Abstract
Application of edible coatings is a suitable method to maintain the quality and reduce post-harvest losses in fresh vegetables and fruits. Pear fruits being climacteric have a short shelf life, and coating is considered as one of the most popular techniques to prolong its shelf life.The present study evaluates the effect of optimized edible coatings containing soy protein isolate (SPI) in combination with additives like hydroxypropyl methylcellulose (HPMC) and olive oil on ‘Babughosha’ Pears (Pyrus communis L.) stored at ambient temperature (28 ± 5 °C and 60 ± 10% RH). Four different coatings optimized by response surface methodology study were used in the present experiment. The results of the present study shows that the optimized edible coatings help retain the firmness of fruits and lowered the moisture loss. The tested combination of coating could also withhold the levels of ascorbic acid, chlorophyll and sugar contents in the treated fruits. Activities of enzymes associated with fruit softening (β-galactosidase, polygalacturonase, pectin methyl esterase) showed delayed peaks. Amongst all treatments, T1 (SPI 5.0%, HPMC 0.40%, Olive oil 1%, Potassium sorbate 0.22%) and T2 (SPI 5.0%, HPMC 0.40%, Olive oil 0.98% Potassium sorbate 0.20%) were found to have pronounced effect on retention of nutritional quality in pears. Observations of shelf-life extension established that T2 (SPI 5.0%, HPMC 0.40%, Olive oil 0.98% Potassium sorbate 0.20%) was successful in extending shelf-life of pear fruits up to 15 days, as compared to 8 days for untreated pear fruits.
Keywords: Edible coating, Postharvest, Pyrus communis L., Shelf-life, Storage
Introduction
Short shelf life of fresh horticulture produce like fruits and vegetables constraints their handling, transportation and marketing. Present day post harvest technology demands efficient, eco-friendly and cost-effective methods to address this problem. Edible coatings are alternative storage methods for fresh products and have attracted increasing attention because of environmental considerations and the trends toward the use of convenience foods (Yaman and Bayindirli 2002). Edible coatings, like modified atmosphere packaging, have been known to retard softening changes in fruits by inhibiting metabolic processes (Park 1999) or lowering migration of moisture and gases, leading to reduced weight loss (Dhall 2013).
Edible films of proteins are quite attractive because of their impressive gas barrier properties and the fact that they supplement the nutritive value of food. Amongst proteins, Soy protein isolate (SPI) is the primary choices for forming a protein edible film. The use of soy protein in the formation of films or coatings on food products has been explored by many researchers (Amal et al. 2010; Gennadios et al. 1997; Lim et al. 2011; Rhim et al. 2000, 2002). However, the inherent hydrophilicity of these films results in poor moisture barrier properties (Bourtoom 2009). Addition of a small amount of other edible materials and additives such as lipids, polysaccharides, emulsifiers and plastisizers can improve the performance of protein based edible coatings in order to delay the ripening of tropical fruits. Gennadios et al. (1997) observed that bicomponent soy protein/fatty acid films have improved functionality as moisture barriers compared to prototype soy protein films. Thus, composite edible coatings combining beneficial properties of a number of components may give desired protective effect to the fruits.
The concentration of each component in the film effects the overall quality of the film. Response surface methodology (RSM) is an effective technique for exploring the relationships between the responses and the independent variables for optimizing a formulation or process (Mirhosseini et al. 2009). RSM has been utilised for optimizing edible coating formulations for fresh-cut pineapples (Azarakhsh et al. 2012), mushroom slices (Taghian Dinani et al. 2014), strawberry (Ribeiro et al. 2007), pear (Javanmard et al. 2012) and other fruits and vegetables.
Babughosha Pear (Pyrus communis L. cv. Babughosha) is among the most economically important fruit tree crop of the temperate zones. Its habitat is distributed in the temperate regions of Europe and West Asia. The fruits are good source of pectin and help in maintaining desirable acid balance in the body (Kaur and Arya 2012). Because of their thin peel, crisp flesh, rich juice, and good taste, they are popular among consumers. However, rapid postharvest physiological changes marked mainly by excessive softening in this variety of pears are responsible for a short ripening period and rapid senescence and pose a challenge for their marketing (Hetong and Yufang 2003). Various edible coatings like zein-oleic acid (Scramin et al. 2011), plant oils (Ju et al. 2000), Shellac, Semperfresh, carboxy methylcellulose (Hussain et al. 2010) have been found to be effective on pears.
The present study aims to determine the effect of optimized coatings of soy protein isolate (SPI), hydroxypropyl methylcellulose (HPMC), olive oil and potassium sorbate on quality parameters and shelf-life of ‘Babughosha’ Pears (P. communis L.).
Materials and methods
Procurement of fruits
Fresh pear fruits were brought from the local fruit market of Anand town, Gujarat and they were sorted for uniform size, shape, colour and maturity stage. Fruits free from any physical injuries or diseases were selected.
Chemicals and reagents
Soy Protein Isolate used in the experiment was purchased commercially. All other chemicals and solvents were of Himedia, Merck, SDFCL (Mumbai, India) and Sigma-Aldrich (MO, USA) procured from local dealers. Soy Protein Isolate is a source of soy protein. It contains more than 90% protein with film forming and gas barrier properties.
Selection of coating solutions
RSM based optimization of edible coating formulations applicable on pear fruits was done in a preceeding experiment conducted in the laboratory (Nandane et al. 2017). The response variables choosen were pH, total soluble solids (TSS), titrable acidity and weight loss %. The optimization procedures was carried out using Design Expert Software Version 8 (Statease Inc., MN, USA). This experiment helped optimize concentrations of SPI, HPMC, Olive oil and potassium sorbate in the edible coating solution to be applied to pear fruits. This work provided the baseline information for the present study. Amongst a number of solutions provided by RSM, four optimized solutions having high desirability value were choosen to study their effect on the overall quality parameters of pear fruit (Table 1, treatments coded as T1, T2, T3 and T4).
Table 1.
RSM optimized coating formulations selected for study of their effect on the overall quality parameters of pear fruit
| Treatments | SPI (%) | HPMC (%) | Olive oil (%) | Potassium Sorbate (%) |
|---|---|---|---|---|
| T1 | 5.00 | 0.40 | 1.00 | 0.22 |
| T2 | 5.00 | 0.40 | 0.98 | 0.20 |
| T3 | 4.99 | 0.40 | 1.00 | 0.24 |
| T4 | 5.00 | 0.39 | 1.00 | 0.28 |
| Control | – | – | – | – |
Preparation of coating solutions
Soy protein isolate, hydroxypropyl methylcellulose, olive oil and potassium sorbate in predetermined quantities (Table 1) were dispersed in distilled water. Glycerol (0.7%) was added as plasticizer to each solution. The solutions were then heated with constant stirring at 80–85 °C temperature for 15 min.
