Abstract
The extraction of phenolic compounds from Nigella sativa seed cake was optimized in terms of % of EtOH, extraction time, extraction temperature and solid to solvent ratio to maximize the phenolic content yield. The optimized conditions were 40% ethanol for 60 min, at 40 °C, 1/14 solid to sample ratio. The LC–MS profiling of the extract was found to contain many important phenolics such as Kaempferol, p-coumaroyl acid derivative, Thymol-O-sophoroside etc. The extract showed significant antioxidant activity with IC50 values 548.5 ± 9.4, 64.3 ± 2.7 μg/ml and 1.85 ± 0.2 mM TE/g in terms of DPPH scavenging activity, TEAC, and FRAP assay respectively. The results also indicated that the extract has a significant anti-inflammatory potential. This was evaluated as a measure of the membrane stabilization ability and protein denaturation inhibition capacity (IC50 values) and the activities were found to be 318.73 ± 6.98 and 150.39 ± 2.61 μg/ml respectively. Moreover, results of the study are promising and invite to further investigate the above activities in order to confirm them in different experimental situations and to consider for possible use in a nutraceutical approach.
Keywords: Nigella sativa seed cake, Box–Behnken experimental design, LC–Q–TOF–MS/MS, Antioxidant capacity, Phenolic compound
Introduction
In the recent years, awareness about the practice of medicinal plants in the treatment and prevention of various diseases has been increased due to their significant results and rarer side effects. According to the statistics of World Health Organization (WHO), 60–80% of the world’s population depends on traditional herbal medicine for their primary healthcare and treatment. Further, WHO has encouraged developing countries to utilize various medicinal plants available to create new health care programs (AlAttas et al. 2016).
N. sativa (Ranunculaceae) is an erect herbaceous annual plant which is widely cultivated in the entire world. It is native to the south and southwest Asia and also known as black cumin. The major therapeutic values of N. sativa seeds are analgesic, immune stimulation, anti-inflammatory, anti-allergic, anti-histaminic, anti-cancer, anti-asthmatic, hypoglycemic, hypotensive, antimicrobial activities(Atta-ur-Rahman et al. 1995; Kanter et al. 2005; Benkaci-ali et al. 2013; Mansour et al. 2013). In addition, traditionally it is used in the treatment of a headache and toothache, congestion of the nose, intestinal worms and promotion of menstruation and production of milk. Apart from the medicinal herbal oil, N. sativa seeds after the oil removal (cake) can serve as a protein, phenolic, and carbohydrate-rich meal that could improve the immune system. In addition, the N. sativa seed cake is reported to have high phenolic content, especially antioxidant fractions (Adam et al. 2009).
Antioxidants play an important role in neutralizing the free radicals by donating an electron to the free radical which follows to less oxidative stress. Free radicals are the unstable molecule and cause to the development of numerous degenerative disease that includes cognitive impairment, cardiovascular disease, and cancer (Prathapan et al. 2011). The antioxidant can play an important role in inhibiting the propagation of free radical reactions. Therefore, this research is directed towards to find out naturally occurring phytochemicals that protect the human body from diseases.
Plant phenolic compounds are considered to be effective free radical scavengers and studied widely for their beneficial effects in reduction of coronary heart disease, prevention of several kinds of cancer, treatment of urinary tract disorders, and anti-inflammatory and anti-oxidant activities. The extraction and profiling of phenolic compounds from natural sources are immensely important as these phytochemicals are the major constituents in dietary supplements, nutraceutical, functional foods and additives to food, pharmaceutical, and cosmetic products (Doshi et al. 2015; Carciochi and Dimitrov 2014). Therefore, it is important to a screening of N. Sativa seed cake extract for biological activity may provide identification of a newer compound. The yield of the extraction process of bio-active compounds from plant materials is greatly influenced by the process parameters (Singh et al. 2014). The extraction process optimization is also worth studying.
Hence, the current work aimed at optimizing the extraction process for a maximum yield of phenolics and studying the phytochemicals profile of the optimized extract. Further, an attempt was made to investigate the antioxidant and anti-inflammatory activity of extract to analyze a possible use in a nutraceutical production.
Materials and methods
Plant material
N. sativa seeds were procured from the Matunga market, in Mumbai, India. The seeds were cleaned manually and then grounded in a coffee grinder. Oil from seeds was extracted in Soxhlet apparatus for 5 h using n-hexane. The defatted seed flour was dried at room temperature, grounded and passed through a 60-mesh sieve. The samples were stored in a desiccator at room temperature away from light, for further analysis.
Chemicals and standards
2,2′-Diphenyl-1-picrylhydrazyl radical (DPPH), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), Gallic acid, Folin–Ciocalteu’s phenol reagent and quercetin were purchased from Sigma–Aldrich Chemical Company (St. Louis, USA). Solvents and other chemicals (analytical grade) were obtained from Merck Company.
Extraction procedure
All experiment was carried out using 1 g seed cake powder. Different combination of four parameters, namely % of ethanol (0–100%), extraction time (60–120 min), temperature (40–60 °C) and solid to the solvent ratio (1:10–1:20), were studied to optimize the extraction of phenolic compounds from the sample. After extraction, the solvent was removed under vacuum and the dried extract was stored at −22 °C until the further use.
Factorial design
A Box–Behnken design (BBD) was constructed to find the optimum extraction conditions to maximize the phenolic content. The software design expert (version 7.0.0, Stat-Ease Inc., Minneapolis, MN U.S.A.) was used for the experimental design, data analysis, and model building. BBD required an experiment number,
where k is a number of independently variable factors and cp is the number of center point.
All the independent variables considered for the Study, % of EtOH (X1), extraction time (X2), extraction temperature(X3) and solid to solvent ratio (X4), were evaluated at three different levels (−1, 0, +1) and coded according to the following equitation,
where xi is the coded value of the independent variable, Xi is the actual value of an independent variable, X0 is the actual value of the independent variable at the center point and ΔX is the step change value of the independent variable. The coded and uncoded levels of the four independent variables are given in Table 1. In total, 29 experiments were performed with five repetitions of the center point (Table 2). All the experiments were carried out in triplicates.
Table 1.
