Abstract
Production of pectinases by Aspergillus niger was successfully carried out through solid state fermentation. Orange pomace was used as substrate to produce pectinases using a wild type of A. niger isolated from a rotten orange texture. Some of the important parameters affecting exo- and endo-pectinases activities such as temperature, moisture, C/N ratio were optimized. The results indicated that the produced pectinases exhibited maximum activity in temperature range of 45–55 °C and the maximum enzyme productivity occurred at 70% moisture content and C/N ratio of 10. The enzyme kinetic was studied using Michaelis–Menten and Logistic model and the equation were fitted to experimental data for both exo- and endo-pectinases activities. In evaluation of kinetic model, it was found that Monod model presented perfectly fitted with experimental data. Monod kinetic parameters for exo-pectinase activities were mM, respectively. The Monod kinetic parameters for endo-pectinase activity were and respectively. Finally, the performances of the produced pectinases were evaluated on natural apple juice. It was confirmed that concentration of soluble sugar, clarity and viscosity of the juice and the yield of extracted juice were significantly improved by the enzymatic hydrolysis activity of pectinases.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2829-8) contains supplementary material, which is available to authorized users.
Keywords: Aspergillus niger, Pectinase, Pomace, Solid state fermentation, Kinetic study
Introduction
Enzyme production via solid state fermentation (SSF) has been proposed as an alternative method to submerged fermentation (SmF). SSF processes are interesting for countries with abundant agricultural and industrial solid wastes (Smits 1998). In Asian countries, application of SSF technology in industrial scale for enzyme production is considered as a reliable fermentation process. Nevertheless, there are still lots of challenges for implementation of SSF technology at large scale due to lack of suitable bioreactor with controlling units and accessories for SSF process which guarantee an acceptable production yield (dos Santos et al. 2004).
Pectinases are the most important groups of enzymes that can be obtained in SSF much effectively than SmF (Maldonado and De Saad 1998). Pectinases are responsible for the hydrolysis of pectinic chain found in the plant cell walls. Pectin is a galacturonic acid-rich polymer in primary cell wall and middle lamella of plant cells which plays an important role as a structural component. It is responsible for maintaining the integrity and safety of plant tissues (MacDougall et al. 2001; Palin and Geitmann 2012). Pectin is plentifully found and characterized in several fruits, vegetables and agricultural residues. If pectin is hydrolyzed by pectinases; then, the plant cell is ruptured and the cell components are released.
Pectinases are widely used in fruit juice industry for the improvement of the quality of extracted juice. The enzyme enhances the process yield and accelerates the clarification of extracted juice (Saxena et al. 2014; Shah et al. 2015).
However pectinases is not recommended to be used for orange juice, because pulp precipitation which forms a two phase configuration in orange juice is unattractive and unmarketable. Thus pectinases should be controlled in orange juice by thermal treatment (Baker and Bruemmer 1972).
Pectinase are also used for oil extraction, degumming of plant fibers in pulp and paper industry; and also involved in specialized food applications like extraction of bioactive compounds (Hoondal et al. 2002; Bisht et al. 2015).
Produced pectinases by Aspergillus species are commercially important for beverage industries. Several forms of pectinases have been produced by Aspergillus species (Antier et al. 1993). In this study, A. niger was isolated from orange which seems to be well adapted to pectic substrate. According to literature (Maldonado and De Saad 1998), it is confirmed that pectin induces pectinase production. Therefore, it would be suitable and preferable to use pectin as substrate for pectinase production. Table 1 summarizes pectin percentages of fruits and citruses peels which are the main sources of pectin in the nature. Citruses are plentiful and locally available in fruit markets. Furthermore, citrus peel can be used in its original form without any specific pretreatment in solid state fermentation.
Table 1.
Composition of pectin in different fruits and vegetables
| Fruits | Pectin content (%) | References |
|---|---|---|
| Citrus pomace | 25–30 | Sharma et al. (2013) |
| Apple pomace | 14 | Canteri-Schemin et al. (2005) |
| Lemon | 3–7 | Aina et al. (2012) |
| Melon peel | 2.8 | Raji et al. (2017) |
| Oranges | 0.5–3.5 | Sharma et al. (2013) |
| Carrot | 1.2–1.5 | Sharma et al. (2013) |
| Banana | 0.7–1.2 | Sharma et al. (2013) |
| Cranberry | 0.8–1.1 | Sharma et al. (2013) |
The structure of an orange has different parts including albedo, flavedo and lamella. All these parts contain a percentage of pectin; but albedo is the most important source of pectin in an orange (Liu et al. 2006). Therefore, selection of a species of orange for pectinase production, it should be remembered that high pectinases production yield can be achieved by species which contains more albedo part.