Coating application
All fruits were washed with distilled water followed by a wash of Sodium hypochlorite (200 ppm for 10 min) for disinfection. The fruits were divided into 5 sets with 14 fruits in each set. Coatings were applied to the fruit surface by dipping technique, with dipping time of 2 min. Fruits treated with only distilled water were considered as control samples. The coated pears were packed in plastic boxes and stored at ambient temperature (28 ± 5 °C and 60 ± 10% relative humidity RH). All analyses were carried out at beginning of experiment (0 day) and thereafter at regular interval of 4 days till the fruits became unacceptable for consumption due to decay or infection.
Determination of quality parameters
Weight loss percentage
The weight of fruits was determined by use of an electronic balance. The difference between the initial and final weight of the fruit was considered as total weight loss and the results were expressed as the percentage of weight loss, as per the standard method of AOAC (2000).
Total soluble solids (TSS) and titratable acidity (TA)
4 grams of fruit sample was homogenized with 40 ml distilled water and the filterate was used to determine total soluble solids (TSS) and titratable acidity (TA). The TSS content of the fruit was determined using digital refractrometer (Atago Co., Tokyo, Japan). A drop of filtrate was placed on the prism glass of the digital refractometer to read TSS of sample according to degree of Brix (°Brix). The reading obtained was multiplied by dilution factor of 10 to obtain the real °Brix. The TA was determined according to the method of Mazumdar and Majumder (2003) by titration of 10 mL of juice with 0.1 N NaOH using phenolphthalein as an indicator and the results were expressed as the percent of malic acid.
Firmness
Firmness was measured by Texture Analyser (TA Plus Lloyd Instruments, Ltd, England). The measurement was carried out using a needle probe to penetrate the pear fruit surface at 1 mm/s crosshead speed and causing 30% sample compression. Maximum force (N) required to penetrate the sample was recorded and used as the firmness of fruits. At least three measurements were made on each pear fruit at different locations and the results were averaged.
Shelf life
The shelf life of a fruit is a period of time which starts from its harvesting and extends up to onset of rotting in the fruits (Mondal 2000). The criteria used to determine onset of rot was visual appearance. The shelf life was calculated by counting the days required for the fruit to reach the last stage of ripening, but up to the stage of their marketability. The last stage of ripening was considered when the fruit became soft and wrinkles appeared on the surface of fruit.
Biochemical analysis
The reducing sugar contents were determined by following the dinitrosalicylic acid method as cited by Thimmaiah (1999). The quantitative analysis of pigments such as total chlorophylls, total carotenoids and lycopene was carried out as per the methods described by Wang et al. (2005). The quantitative analysis of ascorbic acid was performed as per the method of Roe (1954).
Extraction and assay of enzymes
The procedure for extraction and assay of β-Galactosidase was followed in accordance with Biswas (1985). The reaction mixture containing 0.25 mL of sodium acetate buffer (0.1 M, pH 5) and 0.01 mL of p-nitrophenyl-β-d-galactoside (10 mM) was incubated at 55 °C for 30 min. The reaction was initiated by adding appropriate amount of enzyme extract and incubated for 10 min; similarly the blank was prepared without the enzyme extract which was replaced by the buffer. The reaction was terminated by adding 4 mL of NaOH (0.1 M) and the enzyme activity was expressed as U/mg of protein, where one unit (U) was defined as μmol of p-nitrophenol formed per minute.
Extraction and assay for Polygalacturonase (PG) and Invertase activity was carried out according to the procedure described by Srivastava and Dwivedi (2000). Fruit tissue (1 g) was obtained and homogenized in 10 mL of sodium phosphate buffer (20 mM, pH 7.0) containing cysteine–HCl (20 mM), EDTA (20 mM) and Triton X-100 (0.05%). The homogenate was centrifuged at 15,000 g for 30 min at 4 °C in a refrigerated centrifuge (Model: Eppendorf, 5430R). The clear supernatant was used as enzyme extract for assaying activity of the enzymes.
For PG assay, the reaction mixture comprised of 0.2 mL sodium acetate (200 mM, pH 4.5), 0.1 mL NaCl (200 mM), 0.3 mL polygalacturonic acid (PGA, 1% aqueous solution adjusted to pH 4.5) and appropriate amount of enzyme extract in a total volume of 1.0 mL. The reaction mixture was held at 37 °C for 1 h followed by addition of dinitrosalicylic acid (DNS). The reaction was stopped by heating the reaction mixture in a boiling water bath for 5 min. In control tubes the substrate was added after the heat treatment. d-Galacturonic acid was used as the standard and one unit of enzyme activity was defined as the amount of enzyme required to liberate 1 μmol of reducing groups per min under the condition of the enzyme assay. The enzyme activity was expressed as units per mg of protein.
For Invertase activity, the assay mixture contained acetate buffer (100 mM, pH 4.5), sucrose (100 mM) and enzyme preparation in a total volume of 1.0 ml. The reaction mixture was incubated for 1 h at 37 °C. The substrate was added to control tubes after the incubation and colour was developed using DNS. The tubes were boiled on a waterbath for 10 min and the colour was read at 540 nm using spectrophotometer. Amount of reducing sugar released was calculated from a calibration curve drawn using glucose as standard. One unit of invertase activity was defined as μmol of reducing sugars equivalent released per min at 37 °C. The enzyme activity was expressed as units per mg of protein.
The procedure for extraction and assay of PME was adapted from Lohani et al. (2004). The reaction mixture contained 1 mL pectin solution (0.01%, pH 7.5), 0.2 mL NaCl (0.15 M), 0.1 mL bromothymol blue solution (0.01%), 0.2 mL water and appropriate amount of enzyme extract. Absorbance at 620 nm was measured immediately after addition and after 3 min. The difference in initial absorbance and absorbance after 3 min was the measure of PME activity. The enzyme activity was expressed as A620 min−1 mg protein−1.
