Abstract
Vitamin D refers to a group of secosteroid compounds and recognized as the antirachitic vitamin, as it counters rickets, mineral desorption from fully-grown bones (Osteodistrophy), bone, joint disorders, and fragility of bones. On one hand, there is scarcity of vitamin D rich food while on other hand a number of factors negotiate its absorption efficiency in human gastrointestinal tract (GIT). These factors include variations in the physiochemical state of the vitamin D (molecular forms, potency and their physiological linkages), the complexity of food matrix (the amount and type of fatty acids, dietary fibers and presence/absence of vitamin D enhancer and inhibitor), and its interaction of other fat soluble compounds with vitamin D as well as the host-associated factors (age, disease, surgery, obesity, genetic variation etc.). It is hypothesized that the bioavailability of vitamin D in GIT is compromised if there changes within these factors. Present article is intended to review the contribution of these factors anticipated to be influencing vitamin D absorption in GIT.
Keywords: Vitamin D, Ergocalciferol, Cholecalciferol, Vitamin D2, Vitamin D3, Bioavailability, GIT
Introduction
Vitamin D is the generic name for a group of compounds imparting antirachitic functions. Although vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are the two major physiological forms of vitamin D but vitamin D3 is considered as major dietary source for vitamin D. A significant portion of vitamin D (estimated around 80% but varies with sun exposure) is synthesized endogenously in the skin from 7-dehydrocholecalciferol as the function of UV light. While 25-hydroxy cholecalciferol and 1, 25-dihydroxycholecalciferol are source of vitamin D from diet. The liver oil and flesh of fatty fish are the best known source of vitamin D. It can also be obtained from beef liver, dairy product, egg yolk in small quantity. The few sources of plant origin are also reported such as mushrooms and it is present in form of vitamin D2 (ergocalciferol).
Particular population subsets such as neonates, elderly person (not adequately exposed sun radiation), patient (affected by fat metabolism and genetic variations in protein associated to vitamin D uptake in intestine) obligated to get vitamin D from supplements and fortified food in order to meet the recommended daily allowance (RDA) and to retain plasma 25(OH)D level above 30 ng/ml (Wimalawansa 2012). Mechanism of vitamin D absorption and the factors suspected to influence the absorption process have been reviewed by the time. Vitamin D seems to follow league of lipid metabolism in human GIT thus it is assumed that fat does influence the fate of vitamin D. Moreover, vitamin D is diffused in chylomicron which does facilitate its transportation to liver. It seems that the bioavailability of vitamin D is a function of various factors such as absorption, transportation and metabolism.
Bioavailability
It is defined as the proportion of ingested amount (total vitamin in food) which ultimately ends up in the systemic circulation. It is significant to illustrate the major factors limiting the bioavailability of lipophilic bioactive compounds since this information may help in the designing the efficacious excipient foods (Fig. 1).
Fig. 1.
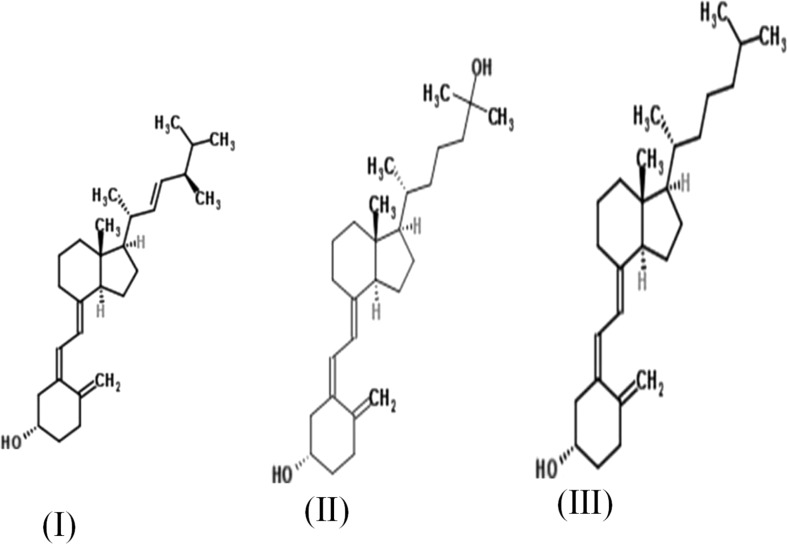
Chemical structures of naturally occuring dietary forms of vitamin D (I) Cholecalciferol, (II) 25(OH) cholecalciferol, and (III) ergocalciferol
Bioavailability of lipophilic agents
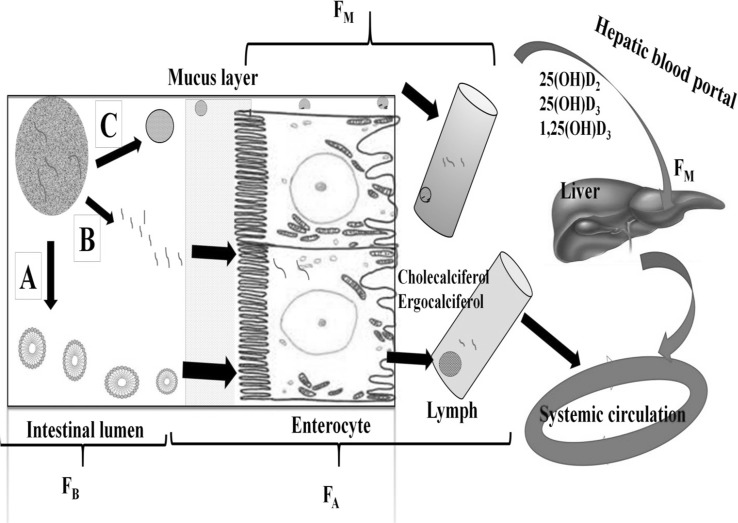
The knowledge about how vitamin D released from food matrix and absorbed in GIT (Fig. 4), can help in designing the efficient delivery system. For any lipophilic agent incorporated in food, the bioavailability (F) could be defined as
where FB: Bioavailability coefficient/proportion of the lipophilic agents which released from food matrix into the gastric juice in GIT, FT: Transport coefficient/ratio of the release lipophilic agent which is transported through the intestinal epithelium, FM: The proportion of the lipophilic agent that reaches the blood circulation without being metabolized, The FM depends on the route taken by lipophilic agent to reach the blood circulation. Before reaching the systemic circulation these lipophilic vitamins transported through the portal vein system and metabolized in liver (Drevon 1991).
Fig. 4.
