Abstract
This study was designed to evaluate antimicrobial activity and chemical composition of four different plant essential oils i.e. Ginger oil (GiO), Black seed oil (BSO), Oregano oil (OO) and Rose oil (RO) against different bacterial and fungal strains. Anti-microbial activities of selected essential oils were determined by the microbiological technique using Agar well diffusion assay. After in vitro study, most of the essential oils showed antimicrobial activity against all the selected pathogens. Among all the tested oils, GiO showed strong antimicrobial activity. GiO showed highest antimicrobial activity against Shigella (119.79%), Enteococcus hirae (110.61%) and Escherichia coli (106.02%), when compared with the tetracycline (50 µg/mL) activity. However, Antifungal activity of GiO was found to be present against Candida albicans and Aspergilluas flavus, when compared with clotrimazole (50 µg/mL) activity. Among all the selected bacteria, BSO showed maximum antimicrobial activity against the E. coli followed by Citrobacter freundii. Moreover, BSO had highest zone of inhibition against the C. ablicans (33.58%). OO indicated that, Shigella had the highest sensitivity (12.6 ± 0.58, 131.25%), followed by E. hirae (19.1 ± 0.61, 96.46%) and Salmonella typhi (15.2 ± 0.27, 83.06%) when compared with tetracycline activity. OO showed poor sensitivity against all the selected fungal strains. Furthermore, Gas Chromatography analysis revealed that, Gingerol (10.86%) was the chief chemical constituents found in GiO followed by α-Sesquiphellandrene (6.29%), Zingiberene (5.88%). While, BSO, OO and RO had higher percentage of p-Cymene (6.90%), Carvacrol (15.87%) and Citronellol (8.07%) respectively. The results exhibited that the essential oils used for this study was the richest source for antimicrobial activity which indicates the presence of broad spectrum antimicrobial compounds in these essential oils. Hence, essential oils and their components can be recommended for therapeutic purposes as source of an alternative medicine.
Keywords: Antimicrobial activity, Gas chromatography, Essential oils, Agar well assay, Bioactive compounds
Introduction
Despite a long periods mankind has searched for cure and treatment of different diseases and has been used several medicinal plant and their derived products. In recent years, considerable efforts have been made to control the spread of pathogens with various strategies, including the use of alternative antimicrobial compounds (Raquel et al. 2012). As the pipeline of development for newer antibiotics look bleak, therefore the focus has been shifted towards the development of non-antibiotic substances such as plant or plant-derived products like essential oils (Sonboli et al. 2004). The essential oils and their principle constituents have shown good fighting potential against drug resistant pathogens (Ahmad and Beg 2001; Chavan et al. 2006). Scientifically these essential oils have been proven extremely potent antimicrobial agents in comparison to antibiotics (Ravi et al. 2010). Due to their antimicrobial activity, these natural ingredients appear as a viable and healthy alternative to synthetic antibiotics for the treatment of different diseases. Essential oils are aromatic oily liquids, which was obtained from various parts of plant by different techniques. Steam distillation method is most commonly used for commercial production of essential oils. Around 3000 essential oils are known, from which 300 are commercially available (Seenivasan et al. 2006; Ravi et al. 2010). A large number of essential oils have been investigated for their antimicrobial properties and these essential oils have showed good capability of controlling the growth of microorganisms related to skin, dental caries and food spoilage against the gram-negative and gram-positive bacteria, fungi and yeasts (Johnson et al. 2013). Active compounds produced by essential oils during secondary vegetal metabolism are usually responsible for such biological activity and due to these bioactive components; these essential oils are effective as antimicrobial agent (Silva et al. 2010; Biljana et al. 2011). The growing interest in the use of essential oils in food and pharmaceutical industries, Ginger oil (GiO), Black seed oil (BSO), Oregano oil (OO) and Rose oil (RO) has become important source of investigation against pathogen causing food borne illnesses (Baratta et al. 1998).
Ginger (Zingiber officinale) oil has long been used as naturopathy due to their potential antimicrobial activity as well as different biological activities (Yuva 2014). The medicinal properties have been mainly used for treating the symptoms of vomiting, diarrhea, light-headedness, blurred vision, dyspepsia, tremors, decrease in body temperature and high blood pressure (Ishiguro et al. 2007). The chemical composition of essential oils of ginger has been identified and quantified by means of GC–MS (Gas Chromatogrpahy–mass spectrometry) or GC (Gas Chromatogrpahy) with flame ionization detector applications and carried out works concerning the composition of essential oils. The essential oil of ginger has been used as a medicine against several problems, such as a cure for swelling, sores and loss of appetite, stomach ache, diarrhea, tooth ache, gingivitis arthritis, asthmatic respiratory disorders and motor diseases, also possessing anti-inflammatory activity. Some of these functional properties are generally attributed to the gingerol (Sultan et al. 2005; Kamaliroosta et al. 2013). Black seed (Nigella sativa L.) oil has been known for centuries in the Middle East, Eastern Europe, Asia and Africa as a natural remedy for many ailments (Mohamed and Eman 2012). In vivo and in vitro studies revealed many pharmacological actions for BSO. These include immune system stimulation, anti-inflammatory properties, anti-cancer activity, anti-microbial activity, anti-parasitic activity, anti-oxidant effect, hypoglycemic effect, galactagogue, carminative and laxative effects (Randhawa and Al-Ghamdi 2002). The oil and its constituents are well documented as antimicrobial agents. The essential oils are complex mixtures of the compounds which mainly contain monoterpenes, sesquiterpene hydrocarbons (Gerige et al. 2009). Oregano (Origanum vulgare) is one of the most commonly known culinary herbs worldwide used for cooking purposes. The dried herbs are used in many processed foods such as alcohol beverages, meat products, snack foods, and milk products. Some of the Origanum spp. is also used as a fragrance component in soaps, detergents, perfumes, cosmetics, flavorings, and pharmaceuticals. Oregano oil has anti-bacterial, anti-fungal, anti-parasitic, anti-microbial and antioxidant properties (Afef et al. 2013). Chemical analysis of the OO revealed the presence of several ingredients, most of which possess important antioxidant and anti-microbial properties. Carvacrol and thymol are the two main phenols, which was principally responsible for the antimicrobial activity (Maria et al. 2015). Traditionally, rose oil has been demonstrated to possess anti-inflammatory, skin protective and anti-aging effects as well as anti-bacterial activities (Milka et al. 2014). Rose oil is mainly used in the perfumery and cosmetics industry as a base component of modern perfumes but it also finds application in the food industry as a flavor additive (Kamran et al. 2014).