Catalase (CAT) activity was assayed according to the method of Xing et al. (2011). The fruit tissue (1 g) was homogenised in 10 ml, 25 mM PBS, pH 7.8, containing 0.8 g/l PVPP and 1 Mm EDTA, then centrifuged at 12,000 g for 20 min at 4 °C. The resulting supernatant was directly used for enzyme assay. For CAT activity assay, the reaction mixture consisted of 2 ml sodium phosphate buffer (50 mM, pH 7.0), 0.5 ml H2O2 (40 mM) and enzyme extract. The decomposition of H2O2 was measured by the decline in absorbance at 240 nm. The CAT specific activity was expressed as U/mg protein, where U = 0.1 * ∆A240 nm per min.
Statistical analysis
The experimental data was analyzed by using SPSS 17.0 software. All analyses performed were carried out in triplicates. The mean and Standard deviation (SD) were calculated. The statistical significance of the data was assessed by one-way analysis of variance (ANOVA) and the means were compared by using Tukey’s HSD test.
Results and discussion
Effect on weight loss and firmness
The results for weight loss percentage indicated that there was a constant increase in weight loss of pear fruits stored at room temperature (Fig. 1a). The weight loss in uncoated fruit was about 8.01% by 8th day of storage, whereas in all treated fruit samples the weight loss ranged from 4.80 to 7.75%. However, by 12th day the weight loss increased to 9.05% in T4. T1 showed lowest weight loss of 6.96% on 12th day of storage. The possible reason for differences in wt. loss values may be due to the water binding ability of potassium sorbate. Another reason may be the possible differences in physiological maturity level of samples even though efforts were made to choose the samples with uniform maturity. Monedero et al. (2009) observed that the presence of lipid decreases the water vapour permeability of SPI-based films. Thus, the composite coating may effectively reduce water loss from fruits and hence the decrease in overall weight loss.
Fig. 1.
Effect of application of edible coatings on a weight loss % and b firmness of pear fruit during storage at ambient conditions (28 ± 5 °C and 60 ± 10% RH). Each value represents a mean of three independent replicates. Vertical bars represent ±SD of means. T1 = SPI 5.0%, HPMC 0.40%, Olive oil 1%, Pot. sorbate 0.22%, T2 = SPI 5.0%, HPMC 0.40%, Olive oil 0.98%, Pot. sorbate 0.20%, T3 = SPI 4.99%, HPMC 0.40%, Olive oil 1.0%, Pot. sorbate 0.24%, T4 = SPI 5.0%, HPMC 0.39%, Olive oil 1.0%, Pot. sorbate 0.28%
In the present study, it was observed that the firmness of fruits reduced as ripening progressed (Fig. 1b). However the rate of loss in firmness was significantly higher in untreated samples as compared to treated samples measured on 8th day of storage. Fruit firmness was monitored for 8 days for those samples which decayed after 8 days, while for other samples it was carried out up to 12 days. But for comparison purpose graph of 8th day is given. The firmness of untreated fruits was as low as 3.96 N. Amongst treated fruits, firmness could be retained in all fruits apart from those treated with T3. The loss in firmness during storage of pear fruits occurs due to break down of enzymes, loss of water and degradation of pectic substances present in the fruit (Nath et al. 2012). Hussain et al. (2010) in their study on pear fruits observed that polysaccharide based edible coatings had the ability to lower cell-wall loosening enzymatic activities owing to ripening delay and hence retained the firmness of pear fruits. Morever, HPMC films incorporated with lipid content have higher capacity of modifying the fruit internal atmosphere and hence maintain the firmness of the fruit (Navarro-Tarazaga et al. 2011). The lipid content also acts as a barrier to moisture loss. In the present study, the edible coating contained HPMC as the polysaccharide and olive oil as lipid component and hence it could successfully decrease softening of pear fruits as compared to control samples.
Effect on TSS and titrable acidity
The titratable acidity and soluble solids are the best acceptable eating quality indicators for pear fruit during storage (Park 2002). Table 2 summarises the TSS and titrable acidity of pear fruits during storage. TSS content increased throughout the storage period in all treatments. In pears, maximum TSS value recorded was around 11.4 °Brix, observed in control fruits on 8th day of storage and in treated fruits on 12th day of storage. Similar results in increase in TSS during storage period were found in previous studies on pears (Elgar et al. 1997; Nath et al. 2012). Increase in TSS during storage might be associated with the transformation of pectic substances and starch hydrolysis and also with dehydration of fruits (Goncalves et al. 2000; Park 2002). However, in treated fruits the rise in TSS was delayed to 12th day of storage. The delay may most likely have occurred as a result of slowing down of metabolic activity and respiration process in the pear fruits coated with the composite coating.
Table 2.
Effect of application of edible coatings on TSS and titrable acidity of pear fruit during storage at ambient conditions (28 ± 5 °C and 60 ± 10% RH)
| Treatments | Days of storage | |||
|---|---|---|---|---|
| 0 day | 4 days | 8 days | 12 days | |
| TSS (°Brix) | ||||
| Control | 11.01 ± 0.012a | 11.23 ± 0.015a | 11.42 ± 0.020a | – |
| T1 | 11.01 ± 0.012a | 11.13 ± 0.006c | 11.24 ± 0.015c | 11.45 ± 0.015a |
| T2 | 11.01 ± 0.012a | 11.16 ± 0.006b | 11.27 ± 0.015c | 11.41 ± 0.010b |
| T3 | 11.01 ± 0.012a | 11.18 ± 0.015b | 11.34 ± 0.021b | – |
| T4 | 11.01 ± 0.012a | 11.21 ± 0.006a | 11.28 ± 0.010c | 11.43 ± 0.01ab |
| Titrable acidity (%malic acid) | ||||
| Control | 0.67 ± 0.00a | 0.50 ± 0.004c | 0.38 ± 0.018d | – |
| T1 | 0.67 ± 0.00a | 0.54 ± 0.004a | 0.50 ± 0.000b | 0.39 ± 0.007b |
| T2 | 0.67 ± 0.00a | 0.55 ± 0.004a | 0.53 ± 0.004a | 0.42 ± 0.012ab |
| T3 | 0.67 ± 0.00a | 0.50 ± 0.004c | 0.44 ± 0.004c | – |
| T4 | 0.67 ± 0.00a | 0.52 ± 0.004b | 0.49 ± 0.007b | 0.43 ± 0.013a |
Values are mean ± SD, n = 3, Values within treatments with different letters (a to e) in a column differ significantly (P ≤ 0.05) with values from higher to lower. T1 = SPI 5.0%, HPMC 0.40%, Olive oil 1%, Pot. sorbate 0.22%, T2 = SPI 5.0%, HPMC 0.40%, Olive oil 0.98%, Pot. sorbate 0.20%, T3 = SPI 4.99%, HPMC 0.40%, Olive oil 1.0%, Pot. sorbate 0.24%, T4 = SPI 5.0%, HPMC 0.39%, Olive oil 1.0%, Pot. sorbate 0.28%
Titratable acidity significantly (p < 0.05) decreased as a function of storage time for all treatments studied. The maximium value of 0.67% was recorded on 0 day and minimum value 0.38% was recorded for control fruits on 8th day. The reduction in acidity during storage might be associated with the conversion of organic acids into sugars and their derivatives or their utilization in respiration (Eccher-Zerbini 2002). Here, the treated fruits had a higher value of titrable acidity throughout the storage period owing to a slower utilisation of the acids. Blaszczyk and Lysiak (2001), Elgar et al. (1997) and Park (2002), reported similar changes in acidity of pear fruits during storage.
