Abstract
Effect of cold carbonated water (CW) washing on the biochemical properties and gel characteristics of mackerel surimi was evaluated. Three washing cycles were performed with different orders of washing medium including T1 (water, water and water), T2 (CW, water and water), T3 (CW, CW and water) and T4 (CW, CW and CW). The results showed that CW washing, especially T4, caused the decrease in pH, Ca2+-ATPase activity and surface hydrophobicity and led to the increase in reactive sulfhydryl content. Regardless of washing treatment, haem protein of surimi decreased significantly compared to unwashed mince. However, carbonated water did not improve haem protein removal. The highest lipid reduction was found in T1 and T2. Gels from all CW washing treatments had a comparable whiteness. Breaking force of CW surimi gel increased with increasing washing cycle (T2 < T3 < T4). Deformations of all surimi gels were not much different (~7 mm). Expressible drip increased with increasing CW washing cycle. Numbers of jointed spherical matrices were found in surimi gel microstructures. With increasing CW washing cycle, densely packed aggregates were formed. Therefore, CW washing with appropriate cycle can be used as an alternative means for mackerel surimi production.
Keywords: Carbonated water, Washing medium, Mackerel, Surimi, Gel properties
Introduction
Surimi is the concentrated myofibrillar proteins which can be obtained from repeatedly washed fish mince. Surimi has a high commercial value and a wide range of applications in seafood production (Mao and Wu 2007). The white fish species have been used for the surimi production intensively leading to the overexploitation of the white fish species. To solve that problem, dark muscle fish species can be used as an alternative raw material for producing surimi (Hultin and Kelleher 2000; Chaijan et al. 2004). Among all dark-fleshed fish species, mackerel (Auxis thazard) is one of the most abundant species commonly caught in Southern Thailand, especially in the Thasala Coast of Nakhon Si Thammarat (Chaijan et al. 2010, 2013). As a local resource, this fish species can be value-added using the surimi technology. However, the characteristics of surimi gel depend on the properties of the myofibrillar proteins, and these are determined by the species and freshness of the fish, as well as the processing parameters (Niwa 1992). It has been reported that small pelagic fish species, such as mackerel, gave the surimi with poor gel characteristics due to the large quantity of lipid and haem proteins in the muscle tissue (Chaijan et al. 2004). Principally, washing is a crucial step in surimi processing. Chaijan et al. (2010) stated that conventional washing with cold water is not applicable for dark-fleshed fish species. Thus, appropriate washing medium should be optimised in order to produce good quality surimi from dark-fleshed fish muscle.
Carbonated water (CW) is simply water which has been injected with CO2 known as carbonation. During carbonation under high pressure, carbonic acid and hydrogen ion can be released. The pH of CW is about 6.0 (Cuomo et al. 2002). Bubbles commonly appear when concentration levels of CO2 are 3–5 times higher than at the saturation equilibrium value and depend of the pre-existing gas–liquid interfaces (Lubetkin and Blackwell 1988; Wilt 1986). Bubbles generated by CW with mild acidic pH could facilitate the leaching process during surimi production. This concept is similar to the air floatation process developed by Lin et al. (2005) in which air incorporating during washing can improve the gel-forming ability of horse mackerel. Additionally, several ions can be found in CW such as Ca2+, Na+, Mg2+, K+, Li+, Cl−, F−, and leading to a high electrical conductivity of CW (1388 μδ) (Cuomo et al. 2002). The presence of those ions may help leaching process more effectively and can participate in setting phenomenon. However, carbonated water with lower pH may cause denaturation of haem pigments and accelerate lipid oxidation. The application of CW as a washing medium for surimi production has not been reported. Thus, the aim of this study was to assess the effect of CW washing on biochemical properties and gel characteristics of mackerel (Auxis thazard) surimi.
Materials and methods
Chemicals
Sodium dodecyl sulfate (SDS) was purchased from Sigma (St. Louis, MO, U.S.A.). Trichloroacetic acid (TCA), sodium chloride and sodium dihydrogen phosphate were obtained from Merck (Darmstadt, Germany). Sodium hydroxide and hydrochloric acid were obtained from Fluka (Buchs, Switzerland). All chemicals were of analytical grade.
Fish sample
Mackerel (Auxis thazard) with an average weight of 95–110 g were caught from Thasala-Nakhon Si Thammarat Coast along the Gulf of Thailand. The fish, off-loaded approximately 12 h after capture, were placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the School of Agricultural Technology, Walailak University, Thasala, Nakhon Si Thammarat within 15 min. The fish was immediately washed, gutted, filleted, skinned and the whole muscles were collected. To prepare fish mince, fish fillets were minced to uniformity using a meat grinder (a 4 mm hole diameter; Panasonic MK-G20MR, Japan). The muscles were kept on ice during preparation.
Surimi preparation
To prepare surimi by conventional washing process (T1), fish mince was washed with cold water (4 °C) (Chaijan et al. 2004) using a washing medium/mince ratio of 3:1 (v/w). The mixture was stirred gently for 10 min in a cold room (4 °C) and the washed mince was filtered with a layer of nylon screen. Washing was performed three times. Dewatering was made by hydraulic press with the final moisture content of 80%. To produce the surimi by CW washing, the method was carried out similar to conventional washing process but different orders of washing medium were applied during 3-washing cycle, including T2 (CW, water and water), T3 (CW, CW and water) and T4 (CW, CW and CW). Commercial CW (Chang®) was purchased from Cosmos Brewery Co. Ltd., Thailand. The initial pH value of cold water and CW was 7.3 and 6.0, respectively. With all washing process used, the yield of 40–42% (based on weight of whole fish raw material) was obtained. Unwashed mince was used as a control. All samples were added with 4% sucrose and 4% sorbitol, mixed well and frozen using an air-blast freezer. The frozen samples were kept at −18 °C until used. The storage time was not more than 1 month.
