Abstract
The effect of polymer degradation was studied on immunomodulatory and antioxidant properties of fucoidan isolated from S. angustifolium. Partially hydrolyzed fucoidans were prepared using 0.01 N hydrochloric acid after incubation for 10 and 15 min in boiling water. FT-IR analysis showed two major peaks at 850 cm−1 corresponding to bending vibration of C–O–S of sulfate and 1256 cm−1 derived from the stretching vibration of S–O. The native fucoidan consisted mainly of carbohydrate (49.4%), sulfate (22.9%), uronic acid (10.3%) and minor amount of protein (4.1%). The hydrolysis reduced the molecular weight of native fucoidan from 421 × 103 g/mol to 104.1 × 103 g/mol after 10 min boiling and 63.9 × 103 g/mol after 15 min boiling, without a significant change in their chemical compositions. Acid degradation increased the specific volume of gyration from 0.84 to 3.32 cm3/g in hydrolyzed fucoidan polymers. Fucoidan with the lowest molecular weight showed the greatest proliferating effect on RAW264.7 cells and induced the macrophage cells to release more nitric oxide (39.0 μmol) at 50 μg/mL. The DPPH radical scavenging activity, ABTS radical scavenging activity and reducing power remarkably increased after hydrolysis. The current results showed that molecular weight has determinant effect on immunomodulatory and antioxidant activities of unrefined fucoidan and thus acid hydrolysis can be applied on commercial scale to obtain fucoidans with more beneficial effects.
Keywords: Sargassum angustifolium, Fucoidan, Hydrolysis, Molecular weight, Immunomodulatory
Introduction
Anionic polysaccharides including sulfated fucans, sulfated galactans and sulfated rhamnans are predominantly found in marine algae. Some of these polysaccharides have been utilized in the food industry due to their peculiar rheological properties (Rhein-Knudsen et al. 2015). Other polysaccharides have shown positive effects in different biological systems, suggesting their applications as promising bioactive compounds (Wijesekara et al. 2011). Fucoidans are a good example of fucose-containing polysaccharides that have been extensively studied from various species. Fucoidans are found in the cell wall of brown seaweeds in a complex matrix along with alginates, proteins and low molecular weight compounds bound with each other through ionic interactions and hydrogen bonding (Skriptsova 2015).
Numerous reports demonstrated that fucoidan may serve as a good candidate for therapeutic applications, since they exhibited in vitro and in vivo biological functions such as antitumor, antivirus, anticoagulant and immunomodulatory effects (Nishino et al. 1991; Cho et al. 2014; Wijesekara et al. 2011). However, the utilization of these sulfated macromolecules in functional foods has been challenging due to inconsistent results on their bioactivities (Ale et al. 2011). This is partly originated from variations in the molecular structure of fucoidans caused by intrinsic factors including differences in species and seaweed growth conditions (Ale et al. 2011). The method of choice in the isolation process is another effective factor that might either undermine the biofunctionality of a fucoidan polymer or keep it intact (Sánchez-Camargo et al. 2016).
Basically, the biological activities of fucoidans are closely related to their structural specifications such as molecular weight, amount and position of sulfates, monosaccharide composition and etc. (Kim et al. 2015). Thus, reaching a deep understanding of the structure-bioactivity relationship of fucoidans enables the identification of structural feature responsible for a biological effect. Accordingly, the potency of a bioactive fucoidan can be changed by structural alterations using various modification means (Jia et al. 2016). By far, most of the fucoidan products available on the market have been manufactured from unrefined aqueous extracts of brown seaweeds. The reasons for unwillingness of fucoidan industry to further purify the extracted fucoidans can be listed as high operational cost, low product yield and time consuming process. Therefore, the aim of current study is to examine the effect of hydrolysis on chemical and molecular properties of unrefined fucoidan polymers and evaluate their relationship with immunomodulatory and antioxidant activities.
Materials and methods
Materials
The brown seaweed S.angustifolium collected from the coast of Persian Gulf, Bushehr, Iran. Samples were extensively washed with tap water to remove impurities and then air-dried at 60 °C for 4 days. The dried raw material was pulverized in a blender and stored at −20 °C before the extraction of fucoidan. The Dulbecco’s Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS), streptomycin and penicillin were purchased from Hyclone (Logan, UT). Other chemicals and reagents were of analytical grade.