Several effective parameters can influence on the activity of obtained pectinase, the most important parameters are; temperature, pH, moisture content and C/N ratio. These parameters were extensively studied with different species of microorganisms as enzyme producers. In this work, we optimized these parameters and the effect of extracted enzyme at optimum condition was tested on the quality of apple juice. Furthermore, enzyme kinetics was studied to determine the produced enzyme’s affinity toward the substrate.
Materials and methods
Microorganism isolation
A wild type Aspergillus niger was isolated from a rotten orange by streaking technique according to the features specified by Najafpour (2015). The screened and isolated strain (see appendix of supplementary material for cultured strain) was cultured and maintained on a solid medium containing (in g per 100 mL distilled water): pectin, 1; yeast, 0.5; peptone, 0.5; MgSO4, 0.01; K2HPO4, 0.2 and agar, 1.5. The cultured media was monthly re-cultivated.
Preparation of spore suspension
Spore suspension was prepared by washing a 5-day incubated plate agar media with isotonic saline solution (Martínez-Trujillo et al. 2011). After sporulation, the spores were added to media to reach a final concentration of spores g−1 of dry solid (Acuña-Argüelles et al. 1995).
Substrate preparation and optimization of process parameters
Fresh orange pomace with initial moisture content of 30% was obtained from a local factory. Then ammonium sulfate and yeast extract (1:1) were added as nitrogen sources as proposed by Phutela et al. (2005). Five grams of sterilized substrates taken in 250 mL Erlenmeyer flasks was used for each experiment. The experiments were carried out in triplicate and the average values were reported. The parameters selected for optimization were temperature (30, 40, 45, 50, 55, 60, 70 °C), moisture content (60, 65, 70, 75 and 80 wt%) and carbon to nitrogen ratio (C/N) (5, 10, 15, 30). Substrate with desired C/N ratio was prepared by supplementation of the orange pomace (with carbon and nitrogen content of 32 and 1%, respectively) with ammonium sulfate and yeast extract as nitrogen sources.
Enzyme extraction
The produced pectinases were extracted by washing the 96 h incubated culture using acetate buffer solution (pH 5). The extracted solution was filtered and centrifuged at 4000 rpm for 20 min to separate suspended spores, and the supernatant was kept at 3 °C for further analysis (Minjares-Carranco et al. 1997).
Enzyme assay
Endo-pectinase activity was assayed in a reaction mixture containing 1 mL of extract and 18 mL of 2% pectin in acetate buffer solution (0.1 M, pH 4.5) and the mixture was incubated at 45 °C for 30 min. Finally, the reduction in viscosity was determined by Ostwald capillary viscometer (distilled water was used as reference). One unit of endo-pectinase activity was defined as the amount of enzyme required to reduce the viscosity by 50% in 1 min (Acuña-Argüelles et al. 1995).
Exo-pectinase activity was assayed in a reaction mixture containing 0.3 mL of suitably diluted enzyme extract, 0.7 mL acetate buffer solution (0.1 M, pH 4.5) and 1 mL of 0.9% pectin in acetate buffer solution. The mixture was incubated at 45 °C for duration of 30 min. Reducing sugars content of the solution were determined by DNS method (Miller 1959). One exo-pectinase unit was defined as the amount of enzyme that catalyses the formation of one of galacturonic acid per minute (Solis-Pereira et al. 1993).
Determination of kinetic parameters
For the determination of kinetic parameters, enzymatic reactions were carried out at the same conditions as described above (for determination of exo- and endo-pectinases activities) but using a constant amount of enzyme concentration (15 and 5.2, % v/v for exo- and endo-pectinases activities, respectively) and different initial pectin concentrations (0.463, 4.63, 13.9, 23.7 and 46.36%, mM for exo-pectinase activity and 5.426, 54.25, 238, 579.89 and 644.3 mM for endo-pectinase activity). Kinetic parameters for exo- and endo-pectinases activities were expressed by determination of the initial velocity at different concentration of substrate; then, Monod and Logistic equation were fitted to experimental data using MATLAB software (2013).