Determination of pH
The pH was measured as described by Benjakul et al. (1997). Sample was homogenised using an IKA Labortechnik homogeniser (Selangor, Malaysia) with 10 volumes of deionised water (w/v), and the pH was measured using a calibrated pH meter (Cyberscan 500, Singapore).
Determination of Ca2+-ATPase activity
The Ca2+-ATPase activity of natural actomyosin (NAM) from surimi and unwashed mince was determined according to the method of Benjakul et al. (1997). NAM prepared as described by Benjakul et al. (1997) was diluted to 2.5–8 mg/ml with 0.6 M KCl, pH 7.0. Diluted NAM solution (1 ml) was added to 0.6 ml of 0.5 M Tris-maleate, pH 7.0. The mixture was added with 1 ml of 0.1 M CaCl2. Deionised water was added to make up a total volume of 9.5 ml. A 0.5 ml of 20 mM adenosine 50-triphosphate (ATP) solution was added to initiate the reaction. The reaction was conducted for 8 min at 25 °C and terminated by adding 5 ml of chilled 15% (w/v) trichloroacetic acid (TCA). The reaction mixture was centrifuged at 3500×g in a RC-5B plus centrifuge (Sorvall, Norwalk, CT, U.S.A.) for 5 min at 25 °C and the inorganic phosphate liberated in the supernatant was measured by the method of Fiske and Subbarow (1925). The Ca2+-ATPase activity was expressed as µmoles inorganic phosphate released (Pi)/mg protein/min. A blank solution was prepared by adding chilled TCA prior to addition of ATP. The protein content in the NAM solution was measured by Biuret method using bovine serum albumin as a standard.
Determination of reactive sulfhydryl (SH) content
Reactive SH content was measured using 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) according to the method of Ellman (1959). The NAM sample (0.5 ml, 4 mg/ml) was added to 4.5 ml of 0.2 M Tris–HCl buffer, pH 6.8. Thereafter, 0.5 ml of 0.1% DTNB solution was added into the mixture and subjected to incubation at 40 °C for 25 min. Absorbance was measured at 412 nm using a Shimadzu UV-2100 spectrophotometer (Shimadzu Scientific Instruments Inc., Columbia, Md., USA). A blank was prepared by replacing the sample with 0.6 M KCl, pH 7.0. SH content was calculated from the absorbance using the molar extinction of 13,600 M−1 cm−1 and was expressed as mol/108 g protein.
Determination of protein surface hydrophobicity
Hydrophobicity of non-solubilised myofibrils was determined using bromophenol blue (BPB) for electrophoresis according to the method of Chelh et al. (2006). To 1 ml of myofibril suspension prepared according to the method described by Martinaud et al. (1997), 200 μl of 1 mg/ml BPB (in distilled water) was added and mixed well. A control, without myofibrils, consisted of the addition of 200 μl of 1 mg/ml BPB (in distilled water) to 1 ml of 20 mM phosphate buffer at pH 6. Samples and control were kept under agitation, at room temperature, during 10 min and then centrifuged for 15 min at 2000×g. The absorbance of the supernatant (diluted 1/10) was measured at 595 nm against a blank of phosphate buffer. The surface hydrophobicity was reported as the amount of BPB bound given by the formula:
where A = absorbance at 595 nm.
Lipid extraction
Lipid was extracted by the method of Bligh and Dyer (1959). Sample (25 g) was homogenised with 200 ml of a chloroform:methanol:distilled water mixture (50:100:50) at the speed of 9500 rpm for 2 min at 4 °C. The homogenate was treated with 50 ml of chloroform and homogenised at 9500 rpm for 1 min. Then, 25 ml of distilled water were added and homogenised again for 30 s. The homogenate was centrifuged at 3000 rpm at 4 °C for 15 min and transferred into a separating flask. The chloroform phase was drained off into the 125 ml Erlenmeyer flask containing about 2–5 g of anhydrous sodium sulfate, shaken very well, and decanted into a round–bottom flask through a Whatman No. 4 filter paper. The solvent was evaporated at 40 °C using an EYELA rotary evaporator N-100 (Tokyo, Japan). The total lipid content was expressed as g/100 g sample and percentage of lipid reduction was calculated relatively to the initial lipid content of unwashed mince.
Determination of total haem protein
Total haem protein was determined according to the method of Chaijan and Undeland (2015). A sample was homogenised with 3 volumes of 0.1 M phosphate buffer, pH 7.0 containing 5% SDS (w/v) at 13,500 rpm for 20 s. The homogenate was subjected to 85 °C for 1 h in a temperature controlled water bath. After cooling for 10 min under running tap water, the solution was centrifuged at 5000×g for 15 min at room temperature (25 °C). The absorbance of the supernatant was read at 535 nm using phosphate buffer as a blank. Total haem protein concentration was quantified using a standard curve based on bovine haemoglobin (0–50 μM) and expressed as µmol of haemoglobin/kg sample.
Gel preparation
To prepare the gels, frozen surimi or unwashed mince samples were thawed at 4 °C until the core temperature reached 0 °C. The samples were then cut into small pieces and the moisture content was adjusted to 80%. Dry NaCl was added to the samples (2.5%, w/w) and chopped for 5 min in a walk-in cold room at 4 °C to obtain the homogeneous sol. The sol was then stuffed into polyvinylidine casing with a diameter of 2.5 cm and both ends of the casing were sealed tightly. The sol was then incubated at 40 °C for 30 min, followed by heating at 90 °C for 20 min (Panpipat and Chaijan 2016). The gels were cooled in iced water and stored for 24 h at 4 °C prior to analysis.