Depigmentation and isolation of fucoidan
The milled seaweed (20 g) was treated with 85% ethanol (EtOH, 200 mL) under constant mechanical stirring overnight at room temperature in order to remove pigments, lipids and low molecular weight compounds. After centrifugation (10 °C, 9000 rpm, 10 min), the residue was separated and depigmentation continued with fresh EtOH for three times. Then, samples were rinsed with acetone and residue dried at room temperature. A 20 g of dried powder was used for extraction with 400 mL of distilled water at 65 °C with for 2 h. The extracts were centrifuged at 9000 rpm for 10 min at room temperature, and the supernatants were concentrated under reduced pressure at 60 °C. Then, 1% CaCl2 was added into supernatant in order to remove alginate from fucoidan. Precipitated alginate was discarded by centrifugation at 9000 rpm for 10 min at room temperature. Finally, the polysaccharides were precipitated by adding EtOH (99%) into the supernatants to obtain the final concentration of 70%. The fucoidan was obtained by the filtration of the solution with a membrane (0.45 m pore size, Whatman International, Maidstone, UK) and washed with EtOH (99%) and acetone for several times, and then dried at room temperature.
Preparation of partially hydrolyzed fucoidans
To produce fucoidans with different molecular weights, 20 mg of fucoidan was initially dissolved in 1 mL of 0.01 N HCl and then partial hydrolysis was performed by heating in boiling water for 10 and 15 min. The reactants were immediately cool down and neutralized with 1 mL of 0.01 N NaOH. The native fucoidan solution was prepared by dissolving 20 mg of fucoidan in 1 mL of 0.01 N HCl without heating which was followed by neutralization with 1 mL of 0.01 N NaOH. All solutions were dialyzed in a membrane (#3247027, Spectrum Laboratories, Compton, CA, USA) against distilled water, and eventually lyophilized.
Determination of chemical compositions
Total carbohydrate was determined by the phenol–sulfuric acid method using glucose as a standard (Dubois et al. 1956). The amount of protein was measured by Folin-phenol reagent using bovine serum albumin as a standard (Lowry et al. 1951). Sulfate content was determined by the BaCl2-gelatin method using K2SO4 as a standard. Uronic acids were analyzed calorimetrically by m-hydroxydiphenyl analysis according to procedure described by Filisetti-Cozzi and Carpita (1991).
Determination of molecular weight of fucoidans
Fucoidans were initially dissolved in distilled water (2 mg/mL) and heated for 30 s in a microwave bomb (#4872; Parr Instrument Co., Moline, IL, USA). A cellulose acetate membrane was immediately used to filter the solution (3.0 μm pore size; Whatman International). A multi-angle laser light scattering system (HELEOS; Wyatt Technology Corp, Santa Barbara, CA, USA) coupled with high performance size exclusion chromatography column (TSK G5000 PW, 7.5 × 600 mm; Toso Biosep, Montgomeryville, PA, USA), UV detector (Waters, 2487) and refractive index detection (Waters, 2414) system (HPSEC-UV-MALLS-RI) was used to analyze the molecular characteristics. An aqueous solution of 0.15 M NaNO3 and 0.02% NaN3 was used as mobile phase at flow rate of 0.4 mL/min. Bovine serum albumin (BSA) was used to determine the volume delays among the UV, MALLS and RI detectors.
ASTRA 5.3 software (Wyatt Technology Corp.) was employed in order to determine the weight average molecular weight (M w), number average molecular weight (M n), radius of gyration (R g) and polydispersity index. The calculation of specific volume of gyration (SV g) was performed based on the following equation (You and Lim 2000):
in which N is Avogadro’s number (6.02 × 1023/mol) and the units for SV g, M w and R g were cm3/g, g/mol and nm respectively.
FT-IR spectroscopy
Fourier transform infrared (FT-IR) was used to detect functional groups of the polysaccharide and recorded on an infrared spectrometer (Tensor 27, Bruker Instruments, USA). One milligram of fucoidan was milled with 300 mg of KBr and pressed into a disk (1-mm thickness and 13-mm diameter) for transmission infrared spectroscopy. FT-IR spectrum was recorded between 500 and 4000 cm−1 at a resolution of 2 cm−1.