The bioavailability of lipophilic agent. Where, F B: bioavailability coefficient/proportion of the lipophilic agents which released from food matrix into the gastric juice in GIT, F T: Transport coefficient/ratio of the release lipophilic agent which is transported through the intestinal epithelium, F M: The proportion of the lipophilic agent that reaches the blood circulation without being metabolized
Vitamin D status in GIT
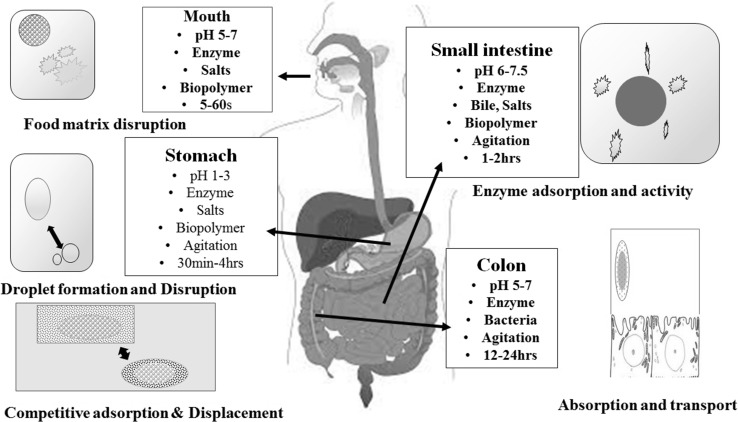
The fate of the vitamin D in GIT is monitored by those factors which have been intimately involved with major lipid (phospholipid and triglycerides) (Tso and Fujimoto 1991). These involve emulsification, dissolution in micelles, diffusion through the stagnant water layer and penetration across enterocytes membranes. The future of vitamin D in GIT seems to be a multistep process including physiochemical as well as enzymatic involvement (Fig. 2). The acidic pH of gastric juice may affect the bioavailability of vitamin D. It is apparent that no data available on the susceptibility of major dietary forms of vitamin D to GIT pH conditions. Further, a hypothesis can be made that protein digestive enzymes (pepsin and trypsin) are also intimately involved in vitamin D absorption as they cleave vitamin D binding proteins present in food and thus facilitate its release. Further, in duodenum digestive enzyme (amylases, lipase and protease) continues the release of vitamin D from food matrix.
Fig. 2.
Schematic diagram of the human digestive system and the various physiochemical and physiological processes involved in digestion and absorption of vitamin D
Mechanism of vitamin D absorption
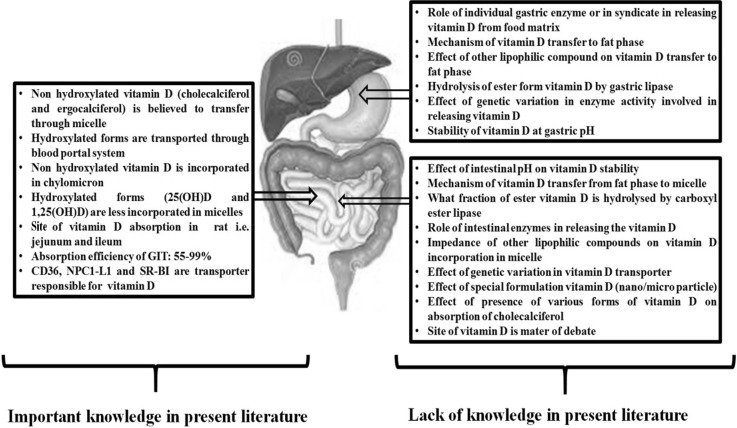
The mechanism of absorption of nonhydroxylated species of vitamin D (i.e. vitamin D2 and vitamin D3) are suspected to be medicated by an unsaturable passive diffusion process. Furthermore, recent studies on human intestinal cell line CaCO2 (Caucasian colon adenocarcinoma) and HEK (Human embryonic kidney) transfected cells clearly demonstrated the intimacy of intestinal cell membrane protein in the absorption of these nonhydroxylated forms at the border side of the enterocytes. Absorption of cholesterol and other lipophilic compounds (tocopherol, carotenoids) is also facilitated by these proteins which are SR-BI (scavenge receptor class B type 1), CD 36 (cluster Determinant 36) and NPC1L1 (Neimann-Pick C1-Like 1).
The observations made from these proteins postulate that there is a mode shift in absorption of vitamin D from protein mediated transport to passive diffusion, depending on the concentration of vitamin D: protein mediated transport at low concentration (dietary concentration of vitamin D) and passive diffusion at high concentration (pharmacological concentration) (Reboul et al. 2011). Further, the difference in vitamin D uptake between jejunum and duodenum clearly indicates the presence of another transporter particularly expressed in the jejunum (Goncalves et al. 2015). Conversely absorption efficiency of hydroxylated forms of vitamin D is significantly higher than that of the nonhydroxylated forms but there is not a single report dealing with uptake mechanism of hydroxylated species of vitamin D. A Figure 3 is depicted to summarize the available literature on vitamin D bioavailability.
Fig. 3.
Summary of important information and lack of information on vitamin D status in GIT
Possible variables influencing absorption of vitamin D
Vitamin D needs to be released from food matrix, in which it is physically or chemically bound, in order to get accessed to enterocytes and get absorbed in human GIT. It is hypothesized that absorption efficiency of vitamin D is controlled by a syndicate of factors.
Status of vitamin D in food
It is believed that the vitamin D, synthesized in skin, alone suffice to meet the daily vitamin D requirement but there are profound evidence that sun exposure does not fulfil RDA of vitamin D which could be due to variation in sun exposure depending on atmospheric components, clothing, skin pigmentation, age, obesity, latitude, season and time of day. This obligates patient to get vitamin D via dietary sources to meet the RDA. The major dietary vitamin D comprised of vitamin D2 and vitamin D3 that could be derived from pharmaceutical supplements, fortified foods or foods from plant or animal origin. Diet and cutaneous in vivo synthesis are being two lateral routes for vitamin D needs to be highly regulated by each in order to avoid the overriding of vitamin D in body. To avoid the overdosing, the 1997 RDA standing committee of the US Department of Agriculture, therefore, have recommended various vitamin D dose depending upon the age i.e. 200 International Unit/day (IU/day) for children, 400 IU/day for adults and 600–800 IU/day for elders (70 years). Further, Food and Nutrition Board declared 2000 IU/day as the upper limit to be safe to consume. Due to lack of representative survey data, it is hard to estimate vitamin D daily intake accurately as the food consumption pattern varies with social economic status. A study carried out to speculate the daily intake of vitamin D via food alone and combining supplements and foods. The daily intake of vitamin D was found to be 153–213.6 IU (African American), 174–278 IU (White American), 158–251.6 IU (Mexican) via food alone while 209.6–330 IU (African American), 258.8–383.2 IU (white American) and 227.6–320.4 IU (Mexican via combining supplements and foods (Calvo et al. 2004). In addition to this, several excellent studies clearly suggest that the daily vitamin D intake via food alone and combining food and supplements, differs with target population and the food consumption pattern (Calvo et al. 2005; Moore et al. 2005).
Molecular forms of vitamin D: vitamin D2, vitamin D3 and 25(OH)D
Vitamin D2, vitamin D3 and hydroxylated vitamin D (25(OH) D3) contribute significantly in dietary vitamin D and in combination referred as total dietary vitamin D. Molecular structure is recalled in Fig. 1. The absorption of vitamin D is facilitated by protein mediated transport depending upon its concentration and the affinity of these transporters which may vary depending upon the molecular forms of vitamin D (Fig. 4).
Bioavailability and potency of various forms of vitamin D
The first report comparing bioavailability of vitamin D2 and vitamin D3 was produced by injecting a high dose of 5000 IU in male subjects and it was observed that they follow same pattern in elevating the serum 25(OH)D level. From this study it can be hypothesized that there is no intestinal discrepancy between absorption efficiency of these two forms of vitamin D. The data produced by further studies followed similar pattern in elevating serum vitamin D level when vitamin D2 and vitamin D3 were administrated through vitamin D fortified orange juice or through vitamin D supplement.