Therefore, this study was carried out to find the antimicrobial potential of four important essential oils against 23 Gram negative bacteria, Gram positive bacterial and fungal strains. This study was undertaken to see the potential effectiveness of selected essential oils as an antimicrobial agents for a specific bacterial and fungal strains. In addition to that, Identification of chemical constituents present in essential oils was also studied using gas chromatography-mass spectrophotometry (GC–MS).
Material and methods
Bacterial strain
All the bacterial and fungal strains such as Rhodococus equi (ATCC 6939), Bacillus cereus (MTCC 430), Enteococcus faecalis (MTCC 439), Staphylococcus aureus (MTCC 96), Listeria monocytogenes (ATCC 19111), Escherichia coli (ATCC 15597), Enterobacter aerogenes (MTCC 111), Cronobacter sakazakii (ATCC 29544), Klebsiella pneumonia (MTCC 109), Pseudomonas aeruginosa (MTCC 741), Citrobacter freundii (MTCC 1658), Clostridium perfringens (MTCC 450), Micrococcus luteus (MTCC 2470), Salmonella typhi (MTCC 733), Vibrio parahaemolyticus (ATCC 17802),Vibrio cholera MTCC (3906), Salmonella enterica (MTCC 733), Shigella (MTCC 1457), Enteococcus hirae (ATCC10541), Listeria ivanovii (ATCC 19119), Listeria innocua (ATCC 33090), Aspergillus niger (MTCC 2196), Aspergillus flavus (MTCC 2798), Candida albicans (ATCC 10231) Penicillium pinophilium (MTCC 2192) and Saccharomyces cerevisiae (MTCC 786) were procured. ATCC (American Type Culture Collection) strains were obtained from LGC Promochem, Banglore, India. While MTCC strains were obtained from Institute of Microbial Technology (IMTECH), Chandigarh, INDIA.
Procurement of essential oils
Selected essential oils (GiO, BSO, OO and RO) were purchased during November 2015 from the local market of Hail, Kingdom of Saudi Arabia. These oils were selected based on literature survey and their use in traditional medicine. Quality of the oils was ascertained to be more than 98% pure.
Culture medium and inoculum preparation
The test organisms were sub-cultured onto fresh plates of Mueller–Hinton agar (Hi Media laboratories) for 24 h and Sabouraud dextrose agar (Hi Media laboratories) for 5–7 days at 37 °C for bacteria and fungi, respectively. Colonies from these plates were suspended in Mueller–Hinton broth and Sabouraud broth to a turbidity matching 0.5 McFarland standard (108 cfu/mL). The media used for antimicrobial assays were Mueller–Hinton agar for bacteria and Sabouraud dextrose agar for fungi. All were incubated appropriately as specified for each organism for a period of 18–24 h (Burt 2004).
Agar well diffusion assay
The antibacterial activities of the selected essential oils were determined by Agar well diffusion assay techniques (Reeves 1989). In this method, 100 μL of standardized inoculum of each test bacterium were spread onto sterile Muller–Hinton Agar and Sabouraud Dextrose Agar for bacteria and fungus respectively. 8 mm diameter well was cut from the agar using a sterile cork-borer; subsequently each well was filled with 100 μL of the essential oils. The plates were kept at room temperature for 1 h to allow proper diffusion of the oil into agar and then incubated at 37 °C for 24 h and 30 °C for 3–5 days respectively. Triplicates were prepared for each sample. The essential oils having antimicrobial activity inhibit the microbial growth and the clear zones were formed. The zone of inhibition was measured in millimeters (Raid et al. 2014). The percentage activities of essential oils were calculated against standard drugs which was considered as 100%.
Gas chromatography mass spectrometry (GC–MS/MS)
The essential oils were analyzed using GC–MS/MS (Thermo Scientific, Triple quadropole MS, TSQ 8000) with two fused silica capillary column TG-5MS (30 m × 0.25 mm × 0.25 µm). Injector and detector temperatures were set at 220 and 250 °C, respectively. One micro-liter oil samples diluted with methanol were injected and analyzed with the column held initially at 50 °C for 1 min and then increased by 5 °C/min up to 280 °C. Helium was employed as carrier gas (1 mL/min). The identification of the different compounds was performed by comparison of their relative retention times and mass spectra with those of authentic reference compounds using NIST (National Institute of Standards and Technology) library database. Identification of chemical constituents present in essential oils was investigated using gas chromatography-mass spectrophotometry (GC–MS).