Effect on reducing sugars
Ripening is accompanied by an increase in the reducing sugar content and invertase activity concomitant with decrease in nonreducing sugar content (Srivastava and Dwivedi 2000). Beaudry et al. (1989) observed that the accumulation of reducing sugars may be due to increased breakdown of starch during ripening. Observations for reducing sugars content in Pear fruits (Table 3) indicated that the value was lowest on 0 day (71.77 mg/g). On 4th day, a peak in reducing sugar content was observed in control fruits with value as high as 175.31 mg/g. A similar trend was observed in treated fruits, but for all treatments the reducing sugar concentration remained lower (157–130 mg/g). The reducing sugar content of pear fruit showed a decline on 8th and 12th day of storage. The levels of sugar reduced drastically in untreated fruits, while in treated fruits the sugar content was retained or reduced slightly, except for T3 wherein a drop similar to control fruits was observed. Similar trends were observed in strawberries (Tanada-Palmu and Grosso 2005). The decrease in concentration of reducing sugars towards end of storage period can be attributed to the fact that as fruit ripening progressed towards senesence there was an overall reduction of substrates and sugar content.
Table 3.
Effect of application of edible coatings on reducing sugar and ascorbic acid of pear fruit during storage at ambient conditions (28 ± 5 °C and 60 ± 10% RH)
| Treatments | Days of storage | |||
|---|---|---|---|---|
| 0 day | 4 days | 8 days | 12 days | |
| Reducing sugars (mg/g F.W.) | ||||
| Control | 71.77 ± 2.21a | 175.31 ± 8.53a | 93.280 ± 4.75d | – |
| T1 | 71.77 ± 2.21a | 130.04 ± 4.07c | 123.46 ± 0.00b | 96.570 ± 1.90b |
| T2 | 71.77 ± 2.21a | 142.44 ± 3.88bc | 130.59 ± 0.95a | 102.60 ± 3.43ab |
| T3 | 71.77 ± 2.21a | 157.09 ± 9.08b | 107.54 ± 0.95c | – |
| T4 | 71.77 ± 2.21a | 140.74 ± 6.17bc | 119.62 ± 1.90b | 105.35 ± 3.29a |
| Ascorbic acid (mg/100 g F.W.) | ||||
| Control | 545.33 ± 28.38a | 382.67 ± 49.08ab | 326.67 ± 8.33b | – |
| T1 | 545.33 ± 28.38a | 490.67 ± 45.88a | 439.33 ± 41.10a | 192.67 ± 11.37b |
| T2 | 545.33 ± 28.38a | 473.33 ± 30.29a | 365.33 ± 17.92b | 240.67 ± 15.01a |
| T3 | 545.33 ± 28.38a | 382.67 ± 26.63ab | 306.00 ± 17.32b | – |
| T4 | 545.33 ± 28.38a | 356.67 ± 53.00b | 326.67 ± 11.01b | 200.67 ± 24.03b |
Values are mean ± SD, n = 3, Values within treatments with different letters (a to e) in a column differ significantly (P ≤ 0.05) with values from higher to lower. T1 = SPI 5.0%, HPMC 0.40%, Olive oil 1%, Pot. sorbate 0.22%, T2 = SPI 5.0%, HPMC 0.40%, Olive oil 0.98%, Pot. sorbate 0.20%, T3 = SPI 4.99%, HPMC 0.40%, Olive oil 1.0%, Pot. sorbate 0.24%, T4 = SPI 5.0%, HPMC 0.39%, Olive oil 1.0%, Pot. sorbate 0.28%
Effect on ascorbic acid content
Initial ascorbic acid content in pears was found to be 545.33 mg/100 g. The results for ascorbic acid content for treated pear fruits as summarised in Table 3 indicated that as ascorbic acid is used up during respiration, this amount of ascorbic acid decreases in fruits of all treatments with increased storage time. In control fruits the abscorbic acid content decreased to 382.67 mg/100 g on 4th day. However, pear fruits treated with T1 and T2 showed better retention of ascorbic acid throughout the storage period, with values of 490.67 mg/100 g and 473.33 mg/100 g on 4th day of storage and 439.33 mg/100 g and 365.33 mg/100 g on 8th day of storage respectively. Amal et al. (2010) observed that coating formulations may reduce oxygen diffusion, slowing down the respiration rate, which ultimately delays the deteriorative oxidation reaction of ascorbic acid of fruit. Similar findings of decrease in ascorbic acid content during storage of pear fruits were reported by Soliva-Fortuny and Martín-Belloso (2003) and Nath et al. (2012).
Effect on chlorophyll, carotenoids and lycopene
The loss of chlorophyll during storage is related to the change of chloroplasts into chromoplasts containing yellow and red carotenoid pigments. Loss of chlorophyll is mediated through several processes involving the action of the enzyme chlorophyllase and photodegradation (Hussain et al. 2010). At the beginning of experiment, the chlorophyll content of pear fruit was noted to be 30.42 μg/g (Table 4). A decline was observed in control and treated fruits with increasing storage time. In control fruits the value of chlorophyll content on 4th day was recorded as 24.53 μg/g. The highest retention of chlorophyll was observed in T2 with value of 29.76 μg/g. Further decline in chlorophyll content was observed on 8th and 12th day. Maximum retention of chlorophyll 24.02 μg/g was observed in T1 on 12th day of storage. Thus, the coatings significantly (p < 0.05) reduced chlorophyll degradation in pear fruits, as compared to untreated fruits. These results are supported by previous studies which state that coating fruit with wax or polymers reduces fruit softening and chlorophyll degradation (Meheriuk and Porritt 1972).