The variation in potency of vitamin D2 and vitamin D3 to enhance the serum vitamin D level could be due to high metabolism and clearance of vitamin D2 than that of vitamin D3 in liver and kidney respectively. In order to avoid the influence of these organs (kidney and liver) intestinal cell line CaCO2 cell were used and similar absorption efficiency was documented for both vitamin D2 and vitamin D3 in these cells.
Hydroxylated versus non hydroxylated vitamin D: Bioavailability and potency
Apart from vitamin D2 and vitamin D3, hydroxylated vitamin D (25(OH) D3) also contributes significantly in dietary vitamin D if food derived from animal origin. Further, the literature reports about the higher bioavailability of vitamin D if it is administrated in hydroxylated form (25(OH)D3) than that of the non hydroxylated forms (vitamin D2 and vitamin D3). However, the relative bioavailability of nonhydroxylated (vitamin D2 and vitamin D3) and hydroxylated (25(OH)D3) forms of vitamin D is well documented. Several reports suggest that the higher bioavailability of hydorxylated vitamin D (25(OH)D3) could be due to greater retention than that of non nonhydroxylated vitamin D (vitamin D2 and vitamin D3).
The difference in their polarity (polar-hydroxylated form and nonpolar-nonhydroxylated form) may contribute to their biological equivalence inconsistency. From these results an assumption can be made that the mechanism absorption and storage for nonhydroxylated and hydroxylated vitamin D significantly varies. The hypothesis was tested on the patient suffering from lipid metabolism by administrating hydroxylated and nonhydroxylated vitamin D and it was found that bioavailability of hydroxylated form (25(OH)D3) is 10 times greater than that of the non hydroxylated forms (vitamin D2 and vitamin D3) vitamin D.
Physiochemical linkage of vitamin D
The patients suffering from vitamin D deficiency are bound to consume vitamin D via pharmaceutical supplements and fortified food. Calcitriol (25-hydroxyvitamin D), calcidiol (1,25- dihydroxyvitamin D), cholecalciferol (previtamin D3), ergocalciferol (previtamin D2) and 7-dehydrocholecalciferol (provitamin D) are the major pharmaceutical supplements available in market with various brand names. Calcitriol (25-hydroxyvitamin D) is considered as most active form while calcidiol (1,25- dihydroxyvitamin D) is an inactive form of vitamin D which helps in storage of vitamin D in tissue. Further, vitamin D2 and vitamin D3 are precursor of the active vitamin D. These various form of vitamin D supplements are supposed to follow the same route in GIT as they share same physiochemical behavior. Nevertheless, there is scarcity of data addressing the relative absorption of these supplements in GIT. These supplements are designed to exhibit high bioavailability of vitamin D. The bioavailability of vitamin D in oil vehicles is greater than that of powder-based vehicles such as starch and cellulose. In contrast to this the bioavailability of vitamin D in lactose capsule is higher than that of oily drop formulation. These supplements are made by changing physical properties (encapsulation of vitamin D) as well as chemical alteration of vitamin (vitamin D ester and salt).
The effect of physical modification such emulsification, encapsulation and nanoparticle of vitamin D is well documented and it was found that these physical modifications make vitamin D more accessible to enterocyte and helpful to penetrate the lipid bilayer thus enhancing the absorption efficiency. But very scant data is available addressing the chemical modification (ester and salt of vitamin D).
Though the absorption of vitamin D is facilitated by protein mediated/passive diffusion transport depending upon the concentration of vitamin D, therefore the variation in these protein transporters and vitamin D receptors may change their structure thus their affinity to vitamin D. But there is no data present addressing this issue. The difference in affinity of VDR Vitamin D Receptor (VDR) between vitamin D2 and vitamin D3 suggests the involvement of some other mechanism of transport which yet to be discovered. Furthermore, there is not a single data produced how concentration of vitamin D3 can affect the affinity of these protein transporters to vitamin D2 and vice versa.
Food matrix and its complexity
Since food is multitude of nutrients and their supportive matrix in which these nutrients are embedded. Before absorption vitamin D need to be released from its matrix and get accessible to enterocytes. An assumption can be made that the bioavailability of vitamin D is affected by amount of ingested food and its complexity.
Amount of ingested food in meal
This factor has not been discussed much in present literature. Recently one study reported the improvement of vitamin D (vitamin D2 and vitamin D3) absorption with the largest meal of day and in consequences about a 50% rise of serum level of 25(OH)D. The data obtained from this study could be result of either high secretion of digestive enzymes (after heavy meal) or some specific food components (Mulligan and Licata 2010). These results were not reproduced when subjects were served vitamin D with and without food (Wagner et al. 2008). More supplementary studies are required to understand the effect of food amount on bioavailability of vitamin D.
Food matrix
Vitamin D needs to be extracted from the food matrix in which it is embedded in order to get bioavailable to enterocytes i.e. need to solubilize in micelles and accessible for absorption. It is essential to determine the bioavailability of food in which vitamin D is delivered. Though several foods (fortified such as bread, milk, meat, orange juice, vegetable oil, other dairy products and naturally rich in vitamin D i.e. meat, fish oil, mushroom etc.) have been used as potential candidate for vitamin D vehicle. But it is remarkable that the bioavailability of these foods is not well reported.
When vitamin D was supplied through meat, its bioavailability was estimated to be 60% as compared to vitamin D supplement. The vitamin D2 from UV radiated mushroom was also tested. The reports on two mushrooms (Lentinula edoes and Agaricus biosporus) suggest the high bioavailability of its vitamin D (Jasinghe et al. 2005; Koyyalamudi et al. 2009) but their bioavailability were not compared with any other food matrix. Furthermore, the 25(OH)D serum level was higher in group 1 and group 2 when 27 subjects, randomized in 3 groups each having 9 persons, were assigned to receive no supplementation (group 3), 14 μg vitamin D2/day (group 2) through pharmaceutical formulation and mushroom containing 14 μg vitamin D2/day (group 1) in lunch for 3 weeks, excluding Saturdays and Sundays (Outila et al. 1999). These results were further supported by a five week single blinded, randomized, placebo controlled study in 26 healthy Caucasian adults with 25(OH)D serum level below 20 ng/ml (Urbain et al. 2011). Further the trials, conducted by serving capsules containing 2000 IU vitamin D2, 2000 IU vitamin D3 or 2000 IU mushroom vitamin D2 (dried white extract of button mushroom or Monterey mushrooms), demonstrate that the mushrooms containing vitamin D2 have similar biological equivalence as supplemental vitamin D2 and vitamin D3 (Keegan et al. 2013).
Results of clinical study demonstrate no significant difference in rise of total 25(OH)D serum levels in healthy subjects who were served orange juice (fortified with 1000 IU of vitamin D2 or 1000 IU of vitamin D3) as compared to ingesting a supplement containing either 1000 IU of vitamin D2 or 1000 IUs of vitamin D3 (Biancuzzo et al. 2010). The high bioavailability of vitamin D in mushroom was confirmed by several studies (Calvo et al. 2013; Ko et al. 2008). It is remarkable that the difference in food matrix does not impose any variation of bioavailability of vitamin D and it was found as effective as vitamin D3 supplements in increasing the 25(OH)D serum level (Natri et al. 2006). These results were supported by another study when vitamin D2 rich yeast were baked in bread (Hohman et al. 2011).