Evaluation of enzyme activity on apple pulp
Apples were sliced into 5 mm cubic size. Equal amount of chopped apples (50 g) were put into two beakers at the same conditions. One mL of extracted pectinase was added to one beaker and 1 mL deactivated pectinase (incubated and heated in boiling water for duration of 5 min) was added to another beaker. Both beakers were kept at 45 °C for duration of 2 h and the produced juices were compared.
Result and discussion
Effect of temperature on enzyme activity
The exo- and endo-pectinases activities of the extracted enzymes were measured at different temperatures in the range of 30–70 °C. The highest enzyme activities for both exo- and endo-pectinase were obtained in the temperature range of 45–55 °C. These results were in accordance with reported data by Acuña-Argüelles et al. (1995) for exo- and endo-pectinase activities of the pectinases produced by A. niger. In addition, Siddiqui et al. (2012) reported that the obtained polygalacturonase from Rhizomucor pusillus was optimally active at 55 °C.
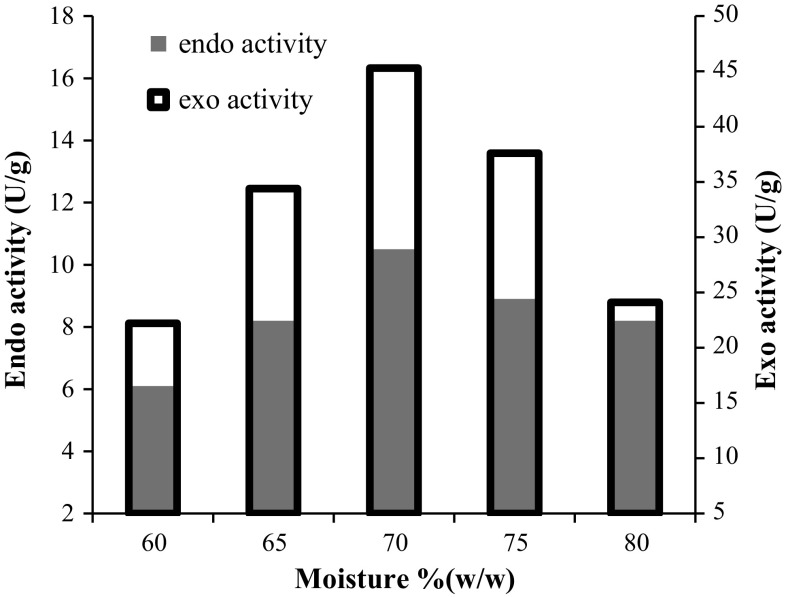
Effect of moisture
Effect of initial moisture content of substrate as an influential parameter in SSF was investigated. The variation of exo- and endo-pectinase activities along with various moisture contents are shown in Fig. 1. As result show, moisture content had profound impact on exo- and endo-pectinase activities. The results indicated that maximum activities were achieved at the initial moisture content of 70% (w/w). Moisture content is related to water activity which is one of the most important parameters for growth of microorganisms and its value increase with raising of water content, however in case of solid state fermentation presence of too much water in the solid may fill the inter particle spaces and as a result oxygen transfer may be limited in the microorganism environment. Although at the moisture content of above 70% the water activity increases; nevertheless, limited availability of oxygen to the microorganism hindered the cell growth and enzyme production. In a similar research, Patil and Dayanand (2006) reported the optimum moisture of 65% for production of pectinases through solid state fermentation of deseeded sunflower head by A. niger, which is nearly in accordance with our obtained results. In addition, they reported the optimum productivity of 34.2 U/g for exo-pectinase activity which is less than exo-pectinase productivity obtained in present work (45 U/g) at optimum moisture content. This is mainly due to the fact that orange pomace has more pectin in its structure than deseeded sunflower head and is more effective in stimulated pectinases production.
Fig. 1.