Table 4.
Effect of application of edible coatings on chlorophyll, carotenoids and lycopene content of pear fruit during storage at ambient conditions (28 ± 5° C and 60 ± 10% RH)
| Treatments | Days of storage | |||
|---|---|---|---|---|
| 0 day | 4 days | 8 days | 12 days | |
| Chlorophyll (μg/g F.W.) | ||||
| Control | 30.42 ± 0.12a | 24.53 ± 0.10e | 23.35 ± 0.21d | – |
| T1 | 30.42 ± 0.12a | 27.33 ± 0.12c | 25.00 ± 0.15c | 24.02 ± 0.12a |
| T2 | 30.42 ± 0.12a | 29.76 ± 0.20a | 27.82 ± 0.34a | 20.66 ± 0.55b |
| T3 | 30.42 ± 0.12a | 25.17 ± 0.12d | 23.89 ± 0.16d | – |
| T4 | 30.42 ± 0.12a | 28.98 ± 0.31b | 26.16 ± 0.16b | 23.62 ± 0.05a |
| Carotenoids (μg/g F.W.) | ||||
| Control | 69.67 ± 0.58a | 138.33 ± 0.58a | 151.33 ± 1.53a | – |
| T1 | 69.67 ± 0.58a | 67.670 ± 0.58d | 103.67 ± 2.89d | 115.33 ± 1.53b |
| T2 | 69.67 ± 0.58a | 66.000 ± 0.00d | 88.330 ± 0.58e | 108.33 ± 1.15c |
| T3 | 69.67 ± 0.58a | 120.00 ± 1.00b | 128.00 ± 0.00b | – |
| T4 | 69.67 ± 0.58a | 96.670 ± 0.58c | 114.67 ± 1.53c | 128.33 ± 0.58a |
| Lycopene (μg/g F.W.) | ||||
| Control | 1.84 ± 0.00a | 2.86 ± 0.018a | 2.19 ± 0.048b | – |
| T1 | 1.84 ± 0.00a | 1.90 ± 0.000e | 2.32 ± 0.018b | 2.27 ± 0.018b |
| T2 | 1.84 ± 0.00a | 2.34 ± 0.000c | 2.54 ± 0.018a | 2.25 ± 0.031b |
| T3 | 1.84 ± 0.00a | 2.44 ± 0.065b | 2.55 ± 0.090a | – |
| T4 | 1.84 ± 0.00a | 2.12 ± 0.000d | 2.48 ± 0.048a | 2.32 ± 0.018a |
Values are mean ± SD, n = 3, Values within treatments with different letters (a to e) in a column differ significantly (P ≤ 0.05) with values from higher to lower. T1 = SPI 5.0%, HPMC 0.40%, Olive oil 1%, Pot. sorbate 0.22%, T2 = SPI 5.0%, HPMC 0.40%, Olive oil 0.98%, Pot. sorbate 0.20%, T3 = SPI 4.99%, HPMC 0.40%, Olive oil 1.0%, Pot. sorbate 0.24%, T4 = SPI 5.0%, HPMC 0.39%, Olive oil 1.0%, Pot. sorbate 0.28%
Wang et al. (2005) have observed that chlorophyll content rapidly decreases and simultaneously there is increased accumulation of carotenoids and lycopene in later stages of fruit ripening. A low carotenoids content of about 69.67 μg/g was observed on 0 day (Table 4). This was followed by increased value with advancing storage time. A gradual increase was observed in treated and untreated fruits, with highest values (151.33 μg/g) of carotenoids observed on 8th day in untreated fruits. For lycopene, a value of 1.84 μg/g was recorded at the beginning and further increase was observed till 4th day and 8th day in untreated and treated fruits respectively (Table 4). Highest value of 2.86 μg/g of lycopene was seen in untreated fruits on 4th day, whereas for treated fruits it ranged from 1.90 to 2.44 μg/g. Thus, it can be summarised that overall there was degradation of chlorophyll and consequent increase of carotenoids and lycopene in pear during ripening. However, the conversion of chlorophyll to carotenoids was delayed due to application of edible coatings and the fruits remained green and fresh for longer time.
Effect on enzyme activity
In a mature fruit, various enzymes such as polygalacturonase (PG), invertase, β-galactosidase and pectin methylesterase (PME) are reported to be contributors to the softening process (Brummell 2006). Effect of coatings on activities of some of these enzymes studied are presented below:
Effect on β-galactosidase and invertase activity
Increase in β-galactosidase activity during ripening has been reported in many fruits, like apples, pear (Ahmed and Labavitch 1980b). In our studies, β-galactosidase activity tend to increse from 0 to 8 days of storage period after which decline in the enzyme activity was observed on 12th day (Fig. 2a). There was an increase of about two to threefold in enzyme activity as fruit ripening progressed. However, the changes in the activity was observed to be similar for treated and untreated fruits. The β-galactosidase activity in all treatments ranged from 0.138 to 0.092 U/mg protein on 8th day in contrast to 0.053 U/mg protein on 0 day.
Fig. 2.