Dietary lipids
In general lipids are most widely used vitamin D delivery medium and considered crucial for fat soluble micronutrients. It facilitates the absorption lipophilic components of food through a multisequence process. First lipids mediate diffusion of these fat soluble food components from food matrix as it behaves as hydrophobic phase within which fat soluble nutrients get solubilized. Then these lipids stimulate the secretion of bile juice resulting into micelle formation. In next step the digestive enzymes catalyze the lipids and release fatty acid, monoglycerides and phospholipid which again generate more micelles available for lipophilic nutrients to solubilize (Hofmann 1963). At last these lipids mediate lipophilic nutrients out the enterocyte avoiding the accumulation of vitamin D in enterocytes thus increasing the vitamin D absorption in GIT. These steps depend on the amount of fat (triglycerides), type of triglycerides, amount of phospholipids, types of phospholipids etc.
Amount of lipids (triglycerides)
This factor is believed to be important because it is hypothesized that vitamin D absorption is facilitated by lipid. Several studies are conducted to verify this hypothesis (Johnson et al. 2005; Weber 1980). In contrast, the absorption rate of vitamin D was found to be decreasing when rats were served with 2.5 mM fatty acid differing in chain length and degree of saturation (Hollander et al. 1978). When the bioavailability of vitamin D was evaluated in two beverages (orange and milk), it was noticed that the lipid content of milk does not significantly affect the bioavailability of vitamin D (Tangpricha et al. 2003). Similar results were obtained in the case of two vitamin D fortified foods (cheddar cheese with 33% fat and low fat cheese 7%) (Wagner et al. 2008). These results were reproduced by two multivitamin supplements (one supplement with vitamin D in tablet and other vitamin D in fish oil) (Holvik et al. 2007). Recently a team of researchers has also disbanded this hypothesis when they observed, the consumption of vitamin D with 2 g fish oil per week did not increase vitamin D absorption (Korkor and Bretzmann 2009). Thus reports available till date clearly neglect the hypothesis that high amount of fat in meal improves the vitamin D bioavailability.
Type of fatty acids
The first report on this factor came from Hollander’s study when the decrease in vitamin D uptake was registered by addition of fatty acids of different chain length and degree of saturation i.e. butyric acid, octanoic acid, oleic acid and linoleic acids (Hollander et al. 1978). In this study the oleic and linoleic acids exhibited greater inhibition of vitamin D absorption as compared to octanoic acid. Authors propose that unlike medium chain fatty acids (do not integrated in micelles), long chain fatty acids impair the vitamin D absorption by increasing the micellar size hence decelerating their diffusion towards the enterocytes. However this result was not reproduced at pharmacological dose of vitamin D when it was ingested with either long chain triacylglycerols (peanut oil) or medium chain triacylglycerol. In contrast to this, the improvement in the serum level of vitamin D3 was registered when it was served in peanut oil (long chain triglycerides) than when it was served in medium chain triglycerides (Holmberg et al. 1990). More clear observations were made in experimental trials conducted to evaluate the bioavailability of vitamin D in monounsaturated and polyunsaturated fatty acids. It was found that monosaturated fatty acid rich diet may increase the efficacy of vitamin D supplements than that of polyunsaturated fatty acids (Niramitmahapanya et al. 2011).
In summary, literature suggests that the species of fatty acids can affect the bioavailability of vitamin D. Nevertheless more research is needed to draw firm conclusion on bioavailability of vitamin D.
Dietary fibers
Dietary fibers have been assumed a key player in deciding the fate of vitamin D in GIT. It affects the bioavailability of vitamin D in following ways:
Impairs the micelle formation
Affects triacylglycerol lipolysis and emulsification of fat soluble food component
Affects the release of lipophilic food nutrient from fat droplet (oil phase)
Increases the viscosity of chyme hence confines the diffusion of lipophilic food nutrients containing micelles to enterocytes
High fiber intake was suspected the reason for the reduced bioavailability of vitamin D for higher prevalence of rickets and osteomalacia in Asian immigrant’s population (Compston et al. 1981). This assumption was supported by a study on relative disappearance of radiolabelled 25(OH)D3 in healthy volunteers served with either higher fiber diet (20 g/day) or normal diet. Result reveals that the mean serum half-life of 3H-25(OH)D3 in the normal diet group was longer (27.5 ± 2.1 day) than that of high fiber diet group (19.2 ± 1.7 day). High clearance of 3H-25(OH)D3 in high fiber diet group could be due to interference of the fiber metabolite in enterohepatic circulation i.e. binding of 3H-25(OH)D3 to dietary fibers.
In contrast, the serum levels of 25(OH)D in two groups (consuming vitamin D fortified low fiber wheat bread (3 g/100 g) and high fiber rye bread (12 g/100 g)) were not significantly different from each other. Conversely, these results could be refuted as volunteers in these groups were allowed to consume other breads. Due to very scant data available on uptake of vitamin D in presence of fibers, it could be too early to conclude the role of fibers. Likewise more dedicated studies are required to understand the effect of fibers on vitamin D bioavailability.
Nutrient status in the host
It can be hypothesized that absorption of vitamin D in GIT is also controlled by nutrient status of host.
Status of vitamin D in the host
The complete vitamin D status of host is comprised of both food derived as well as cutaneous synthesized vitamin D. However, it is very difficult to establish the correlation between the contribution of orally ingested and cutaneous synthesized vitamin D. Due to lipophilic nature vitamin D stored in adipose tissue and release at its need. High intake as well as high de novo synthesis of vitamin D could be toxic, therefore, it can be hypothesized that high intake of oral vitamin D and excessive radiation with UV may cause hypervitaminosis D so there will be feedback inhibition between absorption of orally ingested vitamin D and cutaneous synthesis. Nevertheless, there is not a single study performed to investigate of impact of absorption of vitamin D on cutaneous synthesized vitamin D. The main problem in establishing correlation between dietary and cutaneous synthesized vitamin D is the variation in the surface area of skin exposed and in the frequency and duration of UV exposure.
Interaction with micronutrients
Vitamin E and K follow common pathway used in vitamin D uptake so they may impose competitions for vitamin D absorption in intestine. This assumption was verified by a study on CaCO2 cell line which confirms the role of vitamin E in impairing the vitamin D absorption (reduced 15% at medium concentration of vitamin E and 17% at high concentration of vitamin E) absorption in intestine (Goncalves et al. 2015). Phytosterols, plant sterols traditionally, are applied as functional ingredients to reduce cholesterol absorption in GIT. As vitamin D possesses a steroid structure and parades common absorption pathways as cholesterol, we hypothesized that phytosterols could also hamper vitamin D3 uptake. This assumption was confirmed by an in vitro study on CaCO2 intestinal cell where phytosterol was found to be potential candidate to impair the vitamin D3 absorption by 16–36% (depending on micellar composition) in these cell line (Goncalves et al. 2011).
Concentration of vitamin A was also found to be antagonist to vitamin D absorption (Aburto and Britton 1998). In addition, vitamin A also discriminates dietary D3 (coming from food) and endogenous D3 (synthesized in skin by action of sunlight) in their utilization i.e. negatively affect the utilization of dietary vitamin D3, but not the endogenous vitamin D3 (Johansson and Melhus 2001). Recently a research demonstrated that high concentration of vitamin A reduce bioavailability of vitamin D by 30%. However the mechanisms, how vitamin A hampers the vitamin D absorption, does remain unknown and requires further investigation.