Extraction yield (%) and phenolic content (mg/100 g of extract) as a function of four independent variable
| Level | |||
|---|---|---|---|
| Process variables | Low | Medium | High |
| (% EtOH) | −1 | 0 | +1 |
| (Extraction time) | −1 | 0 | +1 |
| (Extraction temperature) | −1 | 0 | +1 |
| (Sample to solvent ratio) | −1 | 0 | +1 |
| Run | Yield (%) | TPP (mg GAE/g) | ||||
|---|---|---|---|---|---|---|
| 1 | 50 | 90 | 60 | 10 | 39 | 3.877 |
| 2 | 50 | 90 | 50 | 15 | 30 | 4.422 |
| 3 | 50 | 90 | 50 | 15 | 32 | 4.483 |
| 4 | 50 | 60 | 50 | 20 | 21 | 4.222 |
| 5 | 50 | 90 | 50 | 15 | 31 | 4.461 |
| 6 | 50 | 120 | 40 | 15 | 28 | 4.300 |
| 7 | 50 | 90 | 40 | 20 | 21 | 4.118 |
| 8 | 50 | 90 | 40 | 10 | 25 | 3.914 |
| 9 | 50 | 60 | 50 | 10 | 28 | 3.818 |
| 10 | 60 | 90 | 60 | 15 | 29 | 4.316 |
| 11 | 50 | 90 | 50 | 15 | 31 | 4.405 |
| 12 | 60 | 120 | 50 | 15 | 23 | 4.005 |
| 13 | 40 | 90 | 50 | 20 | 20 | 4.214 |
| 14 | 40 | 60 | 50 | 15 | 22 | 4.388 |
| 15 | 60 | 90 | 40 | 15 | 21 | 3.988 |
| 16 | 50 | 120 | 50 | 10 | 30 | 3.670 |
| 17 | 50 | 90 | 60 | 20 | 17 | 4.096 |
| 18 | 50 | 120 | 60 | 15 | 32 | 4.144 |
| 19 | 50 | 120 | 50 | 20 | 24 | 4.088 |
| 20 | 40 | 90 | 50 | 10 | 18 | 3.896 |
| 21 | 40 | 120 | 50 | 15 | 29 | 4.238 |
| 22 | 50 | 60 | 60 | 15 | 29 | 4.294 |
| 23 | 60 | 90 | 50 | 20 | 11 | 3.881 |
| 24 | 40 | 90 | 60 | 15 | 26 | 4.361 |
| 25 | 60 | 60 | 50 | 15 | 26 | 4.044 |
| 26 | 40 | 90 | 40 | 15 | 23 | 4.344 |
| 27 | 50 | 60 | 40 | 15 | 24 | 4.238 |
| 28 | 60 | 90 | 50 | 10 | 32 | 3.696 |
| 29 | 50 | 90 | 50 | 15 | 33 | 4.372 |
Table 2.
Analysis of variance (ANOVA) and Regression coefficient of the predicted quadratic polynomial model
| % Yield | TPP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of square | Mean square | F-value | P value | df | Sum of square | Mean square | F-value | P value | df | |
| Model | 927.43 | 66.245 | 23.459 | <0.0001 | 1.439 | 0.103 | 20.327 | <0.0001 | ||
| 1.33 | 1.33 | 0.47 | 0.5032 | 0.190 | 0.190 | 37.634 | <0.0001 | |||
| 21.33 | 21.33 | 7.55 | 0.0157 | 0.026 | 0.026 | 5.155 | 0.0395 | |||
| 75.00 | 75.00 | 26.56 | 0.0001 | 0.003 | 0.003 | 0.565 | 0.4646 | |||
| 280.33 | 280.33 | 99.27 | <0.0001 | 0.255 | 0.255 | 50.367 | <0.0001 | |||
| 25.00 | 25.00 | 8.85 | 0.0100 | 0.003 | 0.003 | 0.610 | 0.4476 | |||
| 6.25 | 6.25 | 2.21 | 0.1590 | 0.024 | 0.024 | 4.786 | 0.0462 | |||
| 132.25 | 132.25 | 46.83 | <0.0001 | 0.004 | 0.004 | 0.879 | 0.3644 | |||
| 0.25 | 0.25 | 0.09 | 0.7704 | 0.011 | 0.011 | 2.204 | 0.1599 | |||
| 0.25 | 0.25 | 0.09 | 0.7704 | – | 0.000 | 0.011 | 0.9185 | |||
| 81.00 | 81.00 | 28.68 | 0.0001 | – | 0.000 | 0.011 | 0.9185 | |||
| 204.63 | 204.63 | 72.47 | <0.0001 | 0.114 | 0.114 | 22.470 | 0.0003 | |||
| 8.09 | 8.09 | 2.86 | 0.1127 | 0.098 | 0.098 | 19.290 | 0.0006 | |||
| 12.12 | 12.12 | 4.29 | 0.0573 | 0.020 | 0.020 | 3.885 | 0.0688 | |||
| 153.63 | 153.63 | 54.40 | <0.0001 | 0.876 | 0.876 | 173.289 | <0.0001 | |||
| Residual | 39.53 | 2.823 | 14 | 0.071 | 0.0051 | 14 | ||||
| Lack of fit | 34.33 | 3.433 | 2.641 | 0.1810 | 10 | 0.063 | 0.0063 | 3.229 | 0.1349 | 10 |
| Pure error | 5.20 | 1.3 | 4 | 0.008 | 0.0020 | 4 | ||||
| Cor Total | 966.97 | 28 | 1.510 | 28 | ||||||
| R2 = 0.9591; C.V.% = 6.454 | R2 = 0.95311; C.V.% = 1.714 | |||||||||
In order to predict the optimal responses (phenolic compounds), the following second-order polynomial equation was used to fit the experimental data,
Phytochemical analysis
Polyphenols content
Polyphenolic content of the extract was analyzed by Folin–Ciocalteu’s calorimetric method with some modifications (Belwal et al. 2016). The reaction was done by mixing 0.5 ml of diluted (10×) ethanolic extract, 4.5 ml of distilled water and 0.5 ml of Folin–Ciocaltue’s solution. Incubate the mixture at 28 °C for 5 min and then added sodium bicarbonate solution (7% w/v) along with 2 ml of distilled water this solution. The mixture was then kept in dark for 90 min 28 °C and the absorbance was determined at 765 nm using UV–Vis spectrophotometer (Hitachi U-2001, Japan) against the reagent blank. All the experiments were carried out in triplicates and the results were expressed in milligram gallic acid equivalent per g. dry weight (mg GAE/g dw).
Total flavonoid content
Total flavonoid content of the extract was analyzed by the Alcl3 colorimetric method as described by (Belwal et al. 2016). Briefly, 0.5 ml of extract was mixed with 1.5 ml of distilled water and 0.5 ml of 10% (w/v) aluminum chloride. Further, 0.1 ml of potassium acetate (1 M) and 2.8 ml of distilled water was added to this solution. The reaction mixture was then incubated at room temperature for 30 min and the absorbance was recorded at 415 nm using UV–Vis spectrophotometer (Hitachi U-2001, Japan). Flavonoid quantification was done against quercetin as standard and results were expressed as mg quercetin equivalent per 100 g dry weight (mg QE/100 g dw).