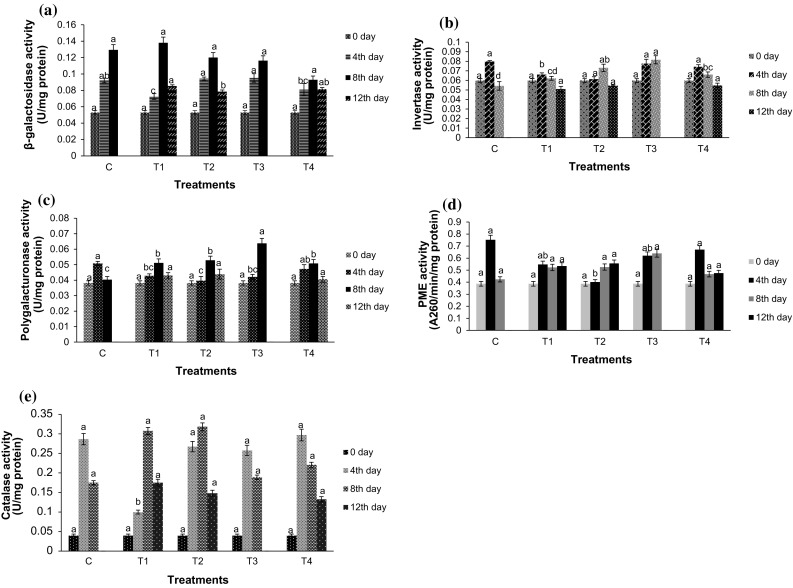
Effect of application of edible coatings on specific activity of a β-galactoside, b Invertase, c Polygalacturonase, d Pectin methyl esterase and e Catalase of pear fruit during storage at ambient conditions (28 ± 5 °C and 60 ± 10% RH). Each value represents a mean of three independent replicates. Vertical bars represent ±SD of means. T1 = SPI 5.0%, HPMC 0.40%, Olive oil 1%, Pot. sorbate 0.22%, T2 = SPI 5.0%, HPMC 0.40%, Olive oil 0.98%, Pot. sorbate 0.20%, T3 = SPI 4.99%, HPMC 0.40%, Olive oil 1.0%, Pot. sorbate 0.24%, T4 = SPI 5.0%, HPMC 0.39%, Olive oil 1.0%, Pot. sorbate 0.28%
Invertase hydrolyse non-reducing sugars like sucrose to reducing sugars, fructose and glucose. The activity of this enzyme is seen to increase during fruit ripening causing degradation of sucrose. Changes in invertase activity of pears during storage is depicted in Fig. 2b. The invertase activity on 0 day was recorded as 0.059 U/mg of protein. On 4th day, invertase activity was found to be highest in untreated fruits recorded as 0.080 U/mg of protein. The invertase activity was significantly (p < 0.05) lower in all treatments. On 8th day, T2 and T3 showed higher enzyme activity with values of 0.073 U/mg of protein and 0.081 U/mg of protein respectively. The enzyme activity decreased in all samples with prolonged storage.
Effect on Polygalacturonase (PG) and Pectin methyl esterase (PME) activity
Pectin-degrading enzymes are closely related to changes in pectins, which play a significant role in the softening changes in fruit and vegetable tissues. An increase in PG activity with a peak at the climacteric stage in mango (Prabha et al. 2000) have been reported. In pear, as in other softening fruits, an increase in cell wall-modifying enzyme activities and the degradation of cell wall polysaccarides have been reported (Ahmed and Labavitch 1980a, b). PG activity in Shellac and Semperfresh coated pears showed a progressive increase during the storage process (Zhou et al. 2011). The PG and PME activity of coated pear fruits are summarised in Fig. 2c, d respectively. In the present study, rise in PG activity was observed during storage period, with a peak in activity evident on 8th day of storage in all treated pear fruits. In control fruits the peak in PG activity (0.051 U/mg of protein) was observed on 4th day of storage. The highest PG activity of 0.063 U/mg of protein was observed in T3 on 8th day. The enzyme activity declined on 12th day of storage in all treatments.
The PME activity in pears was found to increase in all treatments but decreased at a later stage. The PME activity in control fruits was as high as 0.75 U/mg of protein on 4th day of storage and then showed a sharp decline on 8th day. In all treatments, however, the rise in PME activity was found to be relatively lower (0.55–0.67 U/mg of protein) on 4th day and was further retained or further increased on storage. Only exception was T4 wherein the enzyme activity decreased similar to control fruits but the activity was moderately lower than the control fruits throughout. The relatively lower activity of PME in coated fruits contributed to the enhanced retention of brittleness and firmness during their storage as observed in pears (Zhou et al. 2011), strawberries (Gol et al. 2013) and papaya fruit (González-Aguilar et al. 2009).
Effect on Catalase (CAT) activity
Catalase (CAT) is an antioxidant enzyme and its activity usually increases during ripening. The increase in the activity of this enzyme was observed in control and treated fruits (Fig. 2e). However, in T1 and T2 it was observed that the treatments were successful in decreasing enzyme activity, since the peak of activity was observed on 8th day. Of these, the CAT activity in T1 gave best results with lower activity of 0.099 U/mg of protein as compared to 0.287 U/mg of protein activity in control fruits on 4th day of storage. Thereafter it peaked to a value of 0.307 U/mg of protein on 8th day and subsequently decresed by 12th day. In all other treatments, similar pattern of CAT activity was observed wherein after a peak the enzyme activity decreased. However, the time of peak in enzyme activity was variable. Jahnke et al. (1991) observed that, in response to stress, plants normally increase the levels of oxyradical detoxification enzymes including CAT and help prevent damage by oxidation. Many coatings induce rise in the activity of this enzyme, thus promoting its protection.
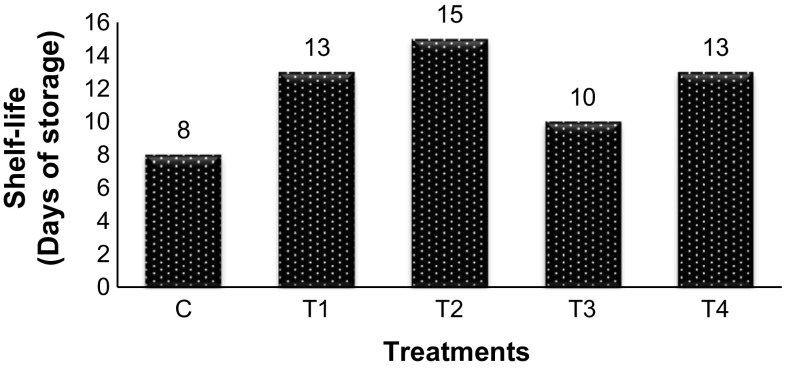
Effect on shelf-life
One of the main purpose of applying edible coatings is to bring about an extension in shelf-life of fruits. In the present study, the shelf-life of ‘Babughosha’ Pears stored at ambient temperature of 28 ± 5 °C was recorded (Fig. 3). The shelf-life of untreated pear fruits was about 8 days at these storage conditions. The degradation in quality of pears is mainly brought about by excessive softening of the fruits due to rapid ripening (Zhou et al. 2011), occurrence of browning (Ju et al. 2000) and mold growth of Penicillium sp. and Botrytis sp. (Hussain et al. 2010). During experiment no such difference was observed with respect to appearance of moulds. In the present study, it was observed that after its onset the process of ripening was rapid. However, in all treated fruits the loss in firmness occurred at a slower rate as compared to control and TSS and sugar content was maintained at a higher level. Browning and mold growth was observed towards end of storage period. Among treated fruits, decay was observed in T3 at 10th day of storage, whereas all other treatments remained acceptable and retained firmness for longer time. Fruits treated with T1 and T4 could be stored up to 13 days and those treated with T2 remained fresh up to 15 days after coating treatment. Thus, the shelf-life of ‘Babughosha’ Pears coated with the optimized coatings could be extended by 5 to 7 days at ambient conditions as compared to control fruits.