Enhancers and inhibitors for vitamin D absorption
Literature reports about various agents which can stimulate or impair the vitamin D absorption in GIT. These agents could be present in food naturally or could be added to improve absorption efficiency of vitamin D. Data from available literature is presented here.
Inhibitors of fat absorption
Person suffering from obesity consumes several antiobesity drugs and fat substitutes to reduce the fat quantity. These drugs and fat substitutes reduce the absorption of triglycerides. Since vitamin D follows the similar league of triglyceride in GIT lumen it can be assumed that these antiobesity drugs could impede vitamin D bioavailability leading to reduction in absorption. The absorption of vitamin D was found to be impaired when vitamin D was served with a fat substitute (olestra: sucrose polyester) was ingested in 102 healthy males and females (James et al. 1997; Schlagheck et al. 1997). The serum level of vitamin D in African-American and Caucasian adolescents was significantly reduced after 1 month supplementation of 400 IU vitamin D with orlistat (Tetrahydrolipstatin: inhibitors of gastric and pancreatic lipases) 120 mg 3 times/day (McDuffie et al. 2002). Furthermore, the cholesterol derivatives from plant origin, used as reduce the cholesterol absorption, may affect the bioavailability of vitamin D. This assumption was confirmed by various studies. The decrease in vitamin D level both in serum as well as in liver was registered in rats when they assigned stanol ester for 13 weeks (Turnbull et al. 1999). Even these results were reproduced for various phytosterols in mice and in vitro and found that phytocholesterols do hinder the micelles formation and its diffusion in enterocytes thus reducing the bioavailability. Though some recent findings of clinical trials have disowned this hypothesis by negating the effect of phytosterol on bioavailability of vitamin D (Gylling et al. 2010; Gylling and Miettinen 1999; Gylling et al. 1999; Hendriks et al. 2003; Korpela et al. 2006; Nguyen et al. 1999). But these conclusions could be refuted as the evaluation was made on the basis of the serum level of 25(OH)D which could be altered by endogenous vitamin D synthesis depending on the several factors i.e. sun exposure and season. Furthermore this hypothesis was again supported by results of two clinical trials in which the serum level of 25(OH)D was observed significantly different in subjects, who assigned plant sterol ester enriched spread. Although these fat reducing agents limit the fat absorption hence hamper the absorption of vitamin D (James et al. 1997; McDuffie et al. 2002) but the exact amount of lipid needed for maximal absorption of vitamin D has not been optimized.
Enhancers of vitamin D
It is thought that the vitamin D delivery in specialized formulation (encapsulated in micro/nano particles, micellar/liposomal form, embedded in β-cyclodextrin and some proteins β-lactoglobulin) may enhance the bioavailability of vitamin D in GIT. Their ability to improve the uptake of vitamin D was tested in various studies. Higher absorption rate of vitamin D in children affected with severe chronic cholestasis was registered when it was administered with tocopherol succinate polyethylene glycol 1000 (TPGS) (Argao et al. 1992). Similar observation was made when vitamin D was served with β-cyclodextrin in rat (Szejtli et al. 1983). It is hypothesized that encapsulated vitamin D remain embedded within non digested nano/microparticles, rather being released. These micro/nanoparticles may transport paracelluarly to the blood portal through tight junction, thus bypassing the liver. This may result in the greater bioavailability for micro/nano encapsulated vitamin D than that of its fortified food and supplement. Limited literature is available on this factor thus it will too early to conclude about the effect of these formulations on the bioavailability of vitamin D and this assumption should be varied by extensive clinical trials.
Physiochemical interaction with GIT secretions
It is assumed that the absorption of vitamin D in GIT remains maximal within a range of salt ionic strength and pH beyond which the vitamin D uptake could be affected. This assumption was addressed in various studies. It was observed that the uptake of vitamin D3 is affected with variation of salt concentration within physiological concentration. For example the progressive reduction in vitamin D3 absorption was noticed as the sodium taurocholate salt concentration was increased above the 5 mM (10 or 15 mM) n (Hollander et al. 1978).
High concentration may facilitate the conversion of monomeric vitamin D into its micellar form thus enhances the solubility of vitamin D3. Since lipophilic compounds such as vitamin D3 have greater penetration power in enterocyte’s membrane in monomer form than that of micellar particles (Simmonds 1974), The high salt concentration may cause the decrease the vitamin D absorption (Simmonds 1974)
The variation in the hydrogen ion concentrations (pH 5.3–8.3) in the perfusate decreases the negative charge of both the micelle and the luminal cell membrane, therefore, reduces the repulsion between the micelles and the cell membrane thus increasing the absorption of vitamin D (Hollander et al. 1978).
Host associated factors
Literature clearly suggests the involvement of various host associated factors which could be suspected players in deciding the bioavailability of vitamin D. Thus several studies have been conducted to optimize recommended dietary allowance (RDA) as the functions of these factors (age and disease obesity).
Age of host
Physiological changes in body function have been witnessed with aging. It is assumed that the age stimulated physiological (age associated GIT functions) changes may directly or indirectly influence vitamin D bioavailability. Aging associated variations in lipoprotein metabolism was suspected for reduction of absorption and postprandial transport of vitamin E (Borel et al. 1997). Several studies have conducted to find out the reason for low vitamin D status in elderly people than that of young adults (Clemens et al. 1986; Ikuma et al. 1996; Russell 1992; Vellas et al. 1991). The first report on this factor was made in 20 elderly women, who had low serum [3H]cholecalciferol than that of younger females. This was explained that GIT of elderly women was less efficient as compared to younger ones (Barragry et al. 1978). Nevertheless, this result was not reproduced in mice study (Hollander and Tarnawski 1984). Conclusive statements regarding changes in serum 25OHD levels in elderly than in young adults could be due to low endogenous vitamin D synthesis in skin, lower sunlight exposure and low dietary intake.