Results and discussion
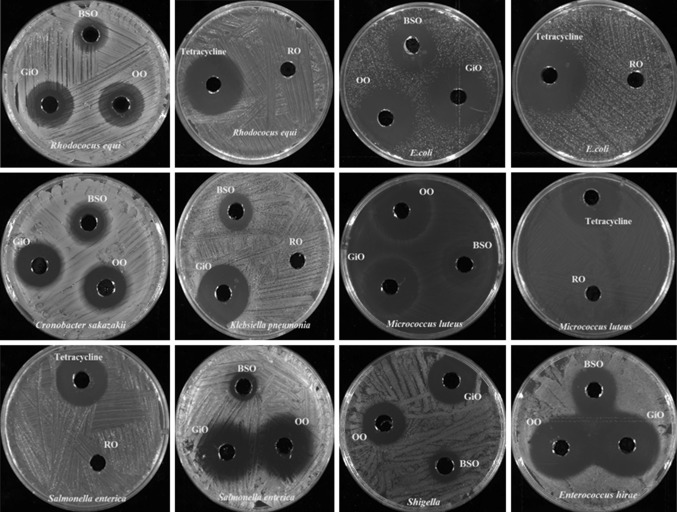
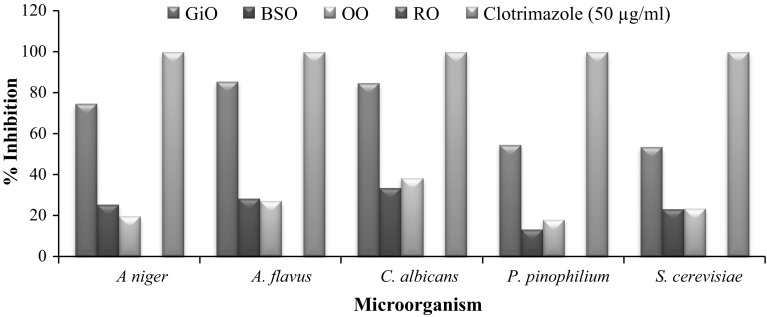
In the present study, evaluation of selected essential oils (GiO, BSO, OO and RO) for their antimicrobial activities and identification of chemical composition were carried out using agar well diffusion technique and Gas chromatography, respectively. The antimicrobial activity was assessed by measuring the zone of inhibition as shown in Fig. 1 and results of antimicrobial activity were presented in Fig. 2 and Table 1, while the identification of chemical constituents were analyzed by relative percentage of the total chromatogram area of oils as presented in (Table 2). To avoid the possible effects of the solvent on the antimicrobial property, commercially available essential oils were not diluted and chemically not altered by any solvent.
Fig. 1.
Antimicrobial activity of Ginger oil (GiO), Black seed oil (BSO), Oregano oil (OO) and Rose oil (RO) against different bacterial strains by using Agar diffusion method
Fig. 2.
Antifungal activity of essential oils using Agar diffusion method
Table 1.
Antibacterial activity of essential oils using Agar diffusion method
| Micro-organism | Zone of inhibition in diameter (mm) and percentage | ||||
|---|---|---|---|---|---|
| GiO | BSO | OO | RO | Tetracycline (50 µg/mL) | |
| Rhodococus equi | 17.2 ± 0.31 (77.13%) | 5.1 ± 0.29 (22.87%) | 15.1 ± 0.52 (67.71%) | NZ | 22.3 ± 0.31 |
| Bacillus cereus | 19.4 ± 0.22 (62.38%) | 7.5 ± 0.16 (24.12%) | 18.4 ± 0.28 (59.16%) | NZ | 31.1 ± 0.36 |
| Enteococcus faecalis | 15.3 ± 0.25 (61.94%) | 4.5 ± 0.29 (18.22%) | 10.7 ± 0.32 (43.32%) | NZ | 24.7 ± 0.27 |
| Bacillus cereus | 19.4 ± 0.22 (62.38%) | 7.5 ± 0.16 (24.12%) | 18.4 ± 0.28 (59.16%) | NZ | 31.1 ± 0.38 |
| Staphylococcus aureus | 17.9 ± 0.31 (66.05%) | 4.6 ± 0.16 (16.94%) | 16.1 ± 0.25 (59.41%) | NZ | 27.1 ± 0.32 |
| Listeria monocytogenes | 5.9 ± 0.19 (29.79%) | 2.0 ± 0.00 (10.10%) | 5.0 ± 0.00 (25.25%) | NZ | 19.8 ± 0.22 |
| Escherichia coli | 22.9 ± 0.16 (106.02%) | 6.8 ± 0.39 (31.48%) | 10.5 ± 0.31 (48.61%) | NZ | 21.6 ± 0.39 |
| Enterobacter aerogenes | 19.4 ± 0.33 (76.98%) | 4.0 ± 0.32 (15.87%) | 8.9 ± 0.24 (35.32%) | NZ | 25.2 ± 0.41 |
| Cronobacter sakazakii | 12.7 ± 0.72 (56.19%) | 4.1 ± 0.19 (18.14%) | 12.0 ± 0.64 (53.09%) | NZ | 22.6 ± 0.37 |
| Klebsiella pneumonia | 12.5 ± 0.61 (66.49%) | 3.5 ± 0.38 (18.62%) | 9.2 ± 0.15 (48.93%) | NZ | 18.8 ± 0.26 |
| Pseudomonas aeruginosa | 16.2 ± 0.47 (83.51%) | 4.2 ± 0.56 (21.65%) | 14.1 ± 0.18 (72.68%) | NZ | 19.4 ± 0.32 |
| Citrobacter freundii | 15.8 ± 0.61 (67.52%) | 7.2 ± 0.27 (30.77%) | 12.5 ± 0.23 (53.42%) | NZ | 23.4 ± 0.41 |
| Clostridium perfringens | 7.1 ± 0.62 (34.30%) | 2.0 ± 0.00 (9.66%) | 5.1 ± 0.25 (24.64%) | NZ | 20.7 ± 0.26 |
| Micrococcus luteus | 24.2 ± 0.24 (82.03%) | 8.1 ± 0.21 (27.46%) | 18.6 ± 0.29 (63.05%) | NZ | 29.5 ± 0.53 |
| Salmonella typhi | 18.1 ± 0.36 (98.91%) | 3.0 ± 0.00 (16.39%) | 15.2 ± 0.27 (83.06%) | NZ | 18.3 ± 0.28 |
| Vibrio parahaemolyticus | 7.0 ± 0.22 | 2.0 ± 0.06 | 5.7 ± 0.19 | NZ | NT |
| Vibrio cholerae | 7.9 ± 0.42 | 2.5 ± 0.07 | 6.2 ± 0.28 | NZ | NT |
| Salmonella enterica | 16.9 ± 0.34 (92.86%) | 2.8 ± 0.15 (15.38%) | 14.5 ± 0.25 (79.67%) | NZ | 18.2 ± 0.31 |
| Shigella | 11.5 ± 0.38 (119.79%) | 2.0 ± 0.17 (20.83%) | 12.6 ± 0.58 (131.25%) | NZ | 9.6 ± 0.48 |
| Enterococcus hirae | 21.9 ± 0.37 (110.61%) | 5.0 ± 0.08 (25.25%) | 19.1 ± 0.61 (96.46%) | NZ | 19.8 ± 0.36 |
| Listeria ivanovii | 7.4 ± 0.66 | 2.5 ± 0.006 | 5.9 ± 0.45 | NZ | NT |
| Listeria innocua | 8.4 ± 0.62 | 2.9 ± 0.24 | 6.55 ± 0.22 | NZ | NT |
Values are expressed as Mean value ± SEM (standard error of means) and Percentage inhibition
* NZ no zone of inhibition
** NT not tested
Table 2.