LS–QToF–MS/MS analysis
LC–MS analysis of extract was analyzed using an Agilent 6200 series Liquid Chromatography system at the Sophisticated Analytical Instrumentation Facility, IIT Powai, Mumbai, India. A zorbax eclipse C18 column (5 μm, 150 mm × 2.1 mm) was used. The solvent system comprised of water 95% (Solvent A): acetonitrile 5% (Solvent B) applying the gradient, 0.02–20, 20–26, 26–30 min, with a flow rate 0.2 ml/min and a column temperature of 25 °C. The injection volume was set at 5 μl with a total run time of 30 min.
An Agilent G6550A ultra high definition Accurate-Mass Quadrupole Time of Flight Mass Spectrophotometer using Agilent Mass Hunter Software version B.05.01 (B5125) was employed. Agilent personal Compound Database Library (PCDL) version B.05.01 build 92 was used to create the custom database. The mass spectrophotometer was operated in 2 GHz and the full scan covered the range from m/z 100 to 1000 m/z in the automatic mode.
Sample ionization was achieved by Electrospray Ionisation (ESI) interface in both positive and negative ion mode. In the negative ion mode, the gas and vaporizer temperatures were set to 250 °C, with a gas flow rate of 13 L/min. The nebulizer was set to 35 psig with a corona current of 20 mA, fragmentor 175 V, skimmer 65 V, Octopole RF 750 V and a capillary voltage of 5500 V.
Antioxidant activity
Antioxidant activity of the extract was measured in terms of DPPH radical scavenging activity, ferric equivalent antioxidant power (FRAP) method and Trolox equivalent antioxidant capacity (TEAC) assay.
DPPH radical scavenging activity of the ethanolic extract was determined according to (Perez-Ramirez et al. 2015) with little modification. About 1 ml of DPPH solution (25 mM) was added to 1.0 ml of test samples at different concentration (5–25 μg/ml of extract and standard) and incubated in dark room for 30 min at 28 °C. The intensity of color developed was measured at 517 nm.
The FRAP assay considers antioxidant as a reductant in a redox-linked calorimetric method using a reduced oxidant, Fe (III). Reduction of a ferric tripyridyltriazine complex to ferrous-(2, 4, 6-tripyridyl-s-triazine), which appear in a blue color is measured in terms of absorbance at 593 nm.
The TEAC assay described by (Alashi et al. 2014) has been used with minor modification. The assay assesses the total radical scavenging ability based on the suppression of absorbance of radical cation. 2, 2′-azinobis 3-ethylbenzothiazoline 6-sulfonate (ABTS) along with potassium persulfate is used for the assay. About 10 µl of diluted extract was added to 990 µl of ABTS solution. The amount of ABTS radical consumed by the extract was determined by means of absorbance at 734 nm, after 30 min of reaction time.
In vitro anti-inflammatory assay
Anti-inflammatory activity of the extract was measured with HRBC membrane stabilization assay with some modification (Kadam and Lele 2014). Whole human blood was freshly collected and mixed with equal volume of Alsever solution. The solution was then centrifuged at 3000 rpm for 10 min. The supernatant was separated and packed cells (sediments) were washed thrice with isosaline. Quantification of red blood cell was done with isosaline (10% v/v). The hypotonicity of the solution triggers the membrane lysis and its in-turn stabilizes human RBC membrane. Different concentration of the extract, reference sample, and control sample was mixed with 1 ml phosphate buffer, 2 ml hyposaline and 0.5 ml HRBC suspension. The mixtures were then incubated at 37 °C for 30 min and centrifuged. The hemoglobin content was obtained by measuring the absorbance at 560 nm.
The percent hemolysis was evaluated by
The hemolysis generated in the control was considered as 100%.
Percent hemolysis was evaluated by inhibition of protein denaturation method (Ullah et al. 2014) with slight modifications. The reaction mixture contained extract and 1% aqueous solution of bovine albumin fraction. The reaction pH was adjusted using small amount HCl. The mixture was then incubated at 37 °C for 20 min followed by heating at 50 °C for 20 min. The tube contents were allowed to cool to room temperature and the absorbance was measured spectrophotometrically at 660 nm. With acetylsalicylic acid (ASA) as a standard drug, the percent inhibition of protein denaturation was calculated as follows:
Statistical analysis
All data are presented as the mean value ± standard deviation (SD) of three replicates. Analyses were performed using the SPSS statistical software package (Version 16.0) and the variance (p < 0.05) of the data was analyzed by a one-way ANOVA test (Duncan’s test).
Results and discussion
Fitting the model
Response surface methodology is an experimental statistical modeling technique, to determine the relationship between experimental and the predicted results. In the present study, Box–Behnken Design was used to obtain a precise model for the optimization of extraction process (Daniel and Cross 2013). Four process variables (% of ethanol (EtOH), extraction time, extraction temperature and sample to solvent ratio) at three levels as presented in Table 1 was used for the study. Extraction experiments were conducted according to the experimental design in order to get the optimum conditions and to analyze the influence of process variable on the extraction yield (%Y), total phenolic (TPC) and flavonoid content (TFC).
The coefficients for an independent variable were obtained by multiple linear regression as shown in Table 2. The positive linear effect of extraction time (), extraction temperature () and sample to solvent ratio () were found to be significant in all responses at p < 0.05 level. However, the quadratic effect of % of EtOH () and sample to solvent ratio () was found to produce significant (p < 0.001) positive effect on % yield, TPC and TFC. The interaction effect of , and was also significant for all the responses. However the interaction between AB and AC significantly (p < 0.05) affected the total phenolic content.
The model showed highly significant and fitted well with the experimental data of Yield, TPC and TFC with less variation around mean (R2 value 0.96, 0.95, 0.97) as presented in Table 2. Moreover, the coefficient of variance (CV), which represents the extent to which the data were dispersed, for optimized bioactive extract was within the acceptable range (1.4–6.4). CV represents standard deviation as a percentage of the mean and hence the high value of CV implies that the variation in the mean value is high and does not satisfactorily develop an appropriate response model, whereas the small value of CV gives a better reproducibility (Daniel and Cross 2013). The lack-of-fit test, that measures the competence of the model, did not result in a significant F-value, reveals that model was sufficiently accurate for predicting the properties of bioactive extract as presented in Table 2.
Effect of extraction variable on extraction yield %
The model obtained had a highly significant limit (p < 0.0001) with experimental data. Analysis of variance (ANOVA) revealed the linear ( and ) and interactive ( and ) effect of model the on % yield. Moreover, quadratic terms such as and 2 were found to be highly significant (Table 2). Based onregression coefficient (β) value, sample to solvent ratio () showed a major positive effect on extraction yield % followed by quadratic term % EtOH (), sample to solvent ratio (), interaction of % EtOH and sample to solvent ratio (), interaction of extraction temperature and sample to solvent ratio (), extraction temperature ().