The effect of moisture content of solid media on production of exo- and endo-pecinases activity by A. niger (at 30 °C)
Effect of C/N ratio
Figure 2 depicts the obtained results which indicate that at C/N ratio of 10, maximum activity of exo- and endo-pectinase was achieved. In a similar research, Kurnar and Reddy (2008) obtained optimum C/N ratio of 5.96 for production of pectinase through solid state fermentation of Manihot utilissima by A. niger. This suggests that, among chemical and physical factors, the C/N ratio is an important parameter that needs to be optimized.
Fig. 2.
The effect of C/N ratio of solid media on production of exo- and endo-pectinase activity by A. niger (at 30 °C)
Enzyme kinetics
Kinetic parameters for exo-pectinase activity were determined by quantifying the initial velocity of galacturonic acid production at different concentration of substrate; then, Monod and Logistic equation were fitted to experimental data. These rate equations are defined, stated as follows:
| 1 |
| 2 |
where s is the concentration of substrate (citrus pectin) in the reaction mixture and , and are constants. Kinetic parameters for exo-pectinase activity were determined by measuring the initial reaction rate of pectinases in production of soluble sugar in solutions contained different concentration of pectin. The kinetic parameters ( and ) for Monod model were respectively. Also the kinetic parameters ( and ) for Logistic model were and respectively. For exo-pectinase, the regression coefficients (R2) for Monod and Logistic models were 0.998 and 0.991, respectively. As can be inferred from the very high regression coefficient Monod model was suitable for describing the kinetics of exo-pectinase activity.
Similarly, kinetic parameters for endo-pectinase activity were determined by quantifying the initial reaction rate of pectinases in reduction of viscosity of different concentration of pectin. The kinetic parameters ( and ) for Monod model were respectively. The kinetic parameter ( and ) for Logistic model were and respectively. For endo-pectinase, the regression coefficients (R2) for Monod and Logistic models were 0.999 and 0.937, respectively. Results indicated that Monod model was very appropriate to describe the kinetics of endo-pectinase activity as implied by the high regression coefficient.
Acuña-Argüelles et al. (1995) reported values for exo- and endo-pectinase activities of the pectinases obtained through solid state fermentation were 2.05 and 270.4 , respectively. Comparing these results with values obtained for exo- and endo pectinases activities in this study were and (6.19 and 194 ), respectively. The obtained results reveal that pectinases obtained in this study have more affinity towards substrate in term of endo-pectinase activity; but it has a bit less affinity towards the substrate in term of exo-pectinase activity. Furthermore, Mutlu et al. (1999) found that the Michaelis–Menten parameters were = 0.0046 pectin % (w v−1) s−1 and = 1.137% w v−1 pectin for endo-pectinase activity of commercial pectinase (Pectinex Ultra SP-L) at 35 °C (R2 = 0.998).
Evaluation of enzyme on the quality of apple juice
Reduction in viscosity of apple juice by enzyme treatment
In fruit juice industries low viscosity of juice accelerates movement of the fluid in the heat exchanger so that pumping energy requirement decreases. In order to decrease the viscosity of crude juice, usually enzymatic treatment is carried out. To quantify, the effect of produced pectinases on juice viscosity, variation in the viscosity of 18 mL of apple juice was considered. The procedure was similar to the method described for endo-pectinase activity measurement. The results showed that the enzymatic treatment of apple juice by the synthesized enzyme reduced the viscosity of apple juice about 7.2%. The reduction in viscosity of various kinds of fruit juices including Pear, Kiwi, Banana and etc., through enzymatic treatment by pectinases were reported in the literature (Sharma et al. 2015).
Enhanced sugar concentration of apple juice
Soluble sugar concentration increases due to action of pectinases on insoluble pectin which is suspended in apple juice. It was quantified by the described method for determination of exo-pectinase activity. The results indicated that suitable amount of enzyme can increase the soluble sugar by 7% (w/v), which implicate that sugar content of apple juice increased by the use of pectinases treatment. The beneficial point is enhancement of natural sugar; which is exactly contributed by its nature without addition of any synthetic sugar.
Pectate formation
Apple pectin is highly methylated. Pectin estrase is a kind of pectinases that strips methoxyl groups from pectin molecules as of the negative charge on pectin chains increases. In the presence of calcium ions calcium pectate is formed which is insoluble and gradually precipitate; finally, the clarified juice is produced. In order to evaluate the effect of extracted pectinase on apple juice, 1 mL of enzyme was mixed with 18 mL of apple juice ansd the mixture was filtered after 1 h (similar procedure was carried out for the deactivated enzymatic extract). The quantitative results revealed that pectate formation increased by 19% (see appendix of supplementary material for additional information).