Compounds identified in the essential oil using Gas chromatography mass spectrophotometry (GC–MS)
| RT | Compound name | Molecular formula | SI | RSI | Area (%) | Identification |
|---|---|---|---|---|---|---|
| GiO | ||||||
| 7.25 | Eucalyptol | C10H18O | 855 | 864 | 1.37 | MS, RI |
| 9.41 | endo-Borneol | C10H18O | 949 | 949 | 1.19 | MS, RI |
| 9.68 | α-Terpineol | C10H18O | 903 | 915 | 0.79 | MS, RI |
| 9.81 | Decanal | C10H20O | 889 | 889 | 2.22 | MS, RI |
| 11.46 | 1-Triethylsilyloxyheptadecane | C23H50OSi | 661 | 775 | 1.11 | MS, RI |
| 13.06 | ç-Elemene | C15H24 | 897 | 906 | 0.63 | MS, RI |
| 13.73 | α-Copaene | C15H24 | 877 | 887 | 1.79 | MS, RI |
| 13.81 | n-Hexadecanoic acid | C16H32O2 | 836 | 856 | 2.38 | MS, RI |
| 13.97 | Alloaromadendrene | C15H24 | 892 | 897 | 4.20 | MS, RI |
| 14.17 | α-Sesquiphellandrene | C15H24 | 891 | 893 | 6.29 | MS, RI |
| 14.52 | Cubenol | C15H26O | 868 | 903 | 1.44 | MS, RI |
| 14.59 | (n)-trans-Nerolidol | C15H26O | 907 | 938 | 0.93 | MS, RI |
| 14.97 | 7-epi-cis-sesquisabinene hydrate | C15H26O | 891 | 905 | 2.87 | MS, RI |
| 15.24 | cis-sesquisabinene hydrate | C15H26O | 848 | 860 | 2.21 | MS, RI |
| 15.64 | 4-(3-Hydroxy-2-methoxyphenyl)-2-butanone | C11H14O3 | 937 | 940 | 3.71 | MS, RI |
| 15.80 | Eudesm-4(14)-en-11-ol | C15H26O | 861 | 874 | 1.40 | MS, RI |
| 18.83 | (-)-Zingiberene | C15H24 | 895 | 930 | 10.86 | MS, RI |
| 20.59 | Linoleic acid, methyl ester | C19H34O2 | 880 | 889 | 14.38 | MS, RI |
| 24.02 | α-Curcumene | C15H22 | 891 | 896 | 4.27 | MS, RI |
| 25.11 | α-Farnesene | C15H24 | 874 | 895 | 4.04 | MS, RI |
| 27.66 | Gingerol | C17H26O4 | 677 | 704 | 7.88 | MS, RI |
| BSO | ||||||
| 5.58 | 2-Thujene | C10H16 | 927 | 947 | 2.78 | MS, RI |
| 6.32 | α-Pinene | C10H16 | 649 | 680 | 1.08 | MS, RI |
| 7.11 | p-Cymene | C10H14 | 918 | 931 | 6.90 | MS, RI |
| 7.64 | ç –Terpinene | C10H16 | 916 | 927 | 1.14 | MS, RI |
| 8.60 | cis-4-methoxy thujane | C11H20O | 915 | 941 | 1.81 | MS, RI |
| 10.52 | Thymoquinone | C10H12O2 | 913 | 913 | 2.07 | MS, RI |
| 11.03 | Anethole | C10H12O | 924 | 929 | 0.96 | MS, RI |
| 11.20 | Carvacrol | C10H14O | 922 | 924 | 0.76 | MS, RI |
| 12.82 | Longifolene | C15H24 | 905 | 918 | 0.50 | MS, RI |
| 14.45 | Apiol | C12H14O4 | 785 | 815 | 0.39 | MS, RI |
| 18.83 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 846 | 847 | 14.51 | MS, RI |
| 20.56 | Linoleic acid, methyl ester | C19H34O2 | 880 | 889 | 13.95 | MS, RI |
| 25.18 | Myristicine | C17H34O2 | 884 | 898 | 1.05 | MS, RI |
| 27.65 | Limonene | C14H28O2 | 867 | 880 | 0.71 | MS, RI |
| OO | ||||||
| 5.63 | 2-Thujene | C10H16 | 923 | 943 | 1.22 | MS, RI |
| 5.71 | α-Pinene | C10H16 | 919 | 927 | 1.88 | MS, RI |
| 6.54 | Thymol | C10H16 | 823 | 833 | 2.52 | MS, RI |
| 7.12 | ç-Terpinene | C10H16 | 917 | 919 | 7.98 | MS, RI |
| 7.65 | p-Cymene | C10H14 | 903 | 919 | 5.46 | MS, RI |
| 8.12 | p-Cymenene | C10H12 | 728 | 870 | 0.84 | MS, RI |
| 8.25 | α-Linalool | C10H18O | 920 | 921 | 4.02 | MS, RI |
| 9.06 | 4-Ethyloctane | C10H22 | 760 | 904 | 1.29 | MS, RI |
| 9.50 | Terpinen-4-ol | C10H18O | 884 | 885 | 2.01 | MS, RI |
| 9.71 | Dodecane | C12H26 | 791 | 876 | 1.84 | MS, RI |
| 11.27 | Carvacrol | C10H14O | 874 | 874 | 15.87 | MS, RI |
| 12.97 | Caryophyllene | C15H24 | 908 | 915 | 2.08 | MS, RI |
| 15.03 | Caryophyllene oxide | C15H24O | 800 | 835 | 2.02 | MS, RI |
| 16.09 | 1-Chloroeicosane | C20H41Cl | 682 | 688 | 0.91 | MS, RI |
| 16.58 | Eutanol G | C20H42O | 798 | 826 | 3.64 | MS, RI |
| 25.80 | Campesterol | C28H48O | 757 | 778 | 0.92 | MS, RI |
| 27.65 | Eugenol | C29H50O | 806 | 815 | 2.55 | MS, RI |
| RO | ||||||
| 8.50 | α-Linalool | C10H18O | 933 | 934 | 1.51 | MS, RI |
| 9.65 | 1-Octanol, 3,7-dimethyl | C10H22O | 905 | 939 | 0.39 | MS, RI |
| 9.97 | α-Citronellol | C10H20O | 893 | 912 | 0.45 | MS, RI |
| 10.15 | Citronellol | C10H20O | 923 | 923 | 8.07 | MS, RI |
| 10.53 | Geraniol | C10H18O | 937 | 937 | 6.63 | MS, RI |
| 11.17 | Linalool, formate | C11H18O2 | 775 | 778 | 0.47 | MS, RI |
| 12.00 | 3-Allyl-2-methoxyphenol | C10H12O2 | 931 | 931 | 0.88 | MS, RI |
| 13.21 | Aristolene | C15H24 | 894 | 898 | 0.55 | MS, RI |
| 20.54 | trans-Geranyl geraniol | C20H34O | 741 | 789 | 3.70 | MS, RI |
| 23.09 | Citronellyl formate | C11H20O2 | 844 | 920 | 1.42 | MS, RI |
| 27.29 | l-Menthone | C10H18O | 867 | 957 | 0.67 | MS, RI |