The best fitted second order polynomial equation after removal of the non-significant variables:
The non-significant value of lack of fit (F-value = 2.64) showed the model is fitted to the special influence of the variables on the response with good prediction (R2 = 0.95) (Table 2).
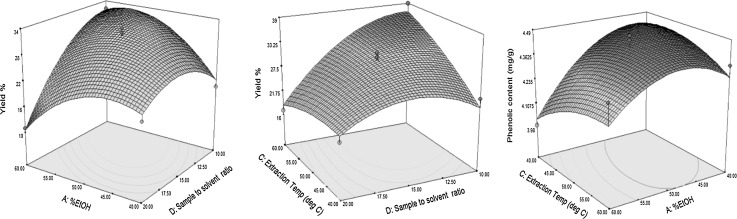
Considering the interactive effect of process variable shown in Fig. 1a, only the interaction between % EtOH and sample to solvent ratio () and extraction temperature and sample to solvent ratio () showed a significant (p < 0.0001) effect on extraction yield.
Fig. 1.
Effect of extraction variable on extraction yield % and phenolic content (mg/g)
Effect of extraction variable on Total phenolic (TP)
Various factors showed significant (p < 0.05) effect on total phenolic (TP) content. These are—linear (, and ) and interactive () (Table 2). Moreover, quadratic term-, and showed significant (p < 0.001) effect on total phenol extraction. Sample to solvent ratio () produced a higher effect on TP content followed by linear terms of sample to solvent ratio () and % of EtOH. These results effects of quadratic terms, % of EtOH () and extraction time (), linear- extraction time () and interaction of % of EtOH and extraction temperature () were confirmed with their regression coefficient (β) values (Table 2).
The fitted the second order polynomial equation showed as
The no-significant value of lac-of-fit (F-value = 3.23) showed that the model is fitted with good prediction (R2 = 0.95) (Table 2).
Among the variables considered, the only interaction of the % of EtOH and extraction temperature showed significant (p < 0.05) effect on total phenolic (TP) content. (Table 2). With increasing extraction temperature and % of EtOH, the TP content significantly increased. At % EtOH (50–55) and extraction temperature (45–50 °C), the extraction of phenolic (TP) content was maximum. These results are in agreement with those in the literature (Tan et al. 2013; Wang et al. 2008) where they reported an increase in the solubility of the phenolic content with increasing extraction temperature. This might be due to the enhanced mass transfer by penetration of solvent into the plant matrix, which increases the viscosity and decreases the surface tension (Al-Farsi and Lee 2008). A similar effect in phenolic content extraction from canola meal was reported (Wang et al. 2008).
As illustrated in Fig. 1, increase in extraction time until 90–100 min resulted in enhancement in phenolic content, after which the phenolic content started decreasing. In addition, when extraction temperature reached a certain threshold (50 °C), the total phenolic (TP) content was declined. This might be due to the degradation of the phenolic compound due to the sample pyrolysis (Singh and Saldaña 2011). Further, the prolonged extraction time might have increased the decomposition of phenolic.
The optimum conditions for extraction to get a maximum yield of phenolics was with 40% ethanol for 60 min, at 40 °C with a solid to sample ratio 1/14. This extract was used for all the further analysis.
Determination of phenolic and flavonoid content
The analysis was carried out to determine the total phenolic content (TP) and total flavonoid content (TF) in ethanolic extract of N. sativa species and the results are presented in Table 4. The TP value is expressed as gallic acid equivalent (mg GAE/ g dw plant material) and TF as quercetin equivalent (mg QE/100 g dw plant material).
Table 4.
The total phenolic and flavonoids content, Antioxidant capacity and In vitro anti-inflammatory assay
| % of inhibition (IC50) | |||||||
|---|---|---|---|---|---|---|---|
| TPC (mg GAE/g dw plant material) | FAC (mg QE/100 g dw plant material) | DPPH IC50 (μg/ml) | TEAC IC50 (μg/ml) | FRAP (mM TE/g) | Stabilization of HRBC (ug/ml) | Inhibition of protein denaturation (ug/ml) | |
| N. sativa | 4.258 ± 0.2 | 8.94 ± 0.1 | 548.5 ± 9.4 | 64.3 ± 2.7 | 1.85 ± 0.2 | 318.73 ± 6.98 | 150.39 ± 2.61 |
| 42.4 ± 0.45 (TE) | 15.7 ± 0.23 (TE) | – | 89.3 ± 1.43 (ASA) | 78.41 ± 1.65 (ASA) | |||
Each value is the mean ± SD of three independent measurements
TPC Total phenolic content, FAC Flavonoid content, GA Gallic acid equivalent, QE Quercetin equivalent, TE Trolox equivalents, ASA Acetylsalicylic acid
The extract of N. sativa contained 4.258 ± 0.2 and 8.94 ± 0.1 mg/100 g dw of total phenolic and flavonoid respectively. Claudia-crina toma et al. (2015) found a similar content of total phenolic content in 70% of ethanolic extract, 4.12 mg caffeic acid equivalent/g dw by using maceration process. Our result is also in line with the finding of Thippeswamy et al. who reported similar results for 80% methanolic extract, 4.1 mg of gallic acid equivalent/g dw by using Soxhlet extraction. In addition, the higher value of total phenolic content (TP) from 95% ethanolic extract by maceration at room temperature were reported as (15.8 mg/g dw) (Al-Bishri et al.). Claudia-crina toma et al (2015) reported that N. sativa has a flavonoid content of 2.01 mg/g in 70% of the ethanolic extract. Ratz-Lyko et al. also reported a significantly higher content of 19.25 mg/g dw quercetin equivalents in a 50% ethanolic extract of seed cake.