Fig. 3.
Effect of application of edible coatings on shelf life of pear fruit during storage at ambient conditions (28 ± 5 °C and 60 ± 10% RH)
Conclusion
The edible coatings containing a combination of SPI, HPMC and Olive oil retained moisture and firmness of the fruits for a longer time. The edible coatings helped lower the metabolic activity and chlorophyll degradation in pears. The optimized coatings T1 (SPI 5.0%, HPMC 0.40%, Olive oil 1%, Potassium sorbate 0.22%) and T2 (SPI 5.0%, HPMC 0.40%, Olive oil 0.98% Potassium sorbate 0.20%) exhibited overall conservation of quality parameters of pear fruits and also helped to extend its post harvest shelf-life at ambient condition. These combination of coatings can thus be applied on ‘Babughosha’ pears for improving their quality and shelf-life at ambient storage conditions.
Acknowledgements
The authors are thankful to DST (SERB-MOFPI) for providing financial assistance under the Research Project (SERB/MOFPI/0020/2012). The authors are also thankful to the Head, B. R. Doshi School of Biosciences, Sardar Patel University and A. D. Patel Institute of Technology for providing necessary facilities for carrying out this research work.
References
- Ahmed AE, Labavitch JM. Cell wall metabolism in ripening fruit II. Changes in carbohydrate-degrading enzymes in ripening ‘Bartlett’ pears. Plant Physiol. 1980;65:1014–1016. doi: 10.1104/pp.65.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AE, Labavitch JM. Cell wall metabolism in ripening fruit I. Cell wall changes in ripening ‘Bartlett’ pears. Plant Physiol. 1980;65:1009–1013. doi: 10.1104/pp.65.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amal S, Atress MM, El-Mogy HE, Aboul-Anean BW. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J Hortic Sci Ornam Plants. 2010;2:88–97. [Google Scholar]
- AOAC . Official methods of analysis of the Association of the Official Analysis Chemists. 17. Washington, DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Azarakhsh N, Osman A, Ghazali HM, Tan CP, Mohd Adzahan N. Optimization of alginate and gellan-based edible coating formulations for fresh-cut pineapples. Int Food Res J. 2012;19:279–285. [Google Scholar]
- Beaudry RM, Severson RF, Black CC, Kays SJ. Banana ripening: implications of changes in glycolytic intermediate concentrations, glycolytic and gluconeogenic carbon flux, and fructose 2, 6-bisphosphate concentration. Plant Physiol. 1989;91:1436–1444. doi: 10.1104/pp.91.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas TK. β-galactosidase activity in the germinating seeds of Vigna sinensis. Phytochemistry. 1985;24:2831–2833. doi: 10.1016/0031-9422(85)80008-X. [DOI] [Google Scholar]
- Blaszczyk J, Lysiak G. Storage properties of Czech pear cultivars ‘Erica’ and ‘Dicolor’. J Fruit Ornam Plant Res. 2001;9:71–76. [Google Scholar]
- Bourtoom T. Edible protein films: properties enhancement. Int Food Res J. 2009;16:1–9. [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Funct Plant Biol. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Dhall RK. Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci Nutr. 2013;53:435–450. doi: 10.1080/10408398.2010.541568. [DOI] [PubMed] [Google Scholar]
- Eccher-Zerbini P (2002) The quality of pear fruit. In:VIII international symposium on pear, vol 596. pp 805–810
- Elgar HJ, Watkins CB, Murray SHF, Gunson A. Quality of ‘Buerre Bose’ and ‘Doyenne du Cornice’ pears in relation to harvest date and storage period. Postharvest Biol Technol. 1997;10:29–37. doi: 10.1016/S0925-5214(96)00058-0. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Hanna MA. Soy protein/fatty acid films and coatings. Inform. 1997;8:622–624. [Google Scholar]
- Gol NB, Patel PR, Rao TVR. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol. 2013;85:185–195. doi: 10.1016/j.postharvbio.2013.06.008. [DOI] [Google Scholar]
- Goncalves ED, Antunes PL, Brackmann A. Controlled atmosphere storage of Asian pears cv. Nijisseiki. Rev Bras Frutic. 2000;22:226–231. [Google Scholar]
- González-Aguilar GA, Valenzuela-Soto E, Lizardi-Mendoza J, Goycoolea F, Martínez-Téllez MA, Villegas-Ochoa MA, Monroy-Garcia I, Ayala-Zavala JF. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J Sci Food Agric. 2009;89:15–23. doi: 10.1002/jsfa.3405. [DOI] [Google Scholar]
- Hetong L, Yufang X. Postharvest softening physiological mechanism of Huanghua pear fruit. Sci Agric Sinica. 2003;36:349–352. [Google Scholar]
- Hussain PR, Meena RS, Dar MA, Wani AM. Carboxymethyl cellulose coating and low-dose gamma irradiation improves storage quality and shelf life of pear (Pyrus communis L., Cv. Bartlett/William) J Food Sci. 2010;75:M586–M596. doi: 10.1111/j.1750-3841.2010.01868.x. [DOI] [PubMed] [Google Scholar]
- Jahnke LS, Hull MH, Long SP. Chilling stress and oxygen metabolizing enzymes in Zea mays and Zea diploperennis. Plant Cell Environ. 1991;14:97–104. doi: 10.1111/j.1365-3040.1991.tb01375.x. [DOI] [Google Scholar]
- Javanmard M, Ojnordi S, Esfandyari M (2012) Effect of edible coating based on whey protein and zataria multiflora bioss extract on the shelf life of ‘shah mive’ pear (Pyrus communis). In: VII international postharvest symposium, pp 427–433
- Ju Z, Duan Y, Ju Z. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol Technol. 2000;20:243–250. doi: 10.1016/S0925-5214(00)00120-4. [DOI] [Google Scholar]
- Kaur R, Arya V. Ethnomedicinal and phytochemical prospectives of Pyrus communis Linn. J Pharmacogn Phytochem. 2012;1:15–20. [Google Scholar]