Antimicrobial activity of essential oils
Essentials oils have been tested for in vitro antimicrobial activity and demonstrated to have potential antimicrobial effect (Fig. 2; Table 1). The results obtained during this study showed that, the zone of inhibition (mm) for the GiO varied from (5.9 ± 0.19 mm) to (24.2 ± 0.24 mm) as compared to standard tetracycline and clotrimazole (18.2 ± 0.31 mm) to (37.5 ± 0.19 mm) respectively. The antimicrobial activity of the GiO was found to be highest against Shigella (119.79%), E. hirae (110.61%) and E. coli (106.02%), when compared with the tetracycline activity. Among all the fungal strains tested, Aspergillus flavus was found to be most sensitive followed by C. albicans and A. niger towards GiO. While, the lowest activity was recorded against L. monocytogenes. Klebsiella pneumoniae showed poor sensitivity to GiO as compare to the other Gram-negative bacteria. Our result showed that GiO stood more effective as an antibacterial agent compared to antifungal activity. Bellik (2014) reported that, GiO were more effective against E. coli, Bacillus subtilis and S. aureus, and least effective against A. niger. This result was in conformity with the previous studies, which reported that GiO exhibited an inhibitory effect against a wide range of pathogenic bacteria and fungi. Their effect was probably due to their active constituents such as Zingeberene, endo-Borneol and Gingerol present in GiO (Amel et al. 2015).
The antimicrobial activity of BSO against different microorganisms has been studied by several research groups. Morsi (2000) reported the antibacterial activity of BSO crude extracts against multi-drug-resistant organisms, including Gram-positive bacteria like S. aureus and Gram-negative bacteria like P. aeruginosa and E. coli. Our results showed that, BSO had more antifungal activity in C. albicans (9.1 ± 0.28 mm) than other fungus like Aspergilluas flavus and A. niger (7.5 ± 0.43 mm and 7.2 ± 0.57 mm) correspondingly. However, in comparison to fungal strains, tested gram positive and gram negative strains showed lesser sensitivity towards BSO. Among all the selected bacteria, BSO had highest antimicrobial activity against the E. coli followed by C. freundii. Listeria monocytogenes had lesser sensitivity (10.10%) compared to tetracycline standard. The presence of biological active compounds in BSO such as α-thujene, α- pinene, limonene, thymoquinone, myristicin etc. contributed the antimicrobial activity (Gerige et al. 2009). Salman et al. (2008) reported that the antimicrobial activity of this oil may be attributed due to the presence of thymoquinone, thymohydroquinone and thymole which possessed antimicrobial activity. Results of Oregano oil showed that, Shigella had the highest sensitivity (12.6 ± 0.58 mm), followed by E. hirae (19.1 ± 0.61 mm) and S. typhi (15.2 ± 0.27 mm) when compared with standard tetracycline zone of inhibition 9.6 ± 0.48 mm, 19.8 ± 0.36 mm and 18.3 ± 0.28 mm respectively. While, among Gram-positive bacteria, L. monocytogenes showed least zone of inhibition (5.0 ± 0.00 mm). OO showed good response against Candida albicans with the zone of inhibition 10.4 ± 0.47 mm. Moreover, A. niger showed slight resistance towards OO when compared with other fungus strains.
Our results showed that the OO had potential antibacterial activity against all the tested micro-organism (Table 1). The highest activity was observed against Shigella with the strongest inhibition zones, followed by S. typhi, when compared with standard tetracycline. Several reports have been attributed the antimicrobial effectiveness of OO was due to thymol, eugenol and carvacrol (Magdalena et al. 2011). Our chemical analysis found that the OO had highest percentage of Carvacrol, thymol and eugenol, which could have played an important role for OO antibacterial activity. RO were ineffective against all the selected strains. Few researchers have been reported that rose oil has very minimal antimicrobial activity. Balkan et al. (2016) and (Mahboubi et al. 2016) had similar reports corresponding to our results. No zone of inhibition recorded from RO, this could be due to source of oil origin, production and probably due to differences in number of active compounds and in their concentrations. Our result showed that GiO stood more potent antimicrobial agents among the selected oils.