LC–ESI–Q–TOF–MS/MS analysis
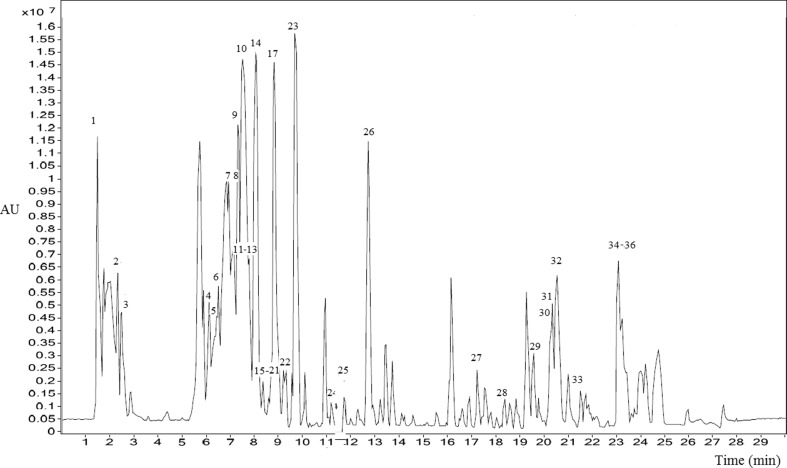
LC–MS consider being an indispensable tool, used in various scientific fields (Abid et al. 2017). In the present work, we used reverse phase HRLC–ESI–Q–TOF–MS/MS to achieve rapid metabolite and peak separation. HRLC coupled with Q–ToF–MS can also detect components with high sensitivity. HRLC–MS total ion chromatogram of N. sativa is presented in Fig. 2. Peaks were identified in reference to the retention times, fragmentation patterns for individual component and by comparison with published libraries (Table 3). Peaks were also authenticated with molecular weight estimates and supplementary data from Phenol-Explorer database. A total of 34 major peaks were detected and the components corresponded to the peaks belonged to various class (Table 3) including phenolic acid, flavonoid, ester, triterpene saponins, alkaloid, vitamin and fatty acid.
Fig. 2.
LC–MS chromatogram for N. sativa seed cake extract (peak 1-36)
Table 3.
List of the detected compounds from ethanol extracts observed from negative ion LC–MS/MS with their retention times, UV spectra and mass spectral data
| Peak no. | Class | Phytochemical compound | RT (min) | Molecular formula | [M+H]+ or [M+Na]+, m/z | Mass | MS/MS fragment ions, m/z | Biological activity |
|---|---|---|---|---|---|---|---|---|
| 1 | Flavonoids | Biochanin A | 1.508 | C16H12O5 | 285.07 | 284.263 | 267, 268, 283 | Gastroprotective |
| 2 | Terpene | Limonen-6-ol, pivalate | 2.4 | C15H24O2 | 236.995 | 236 | 57, 93, 107, 134, 185, 236 | Antioxidant and anti- inflammatory |
| 3 | Turpentine | ß-Pinene | 2.507 | C10H16 | 136.0 | 136 | 53, 69, 93, 121 | Anti-inflammatory, anti-cancer agent, expectorant and bronchodilator properties. |
| 4 | Flavonoid | Kaempferol | 6.119 | C15H10O6 | 287.1074 | 287 | 239, 187, 143 | Antioxidant |
| 5 | Alkaloid | Pyrrolidin-2-one-3ß-(propanoic acid, methyl ester),5- methylene-4α | 6.325 | C16H25NO5 | 311.1649 | 311 | 57, 81, 110, 136, 149, 164, 196, 224, 255, 280, 311 | Unknown |
| 6 | Phenolic | p-coumaroyl acid derivative | 6.451 | C9H8O3R1 | 278.1348 | 278 | 163, 119 | Antioxidant property, Anti-inflammatory activity |
| 7 | di-sacchride | d-Glucose,6-O-α- D-galactopyranosyl | 6.8 | C12H22O11 | 342.1659 | 342 | 60, 73, 85, 110, 126, 182, 212, 261 | Anticoagulant, anti-inflammatory, psychotomimetic and anticancer activities |
| 8 | Sesquiterpenes | Longifolene | 6.953 | C15H24 | 204.1188 | 204 | 55, 67, 79, 94, 107, 119, 133, 147, 161, 175, 189, 204 |
Anti-tumor activity |
| 9 | Sesquiterpenes | ß-Bisabolene | 7.21 | C15H24 | 204.1186 | 204 | 55, 69, 93, 109, 135, 161, 189, 204 |
Anti-ulcer activity |
| 10 | Flavonoids | Apigenin | 7.432 | C15H10O5 | 270.109 | 270.237 | ||
| 11 | Flavonoid | Myricetin-3O-glucoside | 7.712 | C21H20O13 | 481.25 (M+H)+ | 481 | 301, 178, 151 | Antioxidant |
| 12 | Flavonoid | Kaempferol-3-O-glucoside | 7.871 | C27H20O11 | 449.1642 | 449 | 284, 255, 227 | Antioxidant, anti-inflammatory, antimicrobial |
| 13 | Flavonoid | quercetin-3-O-glycuronide | 7.936 | C21H18O13 | 484 (M+H)+ | 479 | 303, 285, 275, 257 | Antioxidant |
| 14 | Phenol | Thymol-O-sophoroside | 8.072 | C22H33O11 | 473.2134 (M+H)+ | 473 | 293, 149 | Anti-microbial activity |
| 15 | Alkaloid | Norargemonine | 8.587 | C20H22NO4 | 342 | 341 | 295, 249 | Anti-malerial |
| 16 | Flavonoid | Kaempferol 3-O-[β-D-glucopyranosyl-(1→2)-β-D-galactopyranosyl-(1→2)- β D-glucopyranoside] | 8.711 | C33H39O21 | 771.1685[MH] | 771 | 609, 447, 285 | Anticancer, Antioxidant, anti-inflammatory, antimicrobial antidiabetic, anti-allergic, cardioprotective |
| 17 | Alkaloid | Nigellidine | 8.825 | C18H19N2O2 | 295.1445[MH] | 295 | 163 | Immune stimulatory, anti-inflammatory, anti-hypertensive, antimicrobial |
| 18 | Phenolic | 2”–O-pentoxide-8-C-hexoside luteolin | 8.925 | 579.2273 | 579 | 459, 429, 357, 327, 309 | ||
| 19 | Flavonoid | kaempferol-3-O-hexose-O-pentoside | 8.926 | 581 | 281 | 449,287 | Anti-inflammatory | |
| 20 | Fatty acid | 10,13-Eicosadienoic acid, methyl ester | 8.953 | C21H38O2 | 322.1231 (M+H)+[–H2O] | 322 | 55, 67, 95, 109, 124, 150, 164, 192, 224, 248, 291, 322 |
Anti-bacterial |
| 21 | Flavonoid | Quercetin-2-O-rutinoside | 8.971 | 611.253(M+H)+ | 611 | 301 | ||
| 22 | Isoquinoline alkaloid | Nigellimine | 9.237 | C12H13NO2 | 203.1153 | 203 | 188, 172, 160, 145, 131, 130, 117, 101 | Antibacterial and antifungal activity |
| 23 | Phenolic | Gentisic acid dipentoside | 9.566 | C17H21O12 | 417.111 | 417 | 153 | Anti-inflammatory, anti-bacterial activity |
| 24 | Phenolic | 1,1-Diphenyl-4- phenylthiobut-3-en-1-ol | 11.207 | C22H20OS | 332.2082 | 332 | 55, 81, 105, 121, 135, 150, 179, 205, 233, 314 | Anti-inflammatory properties |
| 25 | Fatty acid | 1-Heptatriacotanol | 11.729 | C37H76O | 257.0646 | 256 | 55, 81, 95, 147, 161, 190, 257 |
Antioxidant, anticancer, anti-inflammatory and to sex hormone activity |
| 26 | Alkaloid | 2-(4-Nitrobutyryl) cyclooctan one | 12.931 | C12H19NO4 | 241.138 | 241 | 55, 69, 97, 123, 153, 193, 213, 241 |
Anti-tumor activity |
| 27 | 2H- Benzo[f]oxireno[2,3- E]benzofuran-8(9H)- one,9-[[[2-(dimethylar | 17.567 | C19H32N2O3 | 336.2189 | 336 | 58, 81, 109, 149, 173, 204, 233, 278, 336 |