Determination of whiteness
Colourimetric values of the samples were obtained, in triplicate, by using a portable Hunterlab Miniscan/EX instrument (10° standard observers, illuminant D65, Hunter Assoc. Laboratory; VA, U.S.A.). The sample was tightly packed into a plastic plate with the same weight before subjected to colour analysis. The instrument was calibrated to a white and black standard. The tristimulus L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) measurement mode was used as it relates to the human eye response to colour. The whiteness was calculated as follows:
Texture analysis
Texture analysis of the gels was performed using a TA-XT2 texture analyser (Stable Micro Systems, Godalming, Surrey, U.K.). Gels were equilibrated and evaluated at room temperature (28–30 °C). Three cylinder-shaped samples with a length of 2.5 cm were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/deformability) were measured using the texture analyser equipped with a spherical plunger (diameter 5 mm; depression speed 60 mm.min−1) (Panpipat and Chaijan 2016).
Determination of expressible moisture
Expressible moisture of gels was measured according to the method of Ng (1987). A gel sample with a thickness of 0.5 cm was weighed and placed between two pieces of Whatman filter paper No. 1 at the top and three pieces of the same filter paper at the bottom. The standard mass (5 kg) was placed on the top of the sample and maintained for 2 min. The sample was then removed and weighed again. Expressible moisture was expressed as percentage of sample weight.
Determination of gel microstructure
Microstructures of gels were determined using a scanning electron microscope (SEM) (GeminiSEM, Carl Ziess Microscopy, Germany). Samples with a thickness of 2–3 mm were fixed with 2.5% (v/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 2 h. The samples were then rinsed for 1 h in distilled water before being dehydrated in ethanol with serial concentrations of 50, 70, 80, 90 and 100% (v/v). Dried samples were mounted on a bronze stub and sputter-coated with gold. The specimens were observed with an SEM at an acceleration voltage of 10 kV.
Statistical analysis
A completely randomised design was used in this study and the entire experiment was replicated three times. Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple-range test to identify significant differences (p < 0.05) among treatments (Steel and Torrie 1980). Statistical analysis was performed using the Statistical Package for Social Science (SPSS 10.0 for windows, SPSS Inc., Chicago, IL, U.S.A.).
Results and discussion
Biochemical properties of surimi and unwashed mince
Biochemical properties of mackerel unwashed mince and surimi prepared using cold water and CW washing processes including pH, Ca2+-ATPase activity, reactive SH content and surface hydrophobicity are presented in Table 1. The initial pH value of mackerel muscle was 6.11 and the pH of surimi was dependent on the washing medium used. From the results, surimi conventionally washed with water (T1) showed the highest pH value (pH = 6.44) (p < 0.05). With increasing CW washing cycle, pHs of surimi (T2, T3 and T4) were lower than T1 (p < 0.05) which were in the ranges of 6.31–6.10. Final pHs of T3 and T4 surimi were similar to unwashed mince (p > 0.05). However, the pH of all surimi in this study was quite similar to the report of Chaijan et al. (2004) who found the pH value of 6.17 in mackerel (Rastrelliger kanagurta) surimi washed with 0.5% NaCl solution. The results suggested that the final pH of surimi might relate to the pH of medium used and might depend on the leaching efficacy. Both acidic matters and alkaline compounds can be leached out from fish mince during washing process leading to the new equilibrium of muscle pH. Additionally, the adsorption of washing medium into surimi can also alter the pH of resulting surimi. For instance, washing with CW with mild acidic pH rendered the surimi with more acidic pH, especially with increasing CW washing cycle, when compared to cold water washing.
Table 1.
Biochemical properties of mackerel unwashed mince and surimi prepared using cold water and CW washing processes
| Sample | pH | Ca2+-ATPase activity (µmol Pi/mg protein/min) | Reactive SH (mol/108 g protein) | Hydrophobicity (BPB bound (µg)) |
|---|---|---|---|---|
| Unwashed mince | 6.11 ± 0.00ab | 2.87 ± 0.43b | 4.85 ± 0.01a | 107.89 ± 3.63a |
| T1 | 6.44 ± 0.01d | 2.92 ± 0.65b | 5.87 ± 0.04b | 132.90 ± 2.22b |
| T2 | 6.31 ± 0.01c | 2.44 ± 0.40ab | 6.58 ± 0.08c | 130.17 ± 6.87b |
| T3 | 6.12 ± 0.01b | 2.14 ± 0.15ab | 6.71 ± 0.05d | 129.92 ± 3.96ab |
| T4 | 6.10 ± 0.02a | 1.71 ± 0.38a | 6.92 ± 0.02e | 122.87 ± 3.23a |
Values are given as mean ± standard deviation from triplicate determinations. Different letters in the same column indicate significant differences (p < 0.05)
T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW
Ca2+-ATPase activities of NAMs extracted from unwashed mince, conventional surimi and CW washed surimi are shown in Table 1. Water washing (T1) had no effect on Ca2+-ATPase activity when compared to unwashed mince (p > 0.05). However, with increasing CW washing cycle (T2–T4), the Ca2+-ATPase activity tended to decrease suggesting the denaturation or unfolding of myosin molecule. Foegeding et al. (1996) reported that Ca2+-ATPase activity is a sensitive indicator of myosin denaturation. The myofibrillar ATPase activities have been widely used as a measure of actomyosin integrity. From the result, bubbles generated by CW with intensively washing for 3 cycles (T4) led to a greater degree of conformational changes of myosin which can eventually affect the gel-forming ability of resulting surimi. Since gelation of muscle protein involves partial denaturation followed by irreversible aggregation of myosin heads through formation of disulfide bonds and helix-coil transitions of the tail part of the molecules, resulting in a three-dimensional cross-linked network structure (Sun and Holley 2011).