Several mechanisms have been proposed to explain the antimicrobial activity of essential oils. Various compounds with antibacterial activity inhibit growth or cause bacterial deaths or directly affect DNA synthesis. The bacterial cell wall can be affected in various ways, which can be at different stages of synthesis or transport of its metabolic precursors, or by a direct action on its structural organization (Martinez-Martinez 2009). The mechanism of action appears to be predominently on the cell membranes by disrupting its structure thereby causing cell leakage and cell death, secondary actions may be by blocking the membrane synthesis and inhibition of cell respiration. They readily penetrate into the cell membrane and exert their biological effects because of high volatility and liphophilicity of the essential oils (Aligiannis et al. 2001; Oyedemi et al. 2009; lalit et al. 2012). Antimicrobial testing will give us only an idea for presence of antimicrobial compound in essential oils by measuring zone of inhibition. However, GC–MS analysis will be needed for the identification of bioactive compound responsible for the antimicrobial activity.
Analysis of chemical constituents by gas chromatography
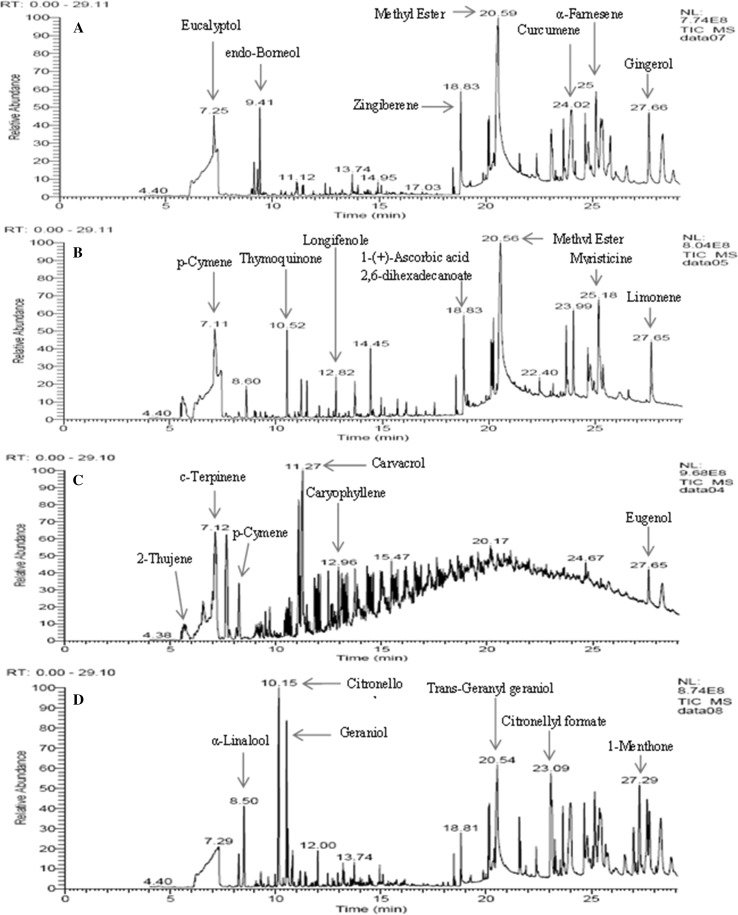
The results of the GC–MS/MS analysis identified various compound present in essential oils (GiO, BSO, OO and RO). The major compound in the methanolic extract were identified and listed in Table 2. Identification was done by direct comparison of the retention times (RT) and mass spectral data of compounds with their respective reference compounds matched with the NIST (National Institute of Standards and Technology) Library. Percentage areas of the each component were measured.
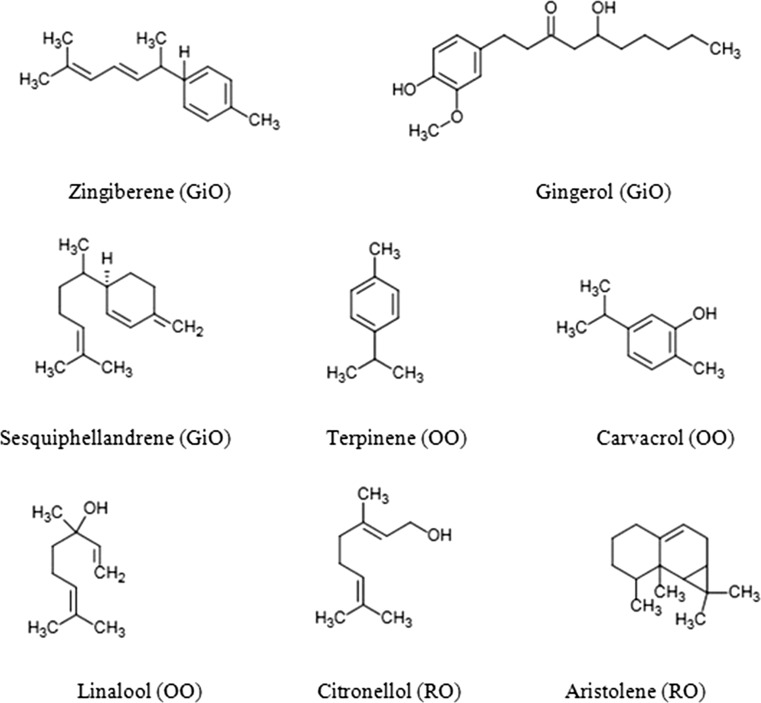
Gas chromatography analysis of GiO identifies 36 peaks (Fig. 4) out of which, major components were found to be Zingiberene (10.86%), Gingerol (7.88%), α-Sesquiphellandrene (6.29%), α-Curcumene (4.27%), Alloaromadendrene (4.20%), α-Farnesene (4.04%), Eucalyptol (1.37%), endo-Borneol (1.19%), Cubenol (1.44%). Zingiberene was the most predominant compounds belonging to the sesquiterpene hydrocarbon of the total extracted essential oil. The specific aroma of ginger was predominantly because of Zingiberene. Our findings were in agreement with previous work carried out by Sultan et al. (2005). GC–MS analysis of BSO identified 29 constituents, out of which l-(+)-Ascorbic acid 2,6-dihexadecanoate (14.51%), p-Cymene (6.90%), 2-Thujene (2.78%), cis-4 methoxy thujane (1.81), α-Pinene (1.08%), c-Terpinene (1.14%), Thymoquinone (2.07%), Anethole (0.96%), Carvacrol (0.76%), Longifolene (0.50%) and Apiol (0.39%) were the major ones. These results were comparable to the qualitative test results obtained from previous studies (Mozaffari et al. 2000; Gerige et al. 2009). Previous author reported, thymoquinone 1.8% and p-cymene 9.0% which was in accordance to our presented results (Dinagaran et al. 2016; Gerige et al. 2009). Other studies also reported, p-cymene 14.8% and thymoquinone 0.6% (Nickavar et al. 2003). OO were analyzed for Gas chromatography determination, 21 major peaks were identified. Carvacrol (15.87%) was the highest, followed by ç-Terpinene (7.98%), p-Cymene (5.46%), α-Linalool (4.02%) and Thymol (2.52%). These percentages were higher than reported from Magdalena et al. (2011). Afef et al. (2013) showed that this species is a rich source of phenolic monoterpenes and carvacrol. Considering that carvacrol-rich essential oils are gaining increasing importance for their considerable antimicrobial activity. Previous studies also showed that carvacrol was high in concentration than thymol (Babili et al. 2011; Milovanovic et al. 2009). Lower concentration of thymol could be due to different extraction techniques due to geographical sources. Our findings also suggested that, in Oregano Oil (OO), carvacrol is present in rich amount than thymol. Volatile oil of RO was analyzed and characterized by very less numbers of monoterpenes and large numbers of sesquiterpenes and aliphatic components. Major component identifies in RO was Citronellol (8.07%), Geraniol (6.63%), Phenylethyl Alcohol (3.18%), trans-Geranylgeraniol (3.70%), Citronellyl formate (1.42%).These results were in comparable with the previous studies (Kiran and Babu 2002; Kamran et al. 2014). Major components with their chemical structure presented in Fig. 3. The GC–MS analysis showed that all the selected essential oils had abundant bioactive compounds which could be of potential use for further studies against pathogenic bacteria.
Fig. 4.
GC–MS chromatogram of a GiO, b BSO, c OO and d RO