Unknown | |
| 28 | Fatty acid | 3-Hydroxydodecanedioic | 18.376 | C16H32O2 | 251.12225 | 246.1438 (M+H)+ | 43, 39, 73, 55, 129, 213, 239, 256 (M) | Antibacterial |
| 29 | Fatty acid | 9-hydroxy-12-oxo-10-octadecenoic acid | 19.793 | C15H35NO | 317.2047 (M+Na)+ | 312.226 | 59, 72, 83, 114, 184, 212, 264, 281 |
Anti-inflammatory activity and antibacterial activity |
| 30 | Flavonoid | Myricetin | 20.19 | C15H19O8 | 319.33 (M+H)+ | 318 | 287, 179, 151 | Antioxidant, antidiabetic, antiatherosclerotic, antithrombic |
| 31 | Flavonoid | Quercetin | 20.332 | C15H10O7 | 302.21 | 302 | 273, 243, 278, 252, 232 | Antioxidant and anti-inflammatory |
| 32 | Phenolic | p-Coumaroyl glucose | 21.527 | C15H18O8 | 326.153 | 326 | 163, 119 | Antioxidant and anti-inflammatory |
| 33 | Vitamin | l-(+)-Ascorbic acid 2,6- dihexadecanoate | 20.547 | C38H68O8 | 652.3022 | 652 | 57, 73, 85, 98, 115, 129, 143, 157, 185, 199, 213, 227, 256, 297, 322, 353 |
Antioxidant, cardio protective, cancer preventive, flavour and anti-infertility |
| 34 | Phenolic | Thermoquinol glucosidase | 21.535 | C15H17O8 | 325.093 | 325 | 168 | Antioxidant and anti-inflammatory |
| 35 | Flavones | Apigenin 7-O-(6’’-malonyl-apiosyl-glucoside) | 23.171 | C29H30O17 | 650 | 649 | 266, 336 | Anti-inflammatory activity |
| 36 | Alkaloid | Magnoflorine | 23.214 | C20H24NO4 | 342.27 | 297, 282, 175 |
As indicated in Table 3, the structural characterization of phenolic acid was mainly based on their MS spectra. Six phenolic compound (peak 6, 14, 18, 23, 24, 32 and 34) (Fig. 2, Table 3) were detected. Thermoquinol glucoside was annotated in peak (34) with a high-resolution mass of 325.093. Similar phenolic glycosidases have been previously reported in Nigella species and Origanum (AlAttas et al. 2016) which are likely to serve as a storage form of thymoquinone. Additionally, peak 6, 14, 18, 23, 24 and 32 showed m/z 287.134, 473.213, 579.227, 411.11, 332.208 and 326.153 amu with predicted molecular formula of C9H8O3R1, C22H33O11, C17H21O12 C22H20OS, and C15H18O8 interpreted asp-coumaroyl acid derivative, thymol-O-sophorodide, 2” –O-pentoxide-8-C-hexoside luteol, gentisic acid dipentoside, 1,1-Diphenyl-4- phenylthiobut-3-en-1-ol and p-coumaroyl glucose respectively.
MS spectral interpretation allowed the identification of four alkaloid peaks (5, 15, 17, 22, 26 and 36,) marked by their high-resolution masses and higher response in positive ionization mode. Further, the peaks were identified as Nigellimine (peak 22) (m/z 203.116) and Magnoflorine (peak 36) (m/z 342.27), an aporphine type of isoquinoline alkaloid with potential antioxidant, antibacterial lipoxygene inhibitory (Rackova et al. 2004), immunosuppressive (Mori et al. 1994) and antifungal activity. This alkaloid class has also been reported in different Nigella species (Kupeli et al. 2002; Benkaci-ali et al. 2013) which exert anti-inflammatory property. Moreover, Peak 5, 15, 17 and 26 showed the mass of m/z 311.16, 340.257, 295.144 and 771.269 amu annotated as Pyrrolidine-2-one-3ß- (propanoic acid, methyl ester),5- methylene-4α, Norargemonine, Nigellidine and 2-(4-Nitrobutyryl) cyclooctan one respectively.
As indicated in (Fig. 2 and Table 3), MS spectral analysis presented four triterpene (Peak 2, 3, 8 and 9) showing fragment ion at m/z 236.995, 136.0705, 204 and 204.11 amu with molecular formula C15H24O2, C10H16, C15H24 and C15H24 annotated as Limonene-6-ol, pivalate, ß-Pinene, Longifolene and ß-Bisabolene respectively. ß-Pinene is a volatile monoterpene that possesses anti-microbial (Liao et al. 2016) and antidepressant-like activity (Guzmán-gutiérrez et al. 2015).
The flavonoids also found in low or even trace amount. MS/MS spectral analysis revealed the presence of Biochanin (peak 1), Apigenin (10 and 30), Myricetin (11 and 30), Kaempferol (4, 12, 16 and 19) and Quercetin (13, 21 and 31) as shown in (Fig. 2, Table 3). Detected flavonoid were mono, di and tri-flavonol glycosides in nature. The nature of sugars was revealed from the elimination of the sugar residue to help structural elucidation by MS/MS i.e., 162 amu (hexose; glucose or galactose), 146 amu (rhamnose) or 132 amu (pentose). Compound 11 and 12 exhibited similar MS spectra characterized by loss of 162 amu fragment (hexosyl radical) to give rise to the ions of their deprotonated glycones as base peak (11 m/z 319 [Myricetin-H]− and 12 m/z 387 [karmpferol-H]−). The presence of Kaempferol 3-O-[β-D-glucopyranosyl-(1→2)-β-D-galactopyranosyl-(1→2)- β D-glucopyranoside] is reported in N. sativa(Kanter et al. 2005). Since in other species, it is present in trace levels, it can be used as a marker to distinguish N. sativa oils or extracts from extracts of its closely related species. Biochanin (Peak 1; m/z 285.07) is an o-methylated iso-flavonoid with potential anti-inflammatory and antioxidant activity (Liu et al. 2016). MS spectra interpretation allowed the detection of apigenin signal (m/z 270.109, C15H10O5) in peak (10 and 35). The presence of this flavonoid class is reported also in Ocimum basilicum var. thyrsiflorum with potential antioxidant activity (Abdelhady and Motaal 2016).