Reactive SH contents of NAMs extracted from unwashed mince, conventional surimi and CW washed surimi are depicted in Table 1. The presence of SH groups in surimi is necessary for gel strengthening because heat treatment can cause the oxidation of SH groups to form disulfide bond. The results showed that the concentration of reactive SH significantly increased after washing (p < 0.05). Additionally, an increase in CW washing cycle led to an increase in reactive SH content. For instance, T4 had the highest reactive SH concentration (6.92 mol/108 g protein). It can be postulated that 3-cycle of CW washing can pronounce the degree of protein unfolding in the way which buried SHs are exposed. Buttkus (1970) explained that myosin molecule contained many SH groups located in the head and tail portions. When myosin molecules underwent conformational changes, the reactive SH groups could be more exposed (Benjakul et al. 1997).
The surface hydrophobicities of unwashed mince, conventional surimi and CW washed surimi are presented in Table 1. Ang and Hultin (1989) highlighted that protein surface hydrophobicity index is used to determine protein molecule unfolding induced by physical treatments such as heat, freezing and pH changes. Unfolding of protein could promote exposure of hydrophobic amino acids, thus modifying its surface hydrophobicity (Careche and Li-Chan 1997). From the technological point of view, a proper change on surface hydrophobicity could have a positive effect on the gel-forming ability of protein systems. From the results, washing with T1, T2 and T3 caused significant increase in surface hydrophobicity of proteins in surimi (p < 0.05). However, amongst surimi, washing with T4 had the lowest surface hydrophobicity. It can be postulated that partial unfolding of proteins may allow the exposure of buried hydrophobic regions to the surface, leading to the increase in surface hydrophobicity. A decrease in surface hydrophobicity of T4 surimi may result from the association between exposed hydrophobic patches via hydrophobic interaction to form aggregates. The presence of aggregates prior to gel may affect the gel properties of resulting surimi.
Lipid reduction and total haem protein of surimi
Lipid is considered to be an undesirable component in surimi because it can interfere with gel forming ability and causes gel rancidity (Kelleher et al. 1994; Chaijan et al. 2004). Moreover, fish lipid contains higher content of unsaturated fatty acids, which is prone to oxidation and may lead to deteriorate of surimi products (Lanier 2000). From the results, lipid content of fresh mackerel mince was 1.33/100 g sample and almost half of the lipid can be removed during washing. It was found that 45.86, 47.28, 42.51 and 40.38% of lipid was removed from the mince after being processed with T1, T2, T3 and T4, respectively (Table 2). From the results, conventional washing (T1) and one-cycle CW washing (T2) exhibited significantly higher potential in lipid reduction compared to two-cycle CW washing (T3) and three-cycle CW washing (T4) (p < 0.05). Lower amount of lipid was depleted by T3 and T4 compared to other treatments. This was probably due to the aggregation of proteins by hydrophobic interaction in which the fat might be entrapped in the aggregates.
Table 2.
Lipid reduction and total haem protein content of mackerel unwashed mince and surimi prepared using cold water and CW washing processes
| Sample | Lipid reduction (%)* | Total haem protein (µmol haemoglobin/kg) |
|---|---|---|
| Unwashed mince | – | 50.16 ± 1.37b |
| T1 | 45.86 ± 0.56b | 30.86 ± 2.25a |
| T2 | 47.28 ± 1.49b | 31.01 ± 2.69a |
| T3 | 42.51 ± 0.30a | 30.93 ± 2.10a |
| T4 | 40.38 ± 0.12a | 31.30 ± 0.64a |
Values are given as mean ± standard deviation from triplicate determinations. Different letters in the same column indicate significant differences (p < 0.05)
T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW
* The initial lipid content of fresh mackerel mince was 1.33% (w/w of wet muscle)
Haem pigments including haemoglobin and myoglobin are known to be the main pro-oxidants in muscle foods. Presence of blood and/or residual haem proteins in surimi can affect negatively several quality parameters such as whiteness, microbial growth, shelf-life and lipid oxidation (Wongwichian et al. 2015). From the result, total haem protein content of surimi, regardless of washing process, was lower than unwashed mince (p < 0.05). The result suggested that some haem pigments can be removed by washing with cold water or CW. However, it is impossible to remove all haem proteins from mackerel mince because interaction between haem proteins and muscle components, especially myofibrillar proteins, has been reported in this species (Chaijan et al. 2010). In addition, mackerel myoglobin had the isoelectric point (pI) of 5.8–5.9 (Yamaguchi et al. 1979). Since the pH of washing media (pH 6.0–7.3) was quite close to pI of myoglobin, the solubility of myoglobin at pHs used in this study might be lower than that at acidic or alkaline pHs. Thus, only ~40% of total haem proteins were removed from mackerel mince in all treatments.