Obesity
Obesity is generally negatively correlated to vitamin D deficiency. This was supported by Liel et al. (1988) work in which they observed improved absorption and higher clearance of vitamin D by obese subjects than that of normal weights. Conversely vitamin D is deposited in adipose tissue and does not released when required (Wortsman et al. 2000) resulting into high supplementation of vitamin D. This was confirmed by a study on elderly subjects served with 700 IU of vitamin D per day for every addition 15 kg of weight above normal subjects (Blum et al. 2008). The low serum level in obese subjects could be due to dilution of vitamin D (ingested or cutaneous vitamin D) in their large fat mass (Drincic et al. 2012). The inference of these findings is that vitamin D stored in fat tissue is not easily available, and obese individuals may oblige larger dose of vitamin D to meet a serum 25OHD level equivalent to that of their normal weight counterparts (Wortsman et al. 2000). The rise in serum 25(OH)D level during weight loss in obese individual verifies this hypothesis (Riedt et al. 2005; Zittermann et al. 2009)
Digestive tube surgery/diseases
Several clinical and experimental animal studies confirmed that vitamin D is most efficiently absorbed when consumed with foods containing fat (Johnson et al. 2005; Weber 1980). Early studies suggested that subjects suffering from impaired GIT i.e. obstructive jaundice (low bile juice release) or pancreatic insufficiency, cystic fibrosis or adult coelic disease and gastric surgery could demonstrate significantly reduced absorption of vitamin D. Reduction of serum level vitamin D3 by 30% was registered in case of Roux-en-Y gastric bypass surgery than before (Aarts et al. 2011). Despite of high dose of vitamin D supplementation (2500–5000 IU/day) vitamin D2 and D3 were not detected in infants and children suffering from extrahepatic biliary atresia (whose portoenterostomy failed to produce bile flow) (Heubi et al. 1990). Similarly in children with cholestasis serum vitamin D2 level remained undetectable instead of high dose of vitamin D supplementations (2500–5000 IU/day) (Heubi et al. 1989). Furthermore, the patients affected with cystic fibrosis were found to be less efficient in vitamin D absorption than that of their normal counterparts (Farraye et al. 2011; Lark et al. 2001). Likewise it is also hypothesized that the vitamin D positively alter the gut microbiota and CD8+ cells which may lead to retain the integrity of the GIT mucosal barrier by controlling the intercellular junctions thus controlling mucosal permeability and increasing the CD8+ cell (Bashir et al. 2016; Kanhere et al. 2016). Additionally some clinical studies also demonstrated the role of vitamin D in inhibiting the various cancers including GIT cancer by modulating cancer stem cell markers and VDR polymorphism and other VDR regulation (Lappe et al. 2017; Li et al. 2017; P Peppelenbosch et al. 2017). Though these studies do verify the involvement of vitamin D in different disease prevention but there is scarcity of data how vitamin D absorption in GIT is influenced under these diseases (Messa et al. 2017; Scragg et al. 2017; Yao et al. 2017). Nevertheless, the deleterious effect of diseases was partially rectified by sunlight exposure or by applying 25(OH)D which does not depend on the fat assimilation in GIT and take portal vein.
Genetic variations
The absorption of vitamin D is monitored by various factors such as vitamin D protein transporter, nuclear vitamin D binding protein, enzyme involved in fat digestion, bile secretion, liver enzyme catalyzing vitamin D. Literature has described the key role of genetic variations in modulating serum level 25(OH)D (Fu et al. 2009). It is clear now that vitamin D is absorbed in enterocyte through protein mediated transport. The expression and activity of these proteins can be modulated by altering the genetic code which may result into complete or partial loss of its activity. Furthermore, variation in genetic code of nearby gene may also interrupt binding of transcription factor leading into absence of these protein transporters. Till date literature lacks data addressing this factor (Hernández-Romano et al. 2009). Similarly any genetic variation in fat digestive enzyme and vitamin D binding protein may also affect the vitamin D absorption.
Research gaps and future prospects
After thorough review of literature the gaps in present literature were identified and these research gaps could be addressed by future dedicated studies. The future research prospects identified from present literature are as follows:
The acidic pH of gastric juice may affect the bioavailability of vitamin D. It is apparent that no data available on the susceptibility of major dietary forms of vitamin D with respect to pH variation in GIT.
It have been observed that various digestive enzymes facilitate the release of vitamin D from food matrix but the role of these enzyme is not fully recognized with respect to bioavailability of vitamin D. The evaluation of effect of enzymes individually or in syndicate and their concentration on vitamin D bioavailability will aid in better understanding their impact on vitamin D uptake.
It has been assumed that vitamin esters are at least partially cleaved by gastric lipase, but this problem in not addressed in present literature. Thus further studies needed to evaluate the effect of gastric lipase on vitamin D ester hydrolysis.
In duodenum digestive enzyme (amylases, lipase and protease) continues the release of vitamin D from food matrix. Vitamin D released from food matrix during digestion need to transfer from oil (naturally retained in dietary lipid) to the fat phase of meal (micelles). But kinetics of vitamin D transfer from food matrix into micelles is not completely understood. More dedicated research is needed to get better understanding about the impact of vitamin D transfer from food oil phase to micelle on the bioavailability of vitamin D.
Since these micelles incorporate lipophilic nutrients in their phospholipid bilayer but there is limited reports on these nutrients (other lipophilic vitamins, phytosterol etc.) addressing how they affect the transfer of vitamin D in micelle. More focused studies, discussing the impact of concentrations of these lipophilic nutrients on vitamin D uptake in GIT, will aid better understanding on bioavailability of vitamin D.
Though within physiological concentration, vitamin D is absorbed in enterocyte via protein mediated transport but there is shift in transport mode from protein medicated to passive diffusion at pharmacological concentration (high dose of vitamin D). The optimal concentration of vitamin D at which this mode shift in vitamin D transport occurs is still unknown.
Difference in vitamin D uptake efficiency in jejunum of rat than that of it ileum clearly suspects about the presence of another transporter particularly expressed in the jejunum (Goncalves et al. 2015). More research on these transporters is required to understand complete mechanism of vitamin D uptake in intestine.
Hydroxylated vitamin D forms do take different path for their absorption by enterocytes but the mechanism of hydroxylated species of vitamin D absorption is still remains unaddressed.
Vitamin D remains embedded in lipid phase of food as well as bound with specific proteins. The various food items differ in their complexity to retain vitamin D. There is limited documents available dealing with effect of food matrix complexity on bioavailability of vitamin D. Conversely more studies comparing bioavailability of vitamin D are required in order to know the effect of complexity of food matrix on vitamin D bioavailability.
Non hydroxylated vitamin D forms are absorbed through protein mediated transporters but their affinity may vary with variation in molecular forms (vitamin D2 or vitamin D3). Effect of concentration of vitamin D2 on the absorption of vitamin D3 and vice versa is still not much discussed.
The mechanism how the esters of vitamin D does effect the absorption of vitamin D is still remained unaddressed
Though vitamin E impairs the vitamin D absorption (reduced 15% at medium concentration of vitamin E and 17% at high concentration of vitamin E) in intestine but the concentration at which vitamin E completely cease the vitamin D absorption is not optimized.
Compressive review of relative bioavailability of vitamin D in different food matrix is essential to evaluate the bioavailability of vitamin D in different food.
Effect of special vitamin D formulations (encapsulated vitamin D in micro/nanoparticle) on bioavailability of vitamin D is not addressed in present literature.
Conclusion
Wide range of food products are available in different parts of the world; each food product displays variations in their matrix due to difference vitamin content, fat content, dietary fibers etc. All these factors contribute difficulty in determining the bioavailability of vitamin D in particular food. The present literature lacks complete knowledge about the mechanism of vitamin D absorption. Although some factors governing fate of vitamin D in GIT is well documented but several factors i.e. genetic variation, dietary fiber, host vitamin D status, effect of nano/microparticle of vitamin D which may influence the bioavailability of vitamin D, are either not addressed or have very little data to conclude. For better understanding about bioavailability of vitamin D more dedicated studies with labeled vitamin D are required addressing the future research prospects mentioned above.