Further, MS spectra revealed the presence of four minor fatty acids (peak 20, 25, 28 and 29) (Fig. 2, Table 3). Briefly, 10, 13-Eicosadienoic acid, methyl ester (peak 20), 1-Heptatriacotanol (peak 25), 3-Hydroxydodecanedioic (Peak 28) and 9-hydroxy-12-oxo-10-octadecenoic acid (peak 29) interpreted with m/z 322.12, 257.06, 251.12 and 317.204 and a predicted formula of C21H38O2, C3H76O, C16H32O2 and C15H35NO respectively. Peak (28 and 29) showed the loss of water molecule(s) masses from their molecular ion peak masses. Importantly, fatty acid derivatives with hydroxyl groups receive a significant attention due to their antimicrobial, cytotoxic and anti-inflammatory activity (Martin-arjol et al. 2010). A similar result was found in other N. sativa species also (Benkaci-ali et al. 2013).
1-(+)-Ascorbic acid 2, 6- dihexadecanoate (Peak 33) was an identified vitamin with the precursor ion at 652.302 and a predicted formula C38H68O8. This vitamin class has been reported to exhibit a potential antioxidant, cardioprotective and anti-cancer property. Lastly, d-Glucose, 6-O-α- D-galactopyranosyl (peak 7) was identified by MS spectra analysis, showing precursor ion at m/z 342.16 and fragment ion at m/z 60, m/z 73, m/z 85, m/z 110, m/z 126, m/z 182, m/z 212 and m/z 261. The similar compound was reported in candid albicans which exhibit antibacterial activity (Kadhim et al. 2016).
In vitro antioxidant activity
Phenolic compounds are considered to be effective in free radical scavenging activity and widely distributed in plants origin, that play an important role in preventing oxidative damages to the cellular component as a consequence of the production of mediator and chemotactic factors which lead to various health problems and degenerative diseases (Morita et al. 2017). The N. sativa seed cake extract was screened for antioxidant property in terms of 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay due to their hydrogen donating ability, ferric ion reducing antioxidant power assay (FRAP) and Trolox equivalent antioxidant capacity (TEAC) method (Table 4).
The extract showed good capacity for scavenging DPPH free radical (IC50: 548.5 ± 9.4 μg/ml). Similar results were observed by (Adam et al. 2009) also, with an IC50 value 624.7 ± 12.77 μg/ml in 70% ethanolic extract. Ratz-Lyko et al. (2013) reported an IC50 of 500 and 350 μg/ml respectively for N. sativa seeds, before and after hydrolysis in 50% ethanolic extract. However, Naidu and Thippeswamy 2005 reported a higher IC50 value, 1.24 mg/dw for methanolic extract. Antioxidant activity of the phenolic compound in quinoa seeds was also promising (Carciochi and Dimitrov 2014).
Concerning with ferric reducing ability, N. sativa seed extract showed a significant antioxidant activity (IC50 = 1.85 ± 0.2 mM TE/mg). (Adam et al. 2009), who carried out the antioxidant investigation by FRAP method, had demonstrated a dose-dependent free radical scavenging capacity of aqueous methanolic extract.
The TEAC result is in agreement with the DPPH scavenging activity results, showing that N. sativa seed exhibit a good antioxidant effect (IC50 = 64.3 ± 2.7 μg/ml). A similar value of IC50:77.7 ± 1.26 μg/ml was reported, in 70% ethanolic extract. However, a much higher value of IC50 (475 μg/ml) was reported for a 50% aqueous-ethanolic extract, suggesting lower antioxidant activity.
The results of the DPPH, FRAP, and TEAC methods are in agreement with the phenolic and flavonoid content, indicating N. sativa seed as having a significant antioxidant activity.
In Vitro anti-inflammatory activity
The anti-inflammatory activity of different compounds or extracts is different and very complex (Alvarez-Suarez et al. 2016; Gasparrini et al. 2017). It involves the breakdown of the lysosomal membrane as well as activation of many antioxidant enzymes. Therefore, in our study, we evaluated the anti-inflammatory potential of the extract by HRBC membrane stabilization assay and protein denaturation inhibition assay. Membrane stabilization of HRBC and protein denaturation were studied to further establish the mechanism of anti-inflammatory activity of N. Sativa seed cake extract(100–500ug/ml). The erythrocyte membrane possesses a similar structure with the lysosomal membrane. Therefore, stabilizing the effect of the extract on HRBC membrane suggests that it can help stabilize the lysosomal membranes also (Kadam and Lele 2014). The results of our study indicated that the components in the extract maintained the tonicity and balance of the membrane and thereby prevented the inhibition of HRBC membrane lysis. The percent membrane stabilization by the extract was studied at different concentration and the results are tabulated in Table 4.
Protein denaturation is one of the major causes of inflammation as it ignites the production of auto-antigens in inflammation (Ullah et al. 2014). From the results obtained, it can be observed that ethanolic extract of N. sativa is capable of regulating autoantigen production and thereby the inhibition of denaturation of proteins which are involved in rheumatic disease (Table 4).
Conclusion
In this study, the extraction of phenolics from the N. sativa seed cake was optimized and the optimized process conditions were 40% ethanol for 60 min, at 40 °C with 1/14 solid to sample ratio. Further, the bioactive composition of the extract was evaluated and making this paper the first one detailing the same. LC–ESI–Q–TOF–MS technique was adopted to reveal the primary and secondary metabolites present in the extract. The extract was found to be a strong antioxidant and anti-inflammatory in nature. Results of the study are promising and invite to further investigate the above activities in order to confirm them in different experimental situations and to consider for possible use in a nutraceutical approach.
Acknowledgements
The authors are grateful to UGC-BSR (Government of India) for providing financial assistance during the course of this investigation.
Compliance with ethical standards
Conflict of interest
Authors have no conflicts of interest.
