiency anemi.
White-flesh peaches can provide not only high nutrition but also a pleasant flavor, which is correlated with the content of sugars and acids. The concentrations of soluble sugars and organic acids had an important impact on fruit flavor and quality, which were also applied on cultivars characterization (Ruan et al. 2013). Both soluble sugars and organic acids showed significant differences among cultivars (p ≤ 0.01) (Table 3). Soluble sugars and organic acids are important components of fruit taste, and together with aromas, they have a strong impact on the overall organoleptic quality of fruits (Ma et al. 2015). Moreover, fructose, glucose and sucrose differed significantly in sweetness. Therefore, it is necessary to determine the contents soluble sugars and organic of white-flesh peach, which might be helpful to discriminate the characteristics of cultivars. Sucrose content (mg g−1) varied from 35.00 (Qingfeng) −106.91 (Cuiyu). The content of sucrose could be used as an indicator of maturity and it continuously increased with fruit growth (Wu et al. 2005), which could be confirmed by the significant correlation between sucrose content and pulp-L* (r = 0.733**), pectin (r = −0.630**) (Table S2). For fructose and glucose, the contents (measured in mg g−1) ranged from 1.35 (Qingfeng) to 12.16 (Jingyan) and from 1.87 (Qingfeng) to 8.33 (Yanhong), respectively.
All fruits were low in organic acids because of the high maturity of peaches (Bae et al. 2014) used in this study. The most abundant acid in the 18 white-flesh peach cultivars was malic acid, whose content ranged from 1.53 mg g−1 (Qingfeng) to 8.54 mg g−1 (Jingmi). Citric acid content (measured in mg g−1) ranged from 0.10 (Dadong) to 2.67 (Jingmi), which was consistent with the result reported by Montevecchi et al. (2012). However, the content of quinic acid (from 1.11 to 5.28 mg g−1) was lower than the data obtained by Versari et al. (2002) in Italy, which might be due to the differences in cultivar, soil condition, and microenvironment. Contents of shikimic acid were low and ranged from 0.01 (Huayu) to 0.55 mg g−1 (Guantao14), signifying the differences among the eighteen cultivars (p ≤ 0.05).
Principal Components Analysis (PCA)
PCA was applied to describe the data set and to detect the most important variables for determining the data structure. The Eigenvalues of the first nine principle components (PCs) were greater than 1.0, and explained 88.78% of the total variance. The first nine PCs accounted for 21.87, 13.61, 12.29, 11.32, 7.93, 7.27, 5.92, 4.75 and 3.79%, respectively (data not shown). The PC1 represented the maximum variation of the data set, and it was positively connected with K, Na, weight and pH, while, crude fiber and Ca showed negative values. Crude fiber had the highest weight (−0.924) should be chosen to be the representative indicators for PC1. Proceeding from positive to negative values of PC2, the content of glucose, fructose and sucrose increased, while ascorbic acid content decreased, in which glucose content with the highest weight (0.888) could be viewed as characteristic indicator for PC2. For PC3, the value of peel-a* was positively connected with it, while peel-b* value and peel-L* valued showed the negative connection. The highest weight could be observed in peel-b* (−0.901). The main influencing indicators of PC4 were SSC/TA, citric acid content (positive) and TA value (negative). Meanwhile, the indicators of moisture content and total pehnolics content were negatively correlated with PC5. Firmness (both peel-firmness and pulp-firmness) and P content were positively correlated with PC6. Additionally, quinic acid content, shikimic acid content and edible rate were the main influencing indicators in the PC7, PC8 and PC9, respectively. According to the weight values of indicators for PCs, TA (−0.835), moisture content (−0.908), pulp-firmness (0.902), quinic content (0.945), shikimic content (0.881) and edible rate (0.907) could be regarded as the characteristic indicators for PC4, PC5, PC6, PC7, PC8 and PC9, respectively.