Whiteness
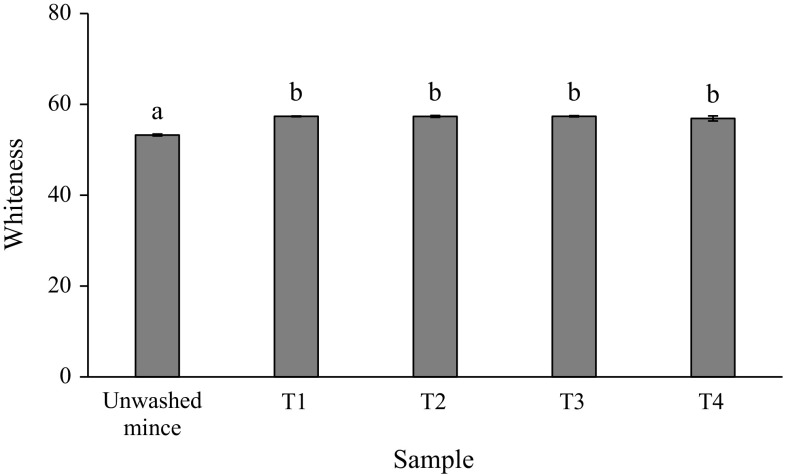
Whiteness of gel from mackerel unwashed mince and surimi is shown in Fig. 1. Whiteness is an important factor determining the quality and acceptability of surimi gel (Vate and Benjakul 2016). From the result, all surimi gels had higher whiteness than unwashed mince gel (p < 0.05). No significantly different in whiteness among surimi gels was found (p > 0.05). This was probably due to the same content of residual haem proteins, such as myoglobin and haemoglobin, in all surimi (Table 2) which can be further oxidised upon thermal gelation to form metmyoglobin/methaemoglobin with the same degree. A whiter colour of surimi gel could be due to a greater removal of haem pigments and a greater removal of fat-soluble pigments like carotenoids after washing process. Chaijan et al. (2013) reported that carotenoid content of dark and ordinary muscles of mackerel (Auxis thazard) was 0.32 and 0.13 mg/g, respectively. The lowest whiteness of unwashed mince gel would also originate from the oxidation of haem proteins to yield met-derivatives, since the highest haem protein content was found in the unwashed mince (Table 2). In addition, the lipid oxidation and Maillard browning reaction can be other factors influencing a lowered whiteness of unwashed mince gel. Since lipid was not being removed in unwashed mince and it can be oxidised during thermal gelation. It has been reported that the aldehydic lipid oxidation products can participate in the Maillard reaction (Chaijan et al. 2007).
Fig. 1.
Whiteness of gels from mackerel unwashed mince and surimi prepared using cold water and CW washing processes. T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW. *Bar indicated standard deviation from triplicate determinations. **Different letters indicate significant differences between treatments (p < 0.05)
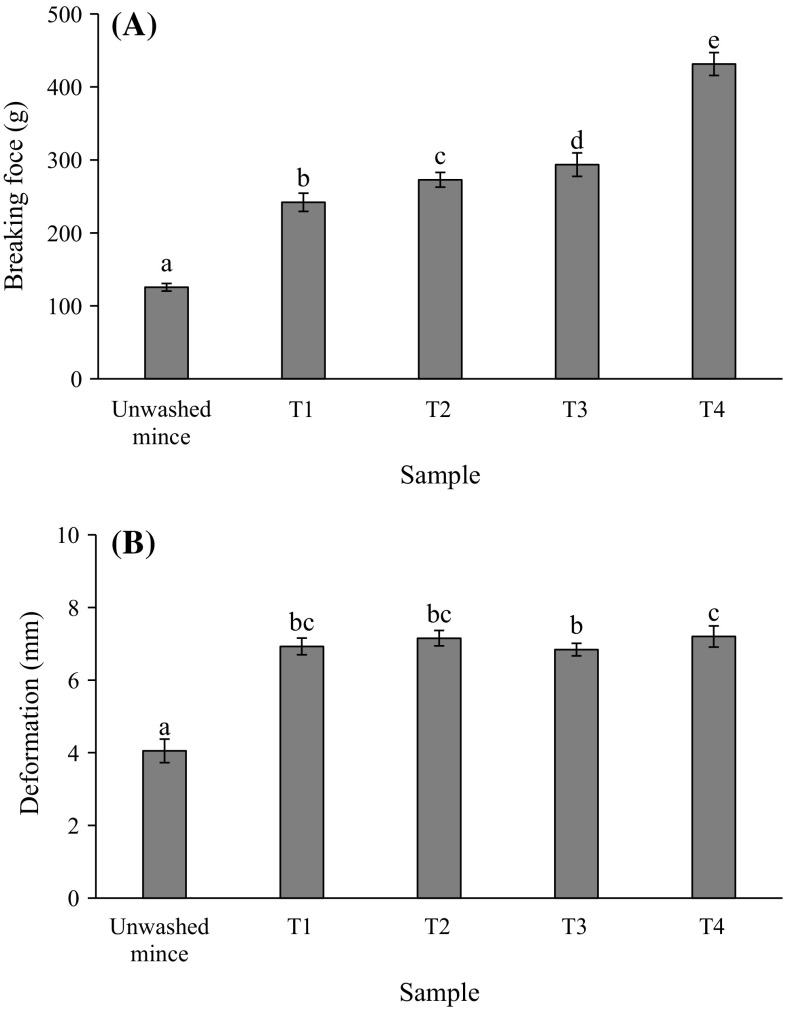
Breaking force and deformation
Breaking force and deformation measurement are usually used to describe the hardness and springiness index for surimi gelation. The breaking force and deformation of mackerel unwashed mince and surimi gels are shown in Fig. 2a, b, respectively. Washing with both cold water and CW rendered the gels with greater breaking force and deformation when compared to unwashed mince (p < 0.05). Washing may remove some sarcoplasmic proteins and lipids which have been reported as interferences in the gel forming ability of myofibrillar proteins (Chaijan et al. 2004). The breaking force of gel increased steadily with increasing CW washing cycle (p < 0.05) and the highest value was found in T4 compared to other treatments (p < 0.05) (Fig. 2a). Although, T4 tended to show the lowest activity of Ca2+-ATPase but it had the highest reactive SH concentration (Table 1) which can form disulfide bond upon heat induced gelation. High temperature during heating led to further oxidation of SH groups with a subsequent disulfide bond formation (Benjakul et al. 2001). Angsupanich et al. (1999) reported that a heat set myosin gel is primarily stabilised by disulfide bonds and hydrophobic interactions. It can be defined that T4 had a higher hardness index compared to the other treatments due to the aggregation of unfolded protein molecules via various interactions, such as disulfide linkages, electrostatic interactions, hydrophobic interactions and hydrogen bonds. In addition, Cuomo et al. (2002) reported that CW contained some ions such as Ca2+, Na+, Mg2+, K+, Cl− and . Ca2+ was the major ion in CW which can be found up to 202 mg/L (Cuomo et al. 2002). Those ions, particularly Ca2+, may participate in the gelation of surimi, since Ca2+ was necessary for the activity endogenous transglutaminase (TGase) (Lee and Park 1998). The setting phenomenon has been attributed to endogenous TGase activity that induces protein cross-linking and gel strengthening. Accordingly, cross-linking ability of myosin induced by TGase during setting at 40 °C could occur to some extent as a result from the presence of Ca2+ in the CW. For the deformation (Fig. 2b), surimi gels had significantly higher deformation than unwashed mince gel (p < 0.05). However, deformations of all surimi gels were not much different (~7 mm). Considering both breaking force and deformation, it can be postulated that gel strength of surimi increased with increasing CW washing cycle.