References
- Aarts E, van Groningen L, Horst R, Telting D, van Sorge A, Janssen I, de Boer H. Vitamin D absorption: consequences of gastric bypass surgery. Eur J Endocrinol. 2011;164:827–832. doi: 10.1530/EJE-10-1126. [DOI] [PubMed] [Google Scholar]
- Aburto A, Britton W. Effects of different levels of vitamins A and E on the utilization of cholecalciferol by broiler chickens. Poult Sci. 1998;77:570–577. doi: 10.1093/ps/77.4.570. [DOI] [PubMed] [Google Scholar]
- Argao EA, Heubi JE, Hollis BW, Tsang RC. da-tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin D in chronic cholestatic liver disease of infancy and childhood. Pediatr Res. 1992;31:146–150. doi: 10.1203/00006450-199202000-00011. [DOI] [PubMed] [Google Scholar]
- Barragry J, France M, Corless D, Gupta S, Switala S, Boucher B, Cohen R. Intestinal cholecalciferol absorption in the elderly and in younger adults. Clin Sci Mol Med. 1978;55:213–220. doi: 10.1042/cs0550213. [DOI] [PubMed] [Google Scholar]
- Bashir M, et al. Effects of high doses of vitamin D3. Eur J Nutr. 2016;55:1479–1489. doi: 10.1007/s00394-015-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancuzzo RM, et al. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr. 2010;91:1621–1626. doi: 10.3945/ajcn.2009.27972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P, et al. Postprandial chylomicron and plasma vitamin E responses in healthy older subjects compared with younger ones. Eur J Clin Invest. 1997;27:812–821. doi: 10.1046/j.1365-2362.1997.1960744.x. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80:1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–316. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- Calvo MS, Babu US, Garthoff LH, Woods TO, Dreher M, Hill G, Nagaraja S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos Int. 2013;24:197–207. doi: 10.1007/s00198-012-1934-9. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Zhouf X-Y, Myles M, Endres D, Lindsay R. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects*. J Clin Endocrinol Metab. 1986;63:656–660. doi: 10.1210/jcem-63-3-656. [DOI] [PubMed] [Google Scholar]
- Compston JE, Merrett AL, Hammett F, Magill P. Comparison of the appearance of radiolabelled vitamin D3 and 25-hydroxy-vitamin D3 in the chylomicron fraction of plasma after oral administration in man. Clin Sci. 1981;60:241–243. doi: 10.1042/cs0600241. [DOI] [PubMed] [Google Scholar]
- Drevon CA. Absorption, transport and metabolism of vitamin E. Free Radic Res Commun. 1991;14:229–246. doi: 10.3109/10715769109088952. [DOI] [PubMed] [Google Scholar]
- Drincic AT, Armas LA, Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- Farraye F, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011;17:2116–2121. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25 (OH) D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Goncalves A, et al. Phytosterols can impair vitamin D intestinal absorption in vitro and in mice. Mol Nutr Food Res. 2011;55:S303–S311. doi: 10.1002/mnfr.201100055. [DOI] [PubMed] [Google Scholar]
- Goncalves A, Roi S, Nowicki M, Dhaussy A, Huertas A, Amiot M-J, Reboul E. Fat-soluble vitamin intestinal absorption: absorption sites in the intestine and interactions for absorption. Food Chem. 2015;172:155–160. doi: 10.1016/j.foodchem.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Gylling H, Miettinen TA. Cholesterol reduction by different plant stanol mixtures and with variable fat intake. Metabolism. 1999;48:575–580. doi: 10.1016/S0026-0495(99)90053-7. [DOI] [PubMed] [Google Scholar]
- Gylling H, Puska P, Vartiainen E, Miettinen TA. Retinol, vitamin D, carotenes and α-tocopherol in serum of a moderately hypercholesterolemic population consuming sitostanol ester margarine. Atherosclerosis. 1999;145:279–285. doi: 10.1016/S0021-9150(99)00078-7. [DOI] [PubMed] [Google Scholar]
- Gylling H, Hallikainen M, Nissinen MJ, Miettinen TA. The effect of a very high daily plant stanol ester intake on serum lipids, carotenoids, and fat-soluble vitamins. Clin Nutr. 2010;29:112–118. doi: 10.1016/j.clnu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Hendriks H, Brink E, Meijer G, Princen H, Ntanios F. Safety of long-term consumption of plant sterol esters-enriched spread. Eur J Clin Nutr. 2003;57:681–692. doi: 10.1038/sj.ejcn.1601598. [DOI] [PubMed] [Google Scholar]
- Hernández-Romano J, Martínez-Barnetche J, Valverde-Garduño V. Polymorphisms in gene regulatory regions and their role in the physiopathology of complex disease in the post-genomic era. Salud Pública de México. 2009;51:s455–s462. doi: 10.1590/S0036-36342009000900011. [DOI] [PubMed] [Google Scholar]
- Heubi JE, Hollis BW, Specker B, Tsang RC. Bone disease in chronic childhood cholestasis. I. Vitamin D absorption and metabolism. Hepatology. 1989;9:258–264. doi: 10.1002/hep.1840090216. [DOI] [PubMed] [Google Scholar]
- Heubi JE, Hollis BW, Tsang RC. Bone disease in chronic childhood cholestasis. II. Better absorption of 25-OH vitamin D than vitamin D in extrahepatic biliary atresia. Pediatr Res. 1990;27:26–31. doi: 10.1203/00006450-199001000-00006. [DOI] [PubMed] [Google Scholar]
- Hofmann A. The function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts. Biochem J. 1963;89:57. doi: 10.1042/bj0890057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman EE, Martin BR, Lachcik PJ, Gordon DT, Fleet JC, Weaver CM. Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J Agric Food Chem. 2011;59:2341–2346. doi: 10.1021/jf104679c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D, Tarnawski H. Influence of aging on vitamin D absorption and unstirred water layer dimensions in the rat. J Lab Clin Med. 1984;103:462–469. [PubMed] [Google Scholar]
- Hollander D, Muralidhara K, Zimmerman A. Vitamin D-3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut. 1978;19:267–272. doi: 10.1136/gut.19.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg I, Aksnes L, Berlin T, Lindbäck B, Zemgals J, Lindeke B. Absorption of a pharmacological dose of vitamin D3 from two different lipid vehicles in man: comparison of peanut oil and a medium chain triglyceride. Biopharm Drug Dispos. 1990;11:807–815. doi: 10.1002/bdd.2510110908. [DOI] [PubMed] [Google Scholar]
- Holvik K, Madar AA, Meyer HE, Lofthus CM, Stene LC. A randomised comparison of increase in serum 25-hydroxyvitamin D concentration after 4 weeks of daily oral intake of 10 μg cholecalciferol from multivitamin tablets or fish oil capsules in healthy young adults. Br J Nutr. 2007;98:620–625. doi: 10.1017/S000711450773074X. [DOI] [PubMed] [Google Scholar]
- Ikuma M, Hanai H, Kaneko E, Hayashi H, Hoshi T. Effects of aging on the microclimate pH of the rat jejunum. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1996;1280:19–26. doi: 10.1016/0005-2736(95)00261-8. [DOI] [PubMed] [Google Scholar]
- James W, Avenell A, Broom J, Whitehead J. A one-year trial to assess the value of orlistat in the management of obesity. Int J Obes. 1997;21:S24–S30. doi: 10.1038/sj.ijo.0800524. [DOI] [PubMed] [Google Scholar]