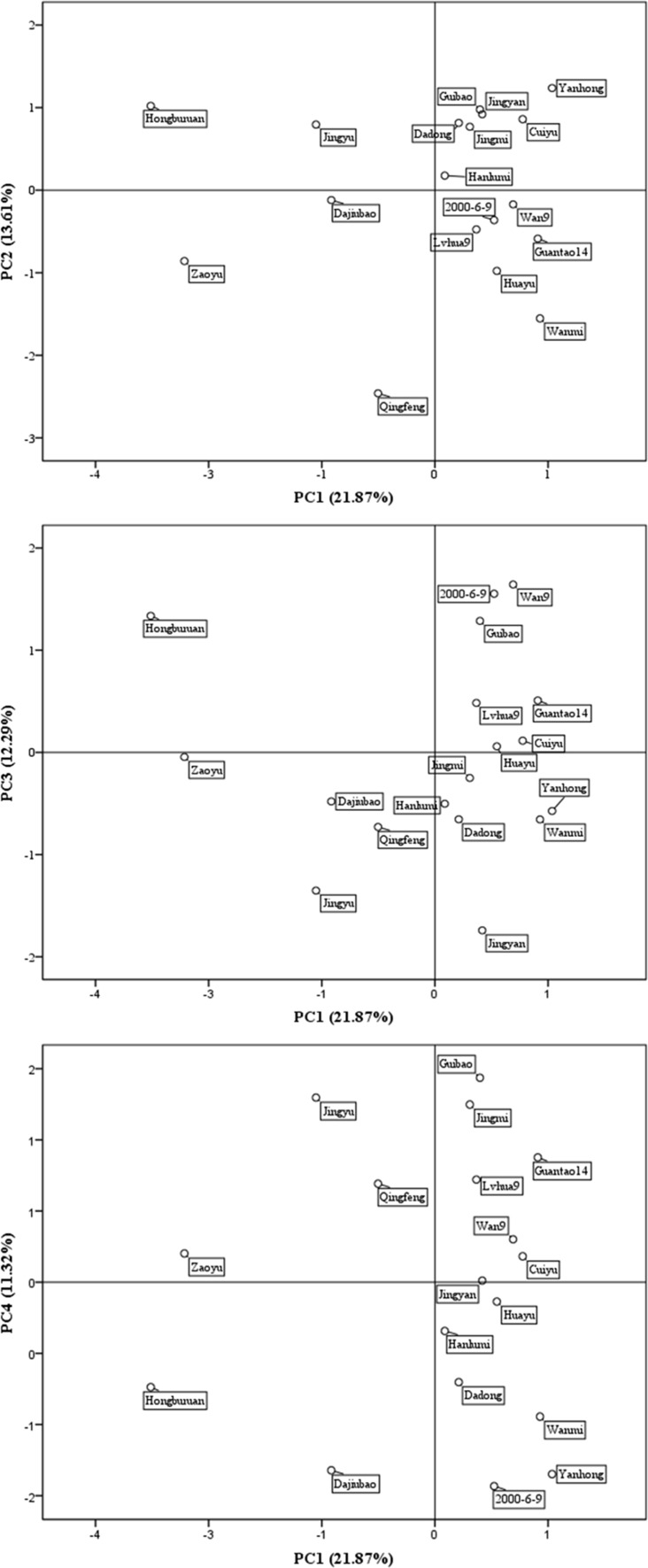
The sample score plots for PC1 versus PC2, PC1 versus PC3, PC1 versus PC4, which could reflect the relationship between quality indicators and cultivars, were shown in Fig. 1, and a number of observations might be made. The plot of the PC1 versus PC2 (Fig. 1a) identified a group containing “Jingmi”, “Jingyu”, “Guibao”, “Cuiyu”, “Dadong”, “Hanlumi” and “Yanhong” samples, which were collocated in the top right-hand quadrant of the PC space, indicating these cultivars were rich in K and soluble sugars. Cultivars located on the positive interval of PC2 presented that fruits had high content of soluble sugar, while cultivars negative interval had high citric acid content. Interestingly, “Qingfeng” located quite some distance away from all of the other cultivars (Fig. 1a), indicating that its composition in terms at least of some of the analytes measured differs significantly from the other cultivars, which might contribute to the high content of crude fiber (0.91 g 100 g−1) and ascorbic acid (57.38 mg 100 g−1). The positive interval of PC3 included about 8 cultivars with high peel-a* value, while, the cultivars with high values of peel-b* and peel-L* located in the negative interval of PC3. “Zaoyu” and “Huayu” almost located on the original line of PC3, indicating their color value close to the average of all the cultivars. However, “Jianyan” with the high value of peel-L* (52.97) and peel-b* (15.35) was located in the bottom right-hand quadrant in Fig. 1b. “Jinmin” and “Guibao” with higher SSC/TA and citric acid content were located on the top of PC4, while “Dajiubao” and “200-6-9” with higher TA content were located on the bottom of PC4 (Fig. 1c). To understand more about the relationship between the different cultivars and the indicators, some other PCs were shown in Figure S1. Therefore, by using the PCA plots, it is possible to suggest reasons for the location of the cultivars on the basis of the quality evaluation indicators.