Improvement of apple juice extraction
Enzymatic activity on natural apple was evaluated according to described method in materials and methods section. The results showed that extraction of juice in the beaker with active enzyme was 13 mL more than another beaker. This improvement in fruit juice extraction is very significant in industrial scale and demonstrates the important role of pectinases in fruit juice industry. The improvement in juice extraction for various kinds of fruits including apricot, pear, plum and etc., through enzymatic treatment by pectinases were reported in the literature (Sharma et al. 2015).
Conclusion
In this study, attempt was made to produce pectinases via SSF with implication of a pectinases producing strain of Aspergillus niger. Furthermore, for maximum enzyme productivities, effective parameters such as temperature, moisture and C/N ratio were defined and the kinetic parameters were determined in optimum condition. In evaluation of kinetic model, it was found that Monod model presented perfectly fitted with experimental data. The Monod kinetic parameters for exo-pectinase activity were mM, respectively. The Monod kinetic parameters for endo-pectinase activity were and respectively. The produced pectinases also exhibited significant activity in natural pectinic environment. It was proved that the concentration of soluble sugar, clarity and viscosity of the juice as well as the juice extraction yield were significantly improved by pectinolytic activity. Therefore, it was concluded that pectinases are useful natural additives for juice industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are gratefully acknowledged Biotechnology Research Lab. Noshirvani University of Technology (Babol, Iran) for the facilities provided to make present work to be successful and useful research.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2829-8) contains supplementary material, which is available to authorized users.
References
- Acuña-Argüelles M, Gutierrez-Rojas M, Viniegra-González G, Favela-Torres E. Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Appl Microbiol Biotechnol. 1995;43:808–814. doi: 10.1007/BF02431912. [DOI] [PubMed] [Google Scholar]
- Aina V, Barau MM, Mamman O, Zakari A, Haruna H, Umar MH, Abba YB. Extraction and characterization of pectin from peels of lemon (citrus limon), grape fruit (citrus paradisi) and sweet orange (citrus sinensis) British J Pharmacol Toxicol. 2012;3(6):259–262. [Google Scholar]
- Antier P, Minjares A, Roussos S, Viniegragonzalez G. New approach for selecting pectinase producing mutants of Aspergillus niger well adapted to solid state fermentation. Biotechnol Adv. 1993;11:429–440. doi: 10.1016/0734-9750(93)90012-C. [DOI] [PubMed] [Google Scholar]
- Baker RA, Bruemmer JH. Pectinase stabilization of orange juice cloud. J Agric Food Chem. 1972;20:1169–1173. doi: 10.1021/jf60184a011. [DOI] [Google Scholar]
- Bisht TS, Sharma SK, Sati RC, Rao VK, Yadav VK, Dixit AK, Sharma AK, Chopra CS. Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. J Food Sci Technol. 2015;52:1543–1551. doi: 10.1007/s13197-013-1155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteri-Schemin MH, Fertonani HCR, Waszczynskyj N, Wosiacki G. Extraction of pectin from apple pomace. Braz Arch Biol Technol. 2005;48:259–266. doi: 10.1590/S1516-89132005000200013. [DOI] [Google Scholar]
- Dos Santos MM, da Rosa AS, Dal’Boit S, Mitchell DA, Krieger N. Thermal denaturation: is solid-state fermentation really a good technology for the production of enzymes? Bioresour Technol. 2004;93:261–268. doi: 10.1016/j.biortech.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Hoondal G, Tiwari R, Tewari R, Dahiya N, Beg Q. Microbial alkaline pectinases and their industrial applications: a review. Appl Microbiol Biotechnol. 2002;59:409–418. doi: 10.1007/s00253-002-1061-1. [DOI] [PubMed] [Google Scholar]