Fig. 2.
Breaking force (A) and deformation (B) of gels from mackerel unwashed mince and surimi prepared using cold water and CW washing processes. T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW. *Bar indicated standard deviation from triplicate determinations. **Different letters indicate significant differences between treatments (p < 0.05)
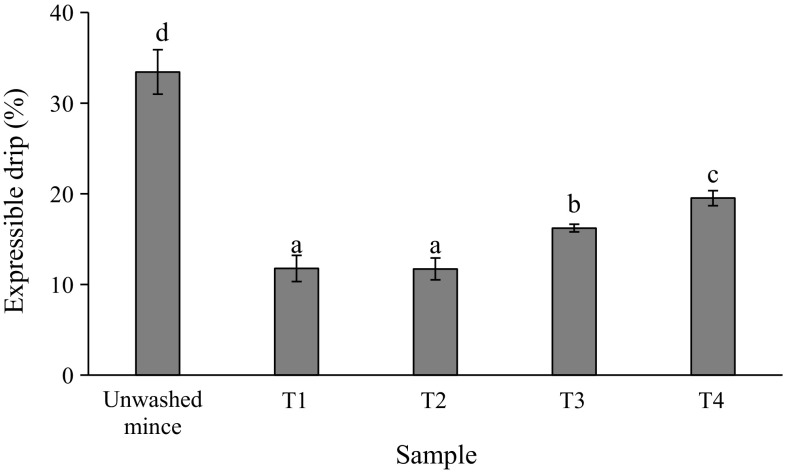
Expressible drip
Expressible drip of gels from unwashed mince and surimi gels are shown in Fig. 3. Different expressible moisture suggested the difference in water holding capacity of gel network. The heat-induced gelation of myosin results in the formation of a three-dimensional network structure that holds water in a less mobile state (Sun and Holley 2011). Water holding capacity is define as the ability of a food matrix to prevent water releases from the three-dimensional structure (Chantrapornchai and McClements 2002). The highest expressible moisture was found in gel of unwashed mince (p < 0.05) and it was related with the lowest strength and deformation of such gel (Fig. 2). However, expressible drip increased with increasing CW washing cycle (p < 0.05) in which T1 and T2 showed the lowest value. The pH value may affect the water holding capacity of surimi gel. Among surimi, T4 had the lowest pH value (6.10) which approached the pI of myofibrillar proteins (pH ~ 5.5) (Foegeding et al. 1996). Theoretically, if the pH value is closed to pI, the agglutination of proteins with released water tends to take place. Also, the highest degree of protein denaturation could be found in T4 surimi as indicated by the lowest Ca2+-ATPase activity with the highest reactive SH group (Table 1). The denatured proteins may form aggregates to some extent before gelation as evidenced by the lowest surface hydrophobicity of T4 surimi (Table 1). Those changes can promote the protein–protein interaction and caused the imbalance between protein–protein interaction and protein–water interaction leading to the formation of hard gel with some water releases (Figs. 2, 3). From the results, it was noted that intensively washing with CW had a detrimental effect on water retention of surimi gel. In the case of unwashed mince, the lowest gel strength was correlated with the lowest water holding capacity of that gel. Actually, the CW washing can cause the unfolding of muscle proteins which can subsequently form the three-dimensional network during thermal gelation by protein–protein interactions via several bonds e.g. hydrophobic interaction, disulfide bond and hydrogen bond. However, the extreme denaturation showed a negative impact on gelation of muscle proteins (Panpipat and Chaijan 2016).
Fig. 3.
Expressible drip of gels from mackerel unwashed mince and surimi prepared using cold water and CW washing processes. T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW. *Bar indicated standard deviation from triplicate determinations. **Different letters indicate significant differences between treatments (p < 0.05)
It can be postulated that the gel network of each treatment was stabilised by different bonds resulting in the different gel strength and water holding capacity. Protein–water interaction stabilised by hydrogen bond or other non-covalent bonds like electrostatic interactions may predominant in T1 and T2 gels leading to the greatest water holding capacity with lowered breaking force. However, protein–protein interaction primarily stabilised by disulfide bonds and hydrophobic interactions could more likely form in T3 and T4 gels leading to the increase in breaking force with the decrease in water holding capacity. Thus, to obtain the good gel, the balance between protein–protein interaction and protein–water interaction of the gel network should be taken place.