Fig. 1.
Varimax rotated principal component loadings. a PC loading 1 versus PC loading 2; b PC loading 1 versus PC loading 3; c PC loading 1 versus PC loading 4
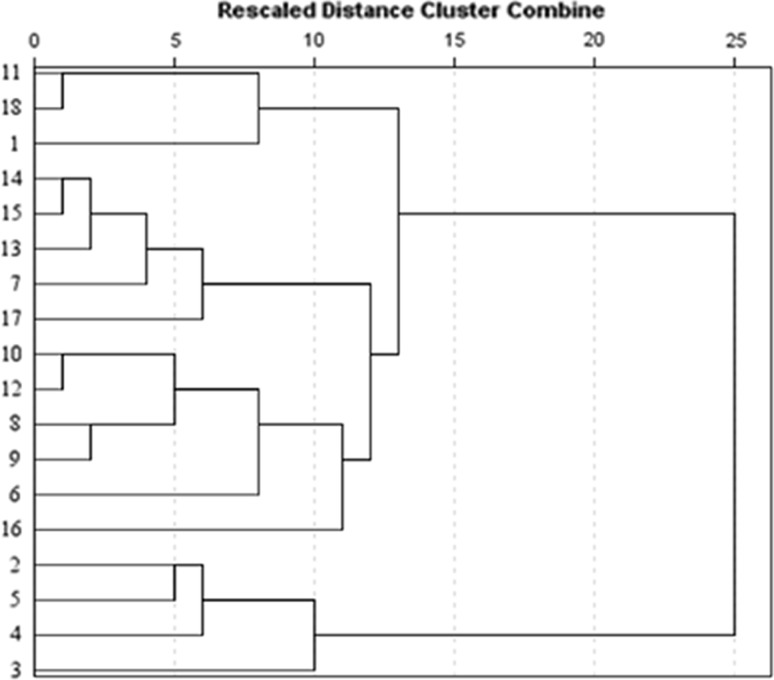
Cluster analysis (CA)
CA was carried out for the different cultivars, grouping samples on the basis of their similarities (Patras et al. 2011). The results obtained by CA were shown as a dendrogram (Fig. 2) in which five well-defined clusters (at the distance of 10) were visible. The first group was clearly discernible which was composed of “Huayu”, “Wanmi” and “Qingfeng”. These cultivars were associated with high value of pulp-L* (PC1 vs. PC8). The second cluster included “Dadong”, “Hanlumi”, “Cuiyu”, “Jingyan” and “Yanhong” because of the high soluble sugar content (Fig. 1a). The third cluster consisted of “Guantao14”, “Wan9”, “Guibao”, “Lvhua9” and “Jingmi”. Cluster five consisted of Zaoyu, Dajiubao, Jingyu and Hongburuan, which were always located on the left-hand quadrant (Fig. 1). However, the fourth cluster included 200-6-9 alone because of the highest firmness (PC1 vs. PC6).
Fig. 2.
Dendrogram of cluster analysis of white-flesh peach cultivars
Conclusion
The quality, including color, physical, chemical and nutritional indicators varied significantly between the different cultivars. The results of the Descriptive statistics of white-flesh peaches could provide basic information for further investigation in different peaches cultivars. For example, “Huayu” and “Wanmi” had the highest fruit weight which may contribute to the production yield. “Jingmi” and “Huayu” may be well appreciated by consumers due to their high soluble solid content. “Dajiubao” and “Qingfeng” were rich in phenolic, which showed anti-oxidation ability, however, it may also cause serious browning during juices production.
Based on PCA analysis, crude fiber content, glucose content, peel-b*, TA, moisture content, pulp-firmness, quinic content, shikimic content and edible rate were chosen for PC1, PC2, PC3,PC4, PC5, PC6, PC7, PC8 and PC9, respectively. “Jingmin”, “Jingyan”, “Guibao”, “Cuiyu”, “Dadongtao”, “Hanlumi” and “Yanhong” were rich in soluble sugars, while, “Lvhua9”, “Guantao14”, “Huayu”, “Wan9”, “2000-6-9” and “Wanmi” were rich in ascorbic acid (PC1 vs. PC2). The method of CA was applied to divide the eighteen cultivars into five clusters. Therefore, PCA and CA can be applied to simplify the quality evaluation process and improve its efficiency, with useful application in selecting peaches with high quality. Information from this study might be useful for promoting white-flesh peach fruits consumption and cultivars characterization.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This work was supported by the Key Technologies R&D Program of China (2012BAD29B03).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2788-0) contains supplementary material, which is available to authorized users.