- Jasinghe VJ, Perera CO, Barlow PJ. Bioavailability of vitamin D2 from irradiated mushrooms: an in vivo study. Br J Nutr. 2005;93:951–956. doi: 10.1079/BJN20051416. [DOI] [PubMed] [Google Scholar]
- Johansson S, Melhus H. Vitamin A antagonizes calcium response to vitamin D in man. J Bone Miner Res. 2001;16:1899–1905. doi: 10.1359/jbmr.2001.16.10.1899. [DOI] [PubMed] [Google Scholar]
- Johnson J, Mistry V, Vukovich M, Hogie-Lorenzen T, Hollis B, Specker B. Bioavailability of vitamin D from fortified process cheese and effects on vitamin D status in the elderly. J Dairy Sci. 2005;88:2295–2301. doi: 10.3168/jds.S0022-0302(05)72907-6. [DOI] [PubMed] [Google Scholar]
- Kanhere M, Chassaing B, Gewirtz AT, Tangpricha V. Role of vitamin D on gut microbiota in cystic fibrosis. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan R-JH, Lu Z, Bogusz JM, Williams JE, Holick MF. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermato-endocrinology. 2013;5:165–176. doi: 10.4161/derm.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Lee B, Lee J, Park HJ. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus) J Agric Food Chem. 2008;56:3671–3674. doi: 10.1021/jf073398s. [DOI] [PubMed] [Google Scholar]
- Korkor AB, Bretzmann C. Effect of fish oil on vitamin D absorption. Am J Kidney Dis. 2009;53:356. doi: 10.1053/j.ajkd.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Korpela R, et al. Safety aspects and cholesterol-lowering efficacy of low fat dairy products containing plant sterols. Eur J Clin Nutr. 2006;60:633–642. doi: 10.1038/sj.ejcn.1602362. [DOI] [PubMed] [Google Scholar]
- Koyyalamudi SR, Jeong S-C, Song C-H, Cho KY, Pang G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J Agric Food Chem. 2009;57:3351–3355. doi: 10.1021/jf803908q. [DOI] [PubMed] [Google Scholar]
- Lappe J, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. Jama. 2017;317:1234–1243. doi: 10.1001/jama.2017.2115. [DOI] [PubMed] [Google Scholar]
- Lark RK, Lester GE, Ontjes DA, Blackwood AD, Hollis BW, Hensler MM, Aris RM. Diminished and erratic absorption of ergocalciferol in adult cystic fibrosis patients. Am J Clin Nutr. 2001;73:602–606. doi: 10.1093/ajcn/73.3.602. [DOI] [PubMed] [Google Scholar]
- Li M-X, et al. Vitamin D and cancer stem cells in the gastrointestinal tract. Curr Med Chem. 2017;24:918–927. doi: 10.2174/0929867324666170214110633. [DOI] [PubMed] [Google Scholar]
- Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH. The effects of race and body habitus on bone mineral density of the radius, hip, and spine in premenopausal women*. J Clin Endocrinol Metab. 1988;66:1247–1250. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- McDuffie JR, Calis KA, Booth SL, Uwaifo GI, Yanovski JA. Effects of orlistat on fat-soluble vitamins in obese adolescents pharmacotherapy. J Hum Pharmacol Drug Therapy. 2002;22:814–822. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- Messa P, Regalia A, Alfieri CM. Nutritional vitamin D in renal transplant patients. Specul Real Nutr. 2017;9:550. doi: 10.3390/nu9060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135:2478–2485. doi: 10.1093/jn/135.10.2478. [DOI] [PubMed] [Google Scholar]
- Mulligan GB, Licata A. Taking vitamin D with the largest meal improves absorption and results in higher serum levels of 25-hydroxyvitamin D. J Bone Miner Res. 2010;25:928–930. doi: 10.1002/jbmr.67. [DOI] [PubMed] [Google Scholar]
- Natri A-M, et al. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J Nutr. 2006;136:123–127. doi: 10.1093/jn/136.1.123. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Dale LC, Von Bergmann K, Croghan IT (1999) Cholesterol-lowering effect of stanol ester in a US population of mildly hypercholesterolemic men and women: a randomized controlled trial. In: Mayo clinic proceedings, vol 12. Elsevier, pp 1198–1206 [DOI] [PubMed]
- Niramitmahapanya S, Harris SS, Dawson-Hughes B. Type of dietary fat is associated with the 25-hydroxyvitamin D3 increment in response to vitamin D supplementation. J Clin Endocrinol Metab. 2011;96:3170–3174. doi: 10.1210/jc.2011-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outila TA, Mattila PH, Piironen VI, Lamberg-Allardt CJ. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am J Clin Nutr. 1999;69:95–98. doi: 10.1093/ajcn/69.1.95. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch MP, Fuhler GM, Verhaar PA, van der Laan JWL. Action and function of vitamin D in digestive tract physiology and pathology. Curr Med Chem. 2017;24:928–936. doi: 10.2174/0929867323666161228145137. [DOI] [PubMed] [Google Scholar]
- Reboul E, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–463. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RM. Changes in gastrointestinal function attributed to aging. Am J Clin Nutr. 1992;55:1203S–1207S. doi: 10.1093/ajcn/55.6.1203S. [DOI] [PubMed] [Google Scholar]
- Schlagheck TG, Kesler JM, Jones MB, Zorich NL, Dugan LD, Davidson MH, Peters JC. Olestra’s effect on vitamins D and E in humans can be offset by increasing dietary levels of these vitamins. J Nutr. 1997;127:1666S–1685S. doi: 10.1093/jn/127.8.1666S. [DOI] [PubMed] [Google Scholar]
- Scragg R, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds W. Absorption of lipids. Baltimore: University Park Press; 1974. [Google Scholar]
- Szejtli J, Gerloczy A, Fonagy A. Improvement of the absorption of 3H-cholecalciferol by formation of its cyclodextrin complex. Pharmazie. 1983;38:100–101. [PubMed] [Google Scholar]
- Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr. 2003;77:1478–1483. doi: 10.1093/ajcn/77.6.1478. [DOI] [PubMed] [Google Scholar]
- Tso P, Fujimoto K. The absorption and transport of lipids by the small intestine. Brain Res Bull. 1991;27:477–482. doi: 10.1016/0361-9230(91)90145-A. [DOI] [PubMed] [Google Scholar]
- Turnbull D, Whittaker MH, Frankos VH, Jonker D. 13-week oral toxicity study with stanol esters in rats. Regul Toxicol Pharmacol. 1999;29:216–226. doi: 10.1006/rtph.1999.1291. [DOI] [PubMed] [Google Scholar]
- Urbain P, Singler F, Ihorst G, Biesalski H-K, Bertz H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur J Clin Nutr. 2011;65:965–971. doi: 10.1038/ejcn.2011.53. [DOI] [PubMed] [Google Scholar]
- Vellas B, Balas D, Albarede J. Effects of aging process on digestive functions. Compr Therapy. 1991;17:46. [PubMed] [Google Scholar]
- Wagner D, Sidhom G, Whiting SJ, Rousseau D, Vieth R. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J Nutr. 2008;138:1365–1371. doi: 10.1093/jn/138.7.1365. [DOI] [PubMed] [Google Scholar]
- Weber F. Absorption mechanisms for fat-soluble vitamins and the effect of other food constituents. Prog Clin Biol Res. 1980;77:119–135. [PubMed] [Google Scholar]
- Wimalawansa SJ. Vitamin D in the new millennium. Curr Osteoporos Rep. 2012;10:4–15. doi: 10.1007/s11914-011-0094-8. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Yao S, Ambrosone CB, Kushi LH (2017) Vitamin D and breast cancer survival—In reply JAMA oncology [DOI] [PubMed]
- Zittermann A, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]