References
- Abdelhady MIS, Motaal AA. A cytotoxic C-glycosylated derivative of apigenin from the leaves of Ocimum basilicum var. thyrsifloru. Braz J Pharmacogn. 2016 [Google Scholar]
- Abid M, Yaich H, Cheikhrouhou S, et al. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J Food Sci Technol. 2017 doi: 10.1007/s13197-017-2727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A, Mohamad R, Ismail M, Ismail N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009;116:306–312. doi: 10.1016/j.foodchem.2009.02.051. [DOI] [Google Scholar]
- Alashi AM, Blanchard CL, Mailer RJ, et al. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014;146:500–506. doi: 10.1016/j.foodchem.2013.09.081. [DOI] [PubMed] [Google Scholar]
- AlAttas S, Zahran F, Turkistany S. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med J. 2016;37:235–244. doi: 10.15537/smj.2016.3.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Farsi MA, Lee CY. Food chemistry optimization of phenolics and dietary fiber extraction from date seeds. Food Chem. 2008;108:977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Alvarez-Suarez JM, Giampieri F, Cordero M, et al. Activation of AMPK/Nrf2 signaling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J Funct Foods. 2016;25:38–49. doi: 10.1016/j.jff.2016.05.008. [DOI] [Google Scholar]
- Atta-ur-Rahman Malik S, Hasan S, et al. Nigellidine—a new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995;36:1993–1996. doi: 10.1016/0040-4039(95)00210-4. [DOI] [Google Scholar]
- Belwal T, Dhyani P, Bhatt ID, et al. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits. Food Chem. 2016;207:115–124. doi: 10.1016/j.foodchem.2016.03.081. [DOI] [PubMed] [Google Scholar]
- Benkaci-ali F, Akloul R, Boukenouche A, De Pauw E. Composition of the essential oil of Nigella sativa seeds extracted by microwave steam distillation. J Essent Oil Bear Plants. 2013;16:781–794. doi: 10.1080/0972060X.2013.813275. [DOI] [Google Scholar]
- Carciochi RA, Dimitrov K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol. 2014;52:4396–4404. doi: 10.1007/s13197-014-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel WW, Cross CL. Biostatistics-a foundation for analysis in the health sciences. 10. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- Doshi P, Adsule P, Banerjee K, Oulkar D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. J Food Sci Technol. 2015;52:181–190. doi: 10.1007/s13197-013-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini M, Forbes-Hernandez TY, Giampieri F, et al. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem Toxicol. 2017;102:1–10. doi: 10.1016/j.fct.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Guzmán-gutiérrez SL, Bonilla-jaime H, Gómez-cansino R, Reyes-chilpa R. Linalool and β -pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–29. doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Kadam D, Lele SS. Anti-inflammatory activity of the fruit extract of Benincasa hispida. J Nutr Ther. 2014;3:178–182. doi: 10.6000/1929-5634.2014.03.04.6. [DOI] [Google Scholar]
- Kadhim MJ, Mohammed GJ, Hussein H. Analysis of bioactive metabolites from Candida albicans using (GC-MS) and evaluation of antibacterial activity. Int J Pharm Clin Res. 2016;8:655–670. [Google Scholar]
- Kanter M, Demir H, Karakaya C, Ozbek H. Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone against acute alcohol-induced gastric mucosal injury in rats. World Gastroenterol. 2005;11:6662–6666. doi: 10.3748/wjg.v11.i42.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupeli E, Kosar M, Yesilada E, et al. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72:645–657. doi: 10.1016/S0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- Liao S, Shang S, Shen M, et al. One-pot synthesis and antimicrobial evaluation of novel 3-cyanopyridine derivatives of (-) - β -pinene. Bioorg Med Chem Lett. 2016 doi: 10.1016/j.bmcl.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang T, Liu X, et al. International immunopharmacology biochanin a protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int Immunopharmacol. 2016;38:324–331. doi: 10.1016/j.intimp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Mansour RS, Nasser AK, Abo NY. The effect of different Nigella sativa L. seed (cake) concentrations on leukocytes counts and some serum immunological parameters in calves. Tikrit J Pure Sci. 2013;18:31–35. [Google Scholar]
- Martin-arjol I, Bassas-galia M, Bermudo E, et al. Identification of oxylipins with antifungal activity by LC–MS/MS from the supernatant of Pseudomonas 42A2. Chem Phys Lipids. 2010;163:341–346. doi: 10.1016/j.chemphyslip.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Mori H, Fuchigami M, Inoue N, et al. Principle of the bark of Phellodendron amurense to suppress the cellular immune response. Planta Med. 1994;60:445–449. doi: 10.1055/s-2006-959529. [DOI] [PubMed] [Google Scholar]
- Morita M, Naito Y, Yoshikawa T, Niki E. Antioxidant capacity of blueberry extracts: peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. J Berry Res. 2017;7:1–9. doi: 10.3233/JBR-170152. [DOI] [PubMed] [Google Scholar]
- Naidu KA, Thippeswamy NB. Antioxidant potency of cumin varieties—cumin, black cumin and bitter cumin—on antioxidant systems. Eur Food Res Technol. 2005;220:472–476. doi: 10.1007/s00217-004-1087-y. [DOI] [Google Scholar]
- Perez-Ramirez IF, Castano-Tostado E, Leon R-D, et al. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015;172:885–892. doi: 10.1016/j.foodchem.2014.09.126. [DOI] [PubMed] [Google Scholar]
- Prathapan A, Lijo Cherian O, Nampoothiri SV, et al. In vitro antiperoxidative, free radical scavenging and xanthine oxidase inhibitory potentials of ethyl acetate fraction of Saraca Ashoka flowers. Nat Prod Res. 2011;25:298–309. doi: 10.1080/14786419.2010.510472. [DOI] [PubMed] [Google Scholar]
- Rackova L, Majekova M, Kostalova D, Stefek M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg Med Chem. 2004;12:4709–4715. doi: 10.1016/j.bmc.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Ratz-Lyko A, Arct J, Pytkowska K, et al. Effect of enzymatic hydrolysis on the antioxidant properties of alcoholic extracts of oilseed cakes. Food Technol Biotechnol. 2013;51:539–546. [Google Scholar]
- Singh PP, Saldaña MDA. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44:2452–2458. doi: 10.1016/j.foodres.2011.02.006. [DOI] [Google Scholar]
- Singh M, Jha A, Kumar A, et al. Influence of the solvents on the extraction of major phenolic compounds (Punicalagin, ellagic acid, and gallic acid) and their antioxidant activities in pomegranate aril. J Food Sci Technol. 2014;51:2070–2077. doi: 10.1007/s13197-014-1267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MC, Tan CP, Ho CW. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int Food Res J. 2013;20:3117–3123. [Google Scholar]
- Ullah HMA, Zaman S, Juhara F, et al. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement Altern Med. 2014;14:346. doi: 10.1186/1472-6882-14-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun B, Cao Y, et al. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]