References
- Bae H, Yun SK, Jun JH, Yoon IK, Nam EY, Kwon JH. Assessment of organic acid and sugar composition in apricot, pulmcot, plum, and peach during fruit development. J Appl Bot Food Qual. 2014;87:24–29. [Google Scholar]
- Bi J, Wang X, Cheng Q, Liu X, Wu X, Wang Q, Lv J. Evaluation indicators of explosion puffing Fuji apple chips quality from different Chinese origins. LWT-Food Sci Technol. 2015;60:1129–1135. doi: 10.1016/j.lwt.2014.10.007. [DOI] [Google Scholar]
- Cano-Salazar J, Lopez ML, Echeverria G. Relationships between the instrumental and sensory characteristics of four peach and nectarine cultivars stored under air and CA atmospheres. Postharvest Biol Technol. 2013;75:58–67. doi: 10.1016/j.postharvbio.2012.08.003. [DOI] [Google Scholar]
- Chinnici F, Spinabelli U, Riponi C, Amati A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J Food Compos Anal. 2005;18:121–130. doi: 10.1016/j.jfca.2004.01.005. [DOI] [Google Scholar]
- CIE (1976) International commission on illumination. Colourimetry: Official recommendation of the international commission on illumination. Paris, France: Bureau Central de la CIE Publication CIE No. (E-1.31)
- Crisosto CH, Crisosto GM. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol Technol. 2005;38:239–246. doi: 10.1016/j.postharvbio.2005.07.007. [DOI] [Google Scholar]
- Dabbo S, Maatallah S, Castagna A, Guizani M, Sghaeir W, Hajlaloui H, Ranieri A. Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Food Hum Nutr. 2016;34(4):1–8. doi: 10.1007/s11130-016-0585-y. [DOI] [PubMed] [Google Scholar]
- Di Vaio C, Marallo N, Graziani G, Ritieni A, Di Matteo A. Evaluation of fruit quality, bioactive compounds and total antioxidant activity of flat peach cultivars. J Sci Food Agric. 2015;95(10):2124–2131. doi: 10.1002/jsfa.6929. [DOI] [PubMed] [Google Scholar]
- Etienne C, Rothan C, Moing A, Plomion C, Bodénès C, Svanella-Dumas L, Cosson P, Pronier V, Monet R, Dirlewanger E. Candiate genes and QTLs for sugar and organic acid content in peach [Prunus persica (L.) Batsch] Theor Appl Genet. 2002;105:145–159. doi: 10.1007/s00122-001-0841-9. [DOI] [PubMed] [Google Scholar]
- Flores P, Hellín P, Fenoll J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012;132:1049–1054. doi: 10.1016/j.foodchem.2011.10.064. [DOI] [Google Scholar]
- Goyeneche R, Roura S, Di Scala K. Principal component and hierarchical cluster analysis to select hurdle technologies for minimal processed radishes. LWT-Food Sci Technol. 2014;57:522–529. doi: 10.1016/j.lwt.2014.02.022. [DOI] [Google Scholar]
- Ibarz A, Pagan A, Tribaldo F, Pagan J. Improvement in the measurement of spectrophotometric data in the m-hydroxydiphenyl pectin determination methods. Food Control. 2006;17:890–893. doi: 10.1016/j.foodcont.2005.06.007. [DOI] [Google Scholar]
- Iglesias I, Echeverria G. Differential effect of cultivar and harvest date on nectarine colour, quality, and consumer acceptance. Sci Hortic. 2009;120:41–50. doi: 10.1016/j.scienta.2008.09.011. [DOI] [Google Scholar]
- Leterme P, Buldgen A, Estrada F, Londoño AM. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006;95:644–652. doi: 10.1016/j.foodchem.2005.02.003. [DOI] [Google Scholar]
- Liu H, Cao J, Jiang W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol Technol. 2015;108:111–118. doi: 10.1016/j.postharvbio.2015.06.012. [DOI] [Google Scholar]
- Ma B, Chen J, Zheng H, Fang T, Ogutu C, Li S, Hang Y, Wu B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015;172:86–91. doi: 10.1016/j.foodchem.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Marini RP, Sowers DL. Peach fruit weight is influenced by crop density and fruiting shoot length but not position on the shoot. J Am Soc Hortic Sci. 1994;119(2):180–184. [Google Scholar]