Fig. 3.
Structure of major compounds of the essential oils of GiO, BSO, OO and RO
Conclusion
The present investigation was aimed to study the antimicrobial activity of (BSO, GiO, OO and RO) against 23 Gram-positive bacteria, Gram-negative bacteria and Fungi. The antimicrobial activity of these tested essential oils, against the micro-organisms may be indicative of the presence of broad spectrum antimicrobial compounds present in these oils. Although this study investigated the in vitro antimicrobial activity, the results substantiate the use of four studied essential oils as antimicrobial agents for the treatment of various bacteria or fungal related diseases. In conclusion, it is suggested that these plants may be useful to discover natural bioactive compounds. More importantly, these can be included in the list of herbal medicines due to their high antimicrobial potential and lesser side effects. Hence, essential oils and their components can be recommended for therapeutic purposes and can be used as an alternative source of medicine.
Acknowledgements
We are grateful to the Department of Food technology, Hamdard University, New Delhi and Department of Clinical Nutrition, College of Applied Medical Sciences, Hail University for providing facilities to carrying out the present study.
References
- Afef B, Hedia C, Maroua J, Abdennacer B, Mohamed B. Essential oil composition and antibacterial activity of Origanum vulgare subsp, glandulosum desf, at different phenological stages. J Med Food. 2013;16:1115–1120. doi: 10.1089/jmf.2013.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–123. doi: 10.1016/S0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem. 2001;49:4168–4170. doi: 10.1021/jf001494m. [DOI] [PubMed] [Google Scholar]
- Amel AS, Fadwa ME, Smah AS, Nazar AO. Antimicrobial Activity of Zingiber officinale (Ginger) oil against bacteria isolated from children throat. Int J Microbiol. 2015;1:01–06. [Google Scholar]
- Babili FE, Bouajila J, Souchard JP, Bertrand C, Bellvert F, Fouraste I, Moulis C, Valentin A. Oregano: chemical analysis and evaluation of its antimalarial, antioxidant and cytotoxic activities. J Food Sci. 2011;76:C512–C518. doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- Balkan CE, Karamese M, Celebi D, Aydogdu S, Dicle Y, Calık Z. The determination of the antibacterial activities of Rose, Thyme, Centaury and Ozone Oils against some pathogenic microorganisms, Kafkas. J Med Sci. 2016;6:18–22. [Google Scholar]
- Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruperto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr J. 1998;13:235–244. doi: 10.1002/(SICI)1099-1026(1998070)13:4<235::AID-FFJ733>3.0.CO;2-T. [DOI] [Google Scholar]
- Bellik Y. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Pac J Trop Dis. 2014;14:40–44. doi: 10.1016/S2222-1808(14)60311-X. [DOI] [Google Scholar]
- Biljana DV, Tatjana D, Danijela S, Jovanka D. Antimicrobial effect of essential oil isolated from eucalyptus globulus Labill, from Montenegro. Czech J Food Sci. 2011;29:277–284. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chavan MJ, Shinde DB, Nirmal SA. Major volatile constituents of Annona squamosa L., bark. Nat Prod Res. 2006;20:54–757. doi: 10.1080/14786410500138823. [DOI] [PubMed] [Google Scholar]
- Dinagaran S, Sridhar S, Eganathan P. Chemical composition and antioxidant activities of black seed oil (NIGELLA SATIVA L.) IJPSR. 2016;7:4473–4479. [Google Scholar]
- Gerige SJ, Yadav MKG, Rao M, Ramanjaneyulu GC–MS analysis of Nigella sativa seeds and antimicrobial activity of its volatile oil. Braz Arch Biol Technol. 2009;52:1189–1192. doi: 10.1590/S1516-89132009000500016. [DOI] [Google Scholar]
- Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, Goto H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007;362:218–223. doi: 10.1016/j.bbrc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Johnson OO, Ayoola GA, Adenipekun T. Antimicrobial activity and the chemical composition of the volatile oil blend from Allium sativum (Garlic Clove) and Citrus reticulata (Tangerine Fruit) Int J Pharm Sci Drug Res. 2013;5:187–193. [Google Scholar]
- Kamaliroostaa ZK, Kamaliroosta L, Elhamirad AH. Isolation and identification of ginger essential oil. J Food Biosci Technol. 2013;3:73–80. [Google Scholar]
- Kamran JN, Ansari SH, Ali M, Najmi AK. Volatile oil composition of Rosa damascene Mill (Rosaceae) J Pharmacogn Phytochem. 2014;2:177–181. [Google Scholar]
- Kiran RO, Babu GD. Essential oil composition of Damask rose (Rosa damascena Mill,) distilled under different pressures and temperatures. Flavour Fragr J. 2002;17:136–140. doi: 10.1002/ffj.1052. [DOI] [Google Scholar]