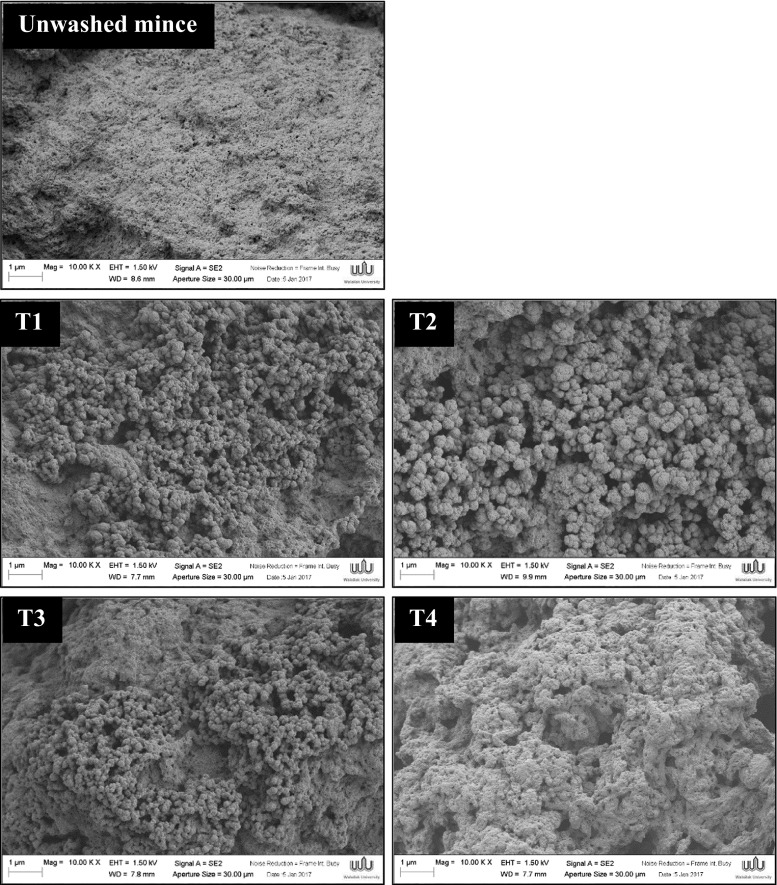
Gel microstructure
The gel microstructures visualised by scanning electron microscope (SEM) are depicted in Fig. 4. During thermal gelation, myosin and other salt-soluble myofibrillar proteins exhibit complex changes in rheological characteristics depending upon specific temperature and pH exposures (Sun and Holley 2011). Good surimi gel can be obtained when a regular aggregated structure with a well-organised three-dimensional network are formed (Benjakul et al. 2005). Unwashed mince gel had a structure with of dense packed aggregates and non-uniform gel network. T1–T4 surimi gels demonstrated more uniform gel networks with homogeneous pores when compared to unwashed mince gel. Furthermore, numbers of jointed spherical matrices were observed in surimi gel microstructures. It could be attributed to the effect of SH groups and the exposed hydrophobic groups on the protein surface which can further form disulfide bonds and hydrophobic interactions. Especially, surimi gel prepared by one-cycle CW washing (T2) exhibited jointed spherical cross-link and more ordered structure. On the other hand, with increasing CW washing cycle (T3 and T4), those jointed spherical networks seemed to disappear but dense packed aggregates tended to instead form. The formation of small number of cavities, high aggregates and non-uniform gel network with irregular pores can be clearly seen in T3 and T4 gels. The highest degree of aggregate can be observed in T4 which may correlate with the highest gel strength with higher degree of expressible drip (Figs. 2, 3).
Fig. 4.
Electron microscopic images of gels from mackerel unwashed mince and surimi prepared using cold water and CW washing processes (magnification: 10,000× EHT: 10 kV). T1 = water, water and water, T2 = CW, water and water, T3 = CW, CW and water and T4 = CW, CW and CW
Conclusion
One-cycle washing with cold CW followed by two-cycle washing with cold water (T2) was the best suited for improvement the total quality of mackerel surimi gel. This condition rendered the surimi with low lipid content, improved gel strength and low released water. With increasing the cycle of cold CW washing, the gel hardening with higher expressible drip was generally experienced. Therefore, cold CW can be used as a novel washing medium for production of mackerel surimi with improved gelling characteristics but number of CW washing cycle seemed to be a crucial factor influencing the biochemical properties and gel-forming ability of mackerel surimi.
Acknowledgements
This work was supported by Walailak University Fund.