- Mokrani A, Krisa S, Cluzet S, Da Costa G, Temasamani H, Renouf E, Mérillon JM, Madani K, Mesnil M, Monvoisin A, Richard T. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016;202:212–220. doi: 10.1016/j.foodchem.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Montevecchi G, Simone GV, Masino F, Bignami C, Antonelli A. Physical and chemical characterization of Pescabivona, a Sicilian white flesh peach cultivar [Prunus persica(L.) Batsch] Food Res Int. 2012;45:123–131. doi: 10.1016/j.foodres.2011.10.019. [DOI] [Google Scholar]
- Pathare PB, Opara UL, Al-Said FA. Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol. 2013;6:36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- Patras A, Brunton NP, Downey G, Downey G, Rawson A, Warriner K, Gernigon G. Application of principal component and hierarchical cluster analysis to classify fruits and vegetables commonly consumed in Ireland based on in vitro antioxidant activity. J Food Compos Anal. 2011;24:250–256. doi: 10.1016/j.jfca.2010.09.012. [DOI] [Google Scholar]
- Posé S, Paniagua C, Cifuentes M, Blanco-Portales R, Quesada MA, Mercado JA. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J Exp Bot. 2013;64(12):3803–3815. doi: 10.1093/jxb/ert210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Lee YH, Hong SJ, Yeoung YR. Sugar and organic acid contents of day-neutral and ever-bearing strawberry cultivars in high-elevation for summer and autumn fruit production in Korea. Hortic Environ Biotechnol. 2013;54(2):214–222. doi: 10.1007/s13580-013-0186-8. [DOI] [Google Scholar]
- Sansavini S, Corelli-Grappadelli L. Yield and light efficiency for high quality fruit in apple and peach high density planting. Int Symp Integr Canopy Rootstock Environ Phys Orchard Syst. 1996;451:559–568. [Google Scholar]
- Singh SR, Lal S, Ahmed N, Srivastava KK, Kumar D, Jan N, Amin A, Malik AR. Determination of genetic diversity in strawberry (Fragaria × ananassa) using principal component analysis (PCA) and single linkage cluster analysis (SLCA) Afr J Biotechnol. 2013;12(24):3774–3782. [Google Scholar]
- Valero C, Crisosto CH, Slaughter D. Relationship between nondestructive firmness measurements and commercially important ripening fruit stages for peaches, nectarines and plums. Postharvest Biol Technol. 2007;44:248–253. doi: 10.1016/j.postharvbio.2006.12.014. [DOI] [Google Scholar]
- Versari A, Castellari M, Parpinello GP, Riponi C, Galassi S. Characterization of peach juices obtained from cultivars redhaven, Suncrest and Maria Marta grown in Italy. Food Chem. 2002;76:181–185. doi: 10.1016/S0308-8146(01)00261-8. [DOI] [Google Scholar]
- Wu BH, Quilot B, Genard M, Kervella J, Li SH. Changes in sugar and organic acid concentrations during fruit maturation in peaches, P. davidiana and hybrids as analyzed by principal component analysis. Sci Hortic. 2005;103:429–439. doi: 10.1016/j.scienta.2004.08.003. [DOI] [Google Scholar]
- Zeballos JL, Abidi W, Giménez R, Monforte AJ, Moreno MA, Gogorcena Y. QTL analysis of fruit quality traits in peach [Prunus persica (L.) Batsch] using dense SNP maps. Acta Hortic. 2015;1084:703–710. doi: 10.17660/ActaHortic.2015.1084.94. [DOI] [Google Scholar]
- Zhang LF, Chen FS, Yang HS, Sun XY, Liu H, Gong XZ, Jiang CB, Ding CH. Changes in firmness, pectin content and nanostructure of two crisp peach cultivars after storage. LWT-Food Sci Technol. 2010;43:26–32. doi: 10.1016/j.lwt.2009.06.015. [DOI] [Google Scholar]
- Zhang L, Chen F, Yang H. Effects of temperature and cultivar on nanostructural changes of water-soluble pectin and chelate-soluble pectin in peaches. Carbohydr Polym. 2012;87:816–821. doi: 10.1016/j.carbpol.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xu P, Zhang H. Pectin in cancer therapy: a review. Trends Food Sci Technol. 2015;44:258–271. doi: 10.1016/j.tifs.2015.04.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.