- Lim R, Stathopoulos CE, Golding JB. Effect of edible coatings on some quality characteristics of sweet cherries. Int Food Res J. 2011;18:1237–1241. [Google Scholar]
- Lohani S, Trivedi PK, Nath P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol. 2004;31:119–126. doi: 10.1016/j.postharvbio.2003.08.001. [DOI] [Google Scholar]
- Mazumdar BC, Majumder K. Methods on physico-chemical analysis of fruit. Delhi: Daya Publishing House; 2003. [Google Scholar]
- Meheriuk M, Porritt SW. Effects of waxing on respiration, ethylene production and other physical and chemical changes in selected apple cultivars. Can J Plant Sci. 1972;52:257–259. doi: 10.4141/cjps72-041. [DOI] [Google Scholar]
- Mirhosseini H, Tan CP, Hamid NS, Yusof S, Chern BH. Characterization of the influence of main emulsion components on the physicochemical properties of orange beverage emulsion using response surface methodology. Food Hydrocoll. 2009;23:271–280. doi: 10.1016/j.foodhyd.2008.02.007. [DOI] [Google Scholar]
- Mondal MF. Production and storage of fruits (in Bangla) Mymsningh: Mrs. Afia Mondal; 2000. p. 312. [Google Scholar]
- Monedero FM, Fabra MJ, Talens P, Chiralt A. Effect of oleic acid–beeswax mixtures on mechanical, optical and water barrier properties of soy protein isolate based films. J Food Eng. 2009;91:509–515. doi: 10.1016/j.jfoodeng.2008.09.034. [DOI] [Google Scholar]
- Nandane AS, Dave RK, Rao TVR. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J Food Sci Technol. 2017;54:1–8. doi: 10.1007/s13197-016-2359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Deka BC, Singh A, Patel RK, Paul D, Misra LK, Ojha H. Extension of shelf life of pear fruits using different packaging materials. J Food Sci Technol. 2012;49:556–563. doi: 10.1007/s13197-011-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Tarazaga ML, Massa A, Pérez-Gago MB. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno) LWT Food Sci Technol. 2011;44:2328–2334. doi: 10.1016/j.lwt.2011.03.011. [DOI] [Google Scholar]
- Park HJ. Development of advanced edible coatings for fruits. Trends Food Sci Technol. 1999;10:254–260. doi: 10.1016/S0924-2244(00)00003-0. [DOI] [Google Scholar]
- Park YM. Relationship between instrumental and sensory analysis of quality factors in apple and pear fruits. Korean J Hortic Sci Technol. 2002;20:394–398. [Google Scholar]
- Prabha TN, Yashoda HM, Prasanna V, Jagadeesh BH, Bimba Jain MV. Carbohydrate metabolism in relation to textural softening during fruit ripening. Trends Carbohydr Chem. 2000;6:89–95. [Google Scholar]
- Rhim JW, Gennadios A, Handa A, Weller CL, Hanna MA. Solubility, tensile, and color properties of modified soy protein isolate films. J Agric Food Chem. 2000;48:4937–4941. doi: 10.1021/jf0005418. [DOI] [PubMed] [Google Scholar]
- Rhim JW, Gennadios A, Handa A, Weller CL, Hanna MA. Sodium dodecyl sulfate treatment improves properties of cast films from soy protein isolate. Ind Crops Prod. 2002;15:199–205. doi: 10.1016/S0926-6690(01)00114-5. [DOI] [Google Scholar]
- Ribeiro C, Vicente AA, Teixeira JA, Miranda C. Optimization of edible coating composition to retard strawberry fruit senescence. Postharvest Biol Technol. 2007;44:63–70. doi: 10.1016/j.postharvbio.2006.11.015. [DOI] [Google Scholar]
- Roe JH. Chemical determination of ascorbic acid, dehydroascorbic acid and diketogluconic acids. Methods Biochem Anal. 1954;1:115–139. doi: 10.1002/9780470110171.ch5. [DOI] [PubMed] [Google Scholar]
- Scramin JA, de Britto D, Forato LA, Bernardes-Filho R, Colnago LA, Assis OB. Characterisation of zein–oleic acid films and applications in fruit coating. Int J Food Sci Technol. 2011;46:2145–2152. doi: 10.1111/j.1365-2621.2011.02729.x. [DOI] [Google Scholar]
- Soliva-Fortuny RC, Martín-Belloso O. Microbiological and biochemical changes in minimally processed fresh-cut conference pears. Eur Food Res Technol. 2003;217:4–9. doi: 10.1007/s00217-003-0701-8. [DOI] [Google Scholar]
- Srivastava MK, Dwivedi UN. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000;158:87–96. doi: 10.1016/S0168-9452(00)00304-6. [DOI] [PubMed] [Google Scholar]
- Taghian Dinani S, Hamdami N, Shahedi M, Keramat J. Optimization of carboxymethyl cellulose and calcium chloride dip-coating on mushroom slices prior to hot air drying using response surface methodology. J Food Process Preserv. 2014;38:1269–1278. doi: 10.1111/jfpp.12088. [DOI] [Google Scholar]
- Tanada-Palmu PS, Grosso CR. Effect of edible wheat gluten-based films and coatings on refrigerated strawberry (Fragaria ananassa) quality. Postharvest Biol Technol. 2005;36:199–208. doi: 10.1016/j.postharvbio.2004.12.003. [DOI] [Google Scholar]
- Thimmaiah SK. Standards methods of bio-chemical analysis. New Delhi: Kalyani publishers; 1999. [Google Scholar]
- Wang ZF, Ying TJ, Bao BL, Huang XD. Characteristics of fruit ripening in tomato mutant epi. J Zhejiang Univ Sci. 2005;6:502–507. doi: 10.1631/jzus.2005.B0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Li X, Xu Q, Yun J, Lu Y, Tang Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper Capsicum annuum L.) Food Chem. 2011;124:1443–1450. doi: 10.1016/j.foodchem.2010.07.105. [DOI] [Google Scholar]
- Yaman Ö, Bayindirli L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT Food Sci Technol. 2002;35:146–150. doi: 10.1006/fstl.2001.0827. [DOI] [Google Scholar]
- Zhou R, Li Y, Yan L, Xie J. Effect of edible coatings on enzymes, cell-membrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem. 2011;124:569–575. doi: 10.1016/j.foodchem.2010.06.075. [DOI] [Google Scholar]