- Lalit KDC, Bhushan AJ, Sheeba S, Hemant S, Mounesh CDK, Pooja AK. Antimicrobial activity of commercially available essential oils against Streptpcoccus mutans. J Contemp Dent Pract. 2012;1(13):71–74. doi: 10.5005/jp-journals-10024-1098. [DOI] [PubMed] [Google Scholar]
- Magdalena ON, Refugio RB, Evelia AF, Alberto GL, Adriana MT, Luz VM. Chemical composition and antimicrobial activity of oregano (Lippia palmeri S. Wats) essential oil. Rev Fitotec Mex. 2011;34:11–17. [Google Scholar]
- Mahboubi M. Rosa damascena as holy ancient herb with novel applications. J Tradit Complement Med. 2016;6:10–16. doi: 10.1016/j.jtcme.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria F, Athanasios K, Ioanna M, Stavros P, Irene T, Virginia P, Ioannis K, Maria P, Elisavet S, Eugenia EB, Athanasios A. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumonia. Microb Ecol Health Dis. 2015;26:2328. doi: 10.3402/mehd.v26.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martınez-Martınez JCYL. Antimicrobial mechanisms of action. Enferm Infecc Microbiol Clin. 2009;27:44–52. doi: 10.1016/j.eimc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Milka MM, Vesselin KK, Dimo SK, Ana MD, Angel SG. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L., essential oil against some oral pathogens. Int J Curr Microbiol Appl Sci. 2014;3:11–20. [Google Scholar]
- Milovanovic MIILJ, Misan AC, Sakac MB, Cabarkapa IS, Saric BM, Matic JJ, Jovanov PT. Evaluation of a GC–MS method for the analysis of oregano essential oil composition. Food Process Qual Saf. 2009;3:75–79. [Google Scholar]
- Mohamed S, Eman H. Comparative antimicrobial activity of some active constituents of N. sativa L. World Appl Sci J. 2012;20:182–189. [Google Scholar]
- Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotic resistant bacteria. Acta Microbiol Pol. 2000;49:63–74. [PubMed] [Google Scholar]
- Mozaffari FS, Ghorbanli M, Babai A, Farzami SM. The effect of water stress on the seed oil of Nigella sativa, L. J Essent Oil Res. 2000;12:36–38. doi: 10.1080/10412905.2000.9712036. [DOI] [Google Scholar]
- Nickavar B, Mojab F, Javidnia K, Amoli MAR. Chemical Composition of the Fixed and Volatile Oils of Nigella sativa L. from Iran. Z. Naturforsch. 2003;58c:629–631. doi: 10.1515/znc-2003-9-1004. [DOI] [PubMed] [Google Scholar]
- Oyedemi SO, Okoh AI, Mabinya LV, Pirochenva G, Afolayan AJ. The proposed mechanism of bactericidal action of eugenol, γ-terpineol and γ-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr J Biotechnol. 2009;8:1280–1286. [Google Scholar]
- Raid AA, Yazeed AS, Ayesha M, Rabbani SK, Janardhan C, Gupta VC. Evaluation of antibacterial activity of crude protein extracts from seeds of six different medical plants against standard bacterial strains. Saudi J Biol Sci. 2014;21:147–151. doi: 10.1016/j.sjbs.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa MA, Al-Ghamdi MS. A review of the pharmaco-therapeutic effects of Nigella sativa. Pak J Med Res. 2002;41:77–83. [Google Scholar]
- Raquel B, Cristina N, Rafael G. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Food Borne Pathog Dis. 2012;9:699–705. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- Ravi KU, Pratibha D, Shoeb A. Screening of antibacterial activity of six plant essential oils against pathogenic bacterial strains. Asian J Med Sci. 2010;2:152–158. [Google Scholar]
- Reeves DS. Antibiotic assays. In: Hawkey PM, Lewis DA, editors. Medical bacteriology, a practical approach. Oxford: IRL Press; 1989. pp. 195–221. [Google Scholar]
- Salman MT, Khan RA, Shukla I. Antimicrobial activity of Nigella sativa Linn. Seed oil against multi drug resistant bacteria from clinical isolates. Nat Prod Radiance. 2008;7:10–14. [Google Scholar]
- Seenivasan P, Manickkam J, Savarimuthu I. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:1–8. doi: 10.1186/1472-6882-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P, Miyata M, Sato DN, Santos AC, Mendes NH, Leite CQ. Rhodococcus equi isolation from sputum of patients with suspected tuberculosis. Mem Inst Oswaldo Cruz. 2010;105:199–202. doi: 10.1590/S0074-02762010000200015. [DOI] [PubMed] [Google Scholar]
- Sonboli A, Salehi P, Yousefzadi M. Anti-microbial activity and chemical composition of the essential oil of Nepeta crispa Willd, from Iran. Z Naturforsch C. 2004;59:653–656. doi: 10.1515/znc-2004-9-1008. [DOI] [PubMed] [Google Scholar]
- Sultan M, Bhatti HN, Iqbal Z. Chemical analysis of essential oil of ginger (Zingiber officinale) Pak J Biol Sci. 2005;8:1576–1578. doi: 10.3923/pjbs.2005.1576.1578. [DOI] [Google Scholar]
- Yuva B. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Pac J Trop Dis. 2014;4:40–44. [Google Scholar]