- Kurnar NK, Reddy DSR. Optimization of pectinase production from Manihot utilissima by Aspergillus niger NCIM 548 using statistical experimental design. Res J Microbiol. 2008;3:9–16. doi: 10.3923/jm.2008.9.16. [DOI] [Google Scholar]
- Liu Y, Shi J, Langrish T. Water-based extraction of pectin from flavedo and albedo of orange peels. Chem Eng J. 2006;120:203–209. doi: 10.1016/j.cej.2006.02.015. [DOI] [Google Scholar]
- MacDougall AJ, Brett GM, Morris VJ, Rigby NM, Ridout MJ, Ring SG. The effect of peptide–pectin interactions on the gelation behaviour of a plant cell wall pectin. Carbohydr Res. 2001;335:115–126. doi: 10.1016/S0008-6215(01)00221-X. [DOI] [PubMed] [Google Scholar]
- Maldonado M, De Saad AS. Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Ind Microbiol Biotechnol. 1998;20:34–38. doi: 10.1038/sj.jim.2900470. [DOI] [PubMed] [Google Scholar]
- Martínez-Trujillo A, Arreguín-Rangel L, García-Rivero M, Aguilar-Osorio G. Use of fruit residues for pectinase production by Aspergillus flavipes FP-500 and Aspergillus terreus FP-370. Lett App Microbiol. 2011;53:202–209. doi: 10.1111/j.1472-765X.2011.03096.x. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Minjares-Carranco A, Viniegra-González G, Augur C. Advances in solid state fermentation. Berlin: Springer; 1997. pp. 347–353. [Google Scholar]
- Mutlu M, Sarıoǧlu K, Demir N, Ercan MT, Acar J. The use of commercial pectinase in fruit juice industry. Part I: viscosimetric determination of enzyme activity. J Food Eng. 1999;41:147–150. doi: 10.1016/S0260-8774(99)00088-6. [DOI] [Google Scholar]
- Najafpour GD. Biochemical engineering and biotechnology. 2. Amsterdam: Elsevier; 2015. [Google Scholar]
- Palin R, Geitmann A. The role of pectin in plant morphogenesis. Biosystems. 2012;109:397–402. doi: 10.1016/j.biosystems.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Patil SR, Dayanand A. Production of pectinase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol. 2006;97:2054–2058. doi: 10.1016/j.biortech.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Phutela U, Dhuna V, Sandhu S, Chadha B. Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz J Microbiol. 2005;36:63–69. doi: 10.1590/S1517-83822005000100013. [DOI] [Google Scholar]
- Raji Z, Khodaiyan F, Rezaei K, Kiani H, Hosseini SS. Extraction optimization and physicochemical properties of pectin from melon peel. Int J Biol Macromol. 2017;98:709–716. doi: 10.1016/j.ijbiomac.2017.01.146. [DOI] [PubMed] [Google Scholar]
- Saxena D, Sabikhi L, Chakraborty SK, Singh D. Process optimization for enzyme aided clarification of watermelon juice. J Food Sci Technol. 2014;51:2490–2498. doi: 10.1007/s13197-012-0720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NNAK, Rahman RA, Shamsuddin R, Adzahan NM. Effects of pectinase clarification treatment on phenolic compounds of pummelo (Citrus grandis l. Osbeck) fruit juice. J Food Sci Technol. 2015;52:5057–5065. doi: 10.1007/s13197-014-1554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Shrivastava A, Sharma S, Gupta R, Kuhad RC. Biotechnology for environmental management and resource recovery. Berlin: Springer; 2013. pp. 107–124. [Google Scholar]
- Sharma HP, Patel H, Sugandha Enzymatic extraction and clarification of juice from various fruits—a review. Crit Rev Food Sci Nutr. 2015;57:1215–1227. doi: 10.1080/10408398.2014.977434. [DOI] [PubMed] [Google Scholar]
- Siddiqui MA, Pande V, Arif M. Production, purification, and characterization of polygalacturonase from Rhizomucor pusillus isolated from decomposting orange peels. Enzyme Res. 2012 doi: 10.1155/2012/138634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JP (1998). Solid-state fermentation: modelling fungal growth and activity, Ph.D. thesis, Wageningen University, The Netherlands
- Solis-Pereira S, Favela-Torres E, Viniegra-González G, Gutiérrez-Rojas M. Effects of different carbon sources on the synthesis of pectinase by Aspergillus niger in submerged and solid state fermentations. Appl Microbiol Biotechnol. 1993;39:36–41. doi: 10.1007/BF00166845. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.