References
- Ang JF, Hultin HO. Denaturation of cod myosin during freezing after modification with formaldehyde. J Food Sci. 1989;54:814–818. doi: 10.1111/j.1365-2621.1989.tb07889.x. [DOI] [Google Scholar]
- Angsupanich K, Edde M, Ledward DA. Effects of high pressure on the myofibrillar proteins of cod and turkey muscle. J Agric Food Chem. 1999;47:92–99. doi: 10.1021/jf980587p. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Seymour TS, Morrissey MT, An H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Ishizaki S, Tanaka M. Differences in gelation characteristics of natural actomyosin from two species of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. J Food Sci. 2001;66:1311–1318. doi: 10.1111/j.1365-2621.2001.tb15207.x. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Thongkaew C, Tanaka M. Effect of frozen storage on chemical and gel-forming properties of fish commonly used for surimi production in Thailand. Food Hydrocoll. 2005;19:197–207. doi: 10.1016/j.foodhyd.2004.05.004. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Buttkus H. Accelerated denaturation of myosin in frozen solution. J Food Sci. 1970;35:558–562. doi: 10.1111/j.1365-2621.1970.tb04808.x. [DOI] [Google Scholar]
- Careche M, Li-Chan ECY. Structural changes in cod myosin after modification with formaldehyde or frozen storage. J Food Sci. 1997;62:717–723. doi: 10.1111/j.1365-2621.1997.tb15443.x. [DOI] [Google Scholar]
- Chaijan M, Undeland I. Development of a new method for determination of total haem protein in fish muscle. Food Chem. 2015;173:1133–1141. doi: 10.1016/j.foodchem.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Chaijan M, Benjakul S, Visessanguan W, Faustman C. Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res Int. 2004;37:1021–1030. doi: 10.1016/j.foodres.2004.06.012. [DOI] [Google Scholar]
- Chaijan M, Benjakul S, Visessanguan W, Lee S, Faustman C. The effect of freezing and aldehydes on the interaction between fish myoglobin and myofibrillar proteins. J Agric Food Chem. 2007;55:4562–4568. doi: 10.1021/jf070065m. [DOI] [PubMed] [Google Scholar]
- Chaijan M, Panpipat W, Benjakul S. Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem. 2010;121:85–92. doi: 10.1016/j.foodchem.2009.12.007. [DOI] [Google Scholar]
- Chaijan M, Klomklao S, Benjakul S. Characterisation of muscles from Frigate mackerel (Auxis thazard) and catfish (Clarias macrocephalus) Food Chem. 2013;139:414–419. doi: 10.1016/j.foodchem.2013.01.111. [DOI] [PubMed] [Google Scholar]
- Chantrapornchai W, McClements DJ. Influence of NaCl on optical properties, large-strain rheology and water holding capacity of heat-induced whey protein isolate gels. Food Hydrocoll. 2002;16:467–476. doi: 10.1016/S0268-005X(01)00124-2. [DOI] [Google Scholar]
- Chelh I, Gatellier P, Santé-Lhoutellier V. Technical note: a simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006;74:681–683. doi: 10.1016/j.meatsci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cuomo R, Grasso R, Sarnelli G, Capuano G, Nicolai E, Nardone G, Ierardi E. Effects of carbonated water on functional dyspepsia and constipation. Eur J Gastroenterol Hepatol. 2002;14:991–999. doi: 10.1097/00042737-200209000-00010. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Foegeding EA, Lanier TC, Hultin HO. Characteristics of edible muscle tissues. In: Fennema OR, editor. Food chemistry. New York: Marcel Dekker; 1996. pp. 880–942. [Google Scholar]
- Hultin HO, Kelleher SD. Surimi processing from dark muscle fish. In: Park JW, editor. Surimi and surimi seafood. New York: Marcel Dekker; 2000. pp. 59–77. [Google Scholar]
- Kelleher SD, Hultin HO, Wilhelm KA. Stability of mackerel surimi prepared under lipid-stabilising processing conditions. J Food Sci. 1994;59:269–271. doi: 10.1111/j.1365-2621.1994.tb06945.x. [DOI] [Google Scholar]
- Lanier TC. Surimi gelation chemistry. In: Park JW, editor. Surimi and surimi seafood. New York: Marcel Dekker; 2000. pp. 237–265. [Google Scholar]
- Lee N, Park JW. Calcium compounds to improve gel functionality of Pacific whiting and Alaska pollock surimi. J Food Sci. 1998;63:969–974. doi: 10.1111/j.1365-2621.1998.tb15835.x. [DOI] [Google Scholar]
- Lin SB, Chen LC, Chen HH. The change of thermal gelation properties of horse mackerel mince led by protein denaturation occurring in frozen storage and consequential air floatation wash. Food Res Int. 2005;38:19–27. doi: 10.1016/j.foodres.2004.08.001. [DOI] [Google Scholar]
- Lubetkin S, Blackwell M. The nucleation of bubbles in supersaturated solutions. J Colloid Interface Sci. 1988;26:610–615. doi: 10.1016/0021-9797(88)90161-0. [DOI] [Google Scholar]
- Mao L, Wu T. Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyn godonidellus) influenced by chitosan. J Food Eng. 2007;82:128–134. doi: 10.1016/j.jfoodeng.2007.01.015. [DOI] [Google Scholar]
- Martinaud A, Mercier Y, Marinova P, Tassy C, Gatellier P, Renerre M. Comparison of oxidative processes on myofibrillar proteins from beef during maturation and by different model oxidation systems. J Agric Food Chem. 1997;45:2481–2487. doi: 10.1021/jf960977g. [DOI] [Google Scholar]
- Ng CS. Measurement of free and expressible drips. In: Hasegawa H, editor. Manual on analytical methods and procedure for fish and fish products laboratory. Singapore: Southeast Asian Fisheries Development Center; 1987. [Google Scholar]
- Niwa E. Chemistry of surimi gelation. In: Lanie TC, Lee CM, editors. Surimi technology. New York: Marcel Dekker; 1992. pp. 389–427. [Google Scholar]
- Panpipat W, Chaijan M. Potential production of healthier protein isolate from broiler meat using modified acid-aided pH shift process. Food Bioprocess Technol. 2016;9:1259–1267. doi: 10.1007/s11947-016-1716-z. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principle and procedure of statistics. New York: MacGraw-Hill; 1980. [Google Scholar]
- Sun XD, Holley RA. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr Rev Food Sci Food Saf. 2011;10:33–51. doi: 10.1111/j.1541-4337.2010.00137.x. [DOI] [Google Scholar]
- Vate NK, Benjakul S. Effect of the mixtures of squid ink tyrosinase and tannic acid on properties of sardine surimi gel. J Food Sci Tech. 2016;53:411–420. doi: 10.1007/s13197-015-1974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt PM. Nucleation rates and bubble stability in water-carbon dioxide solutions. J Colloid Interface Sci. 1986;112:530–538. doi: 10.1016/0021-9797(86)90122-0. [DOI] [Google Scholar]
- Wongwichian C, Klomklao S, Panpipat W, Benjakul S, Chaijan M. Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem. 2015;174:279–285. doi: 10.1016/j.foodchem.2014.11.071. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Takeda N, Ogawa K, Hashimoto K. Properties of mackerel and sardine myoglobins. Bull Jpn Soc Sci Fish. 1979;45:1335–1339. doi: 10.2331/suisan.45.1335. [DOI] [Google Scholar]