RAW264.7 macrophage proliferation and nitric oxide production assays
RAW264.7 macrophage cell lines (ATCC) were seeded in a 96-well microplate (1 × 104 cells/well, 100-μL volume) with RPMI-1640 medium containing 10% FBS. The cells were incubated with 100 μL of the fucoidans (10, 25 and 50 μg/mL) in triplicate. The incubation of cell cultures was carried out in a humidified atmosphere with 5% CO2 at 37 °C for 72 h. Then, the WST-1 solution (20 μL) was added to the wells and the solution was further incubated for 4 h at 37 °C. The optical density was measured at 450 nm using a microplate reader (EL-800; BioTek Instruments, Winooski, VT, USA). The absorbance (A) was translated into a macrophage proliferation ratio, (%) = A t/A c × 100, where A t and A c are the absorbance of the test group and control group, respectively.
The nitric oxide (NO) released by macrophages into culture supernatants was measured as an indicator of immunoenhancing activity of alginates. The RAW264.7 macrophage cells were seeded in a 96-well plate (1 × 105 cells/well) and incubated with alginates (10, 25 and 50 μg/mL) and lipopolysaccharide (LPS, 1 μg/mL; Sigma-Aldrich, MO, USA) at 37 °C for 18 h. The Griess reagent was used to quantify the amount of NO production (Green et al. 1982). The NaNO2 (1–200 μM in culture medium) was used as standard to determine the amount of NO produced by macrophages.
Antioxidant activities
DPPH radical scavenging activity assay
The DPPH radical scavenging activity of native and hydrolyzed fucoidans was conducted according to the method of Brand-Williams et al. (1995) with slight modifications. Briefly, 100 µL of different concentrations of fucoidan samples (1–3 mg/mL) or ascorbic acid (100 µg/mL) were added to 100 µL 2,2-diphenylpicrylhydrazyl (DPPH) solution. The mixture was then incubated at 25 °C in the dark for 30 min. The absorbance was read at 517 nm by UV–Vis spectrometer. The ability to scavenge the DPPH radicals was calculated according to the following equation:
where Ac is the absorbance of the control (100 μL of ethanol with 100 μL of the DPPH solution) and As is the absorbance of the sample.
ABTS radical scavenging activity assay
Free radical scavenging activity of the native and partially hydrolyzed fucoidans was examined using ABTS radicals (Re et al. 1999a, b). ABTS was dissolved in water (7 mM) and diluted with ethanol to an absorbance of 0.70 at 734 nm. Then, 0.5 mL of different concentrations of fucoidans (0.062, 0.125 and 0.250 mg/mL) or ascorbic acid (100 µg/mL) were added to 1.5 mL of 0.7 mM ABTS solution. The solution was kept at room temperature for 20 min and the absorbance was read at 734 nm. The ability to scavenge the ABTS radical was calculated according to the following equation:
where Ac is the absorbance of the control (0.5 ml of ethanol with 1.5 ml of the ABTS solution) and As is the absorbance of the sample.
Reducing power
The reducing power of the fucoidan samples was measured by the method described by Oyaizu (1986). An aliquot of each fucoidan sample (500 μL) with concentrations ranging from 1 to 3 mg/mL was mixed with 500 μL of sodium phosphate buffer (0.2 M, pH 6.6) and 500 μL of 1% potassium ferricyanide. Ascorbic acid was used as a commercial antioxidant (100 µg/mL). After incubation at 50 °C for 20 min, 500 μL of 10% TCA was added and the mixture was centrifuged at 8000 rpm for 10 min. The supernatant (1.0 mL) was incubated in the presence of distilled water (1.0 mL) and 0.1% ferric chloride (200 μL) for 10 min. The absorbance was read at 700 nm.
Statistical analysis
All tests were carried out in triplicate and the results were presented as the mean value with standard deviation. Differences were statistically tested by one-way analysis of variance (ANOVA), and Duncan’s multiple-range test using Statistical Analysis System (SAS Institute, Cary, NC, USA).
Results and discussion
FT-IR spectroscopy of fucoidan
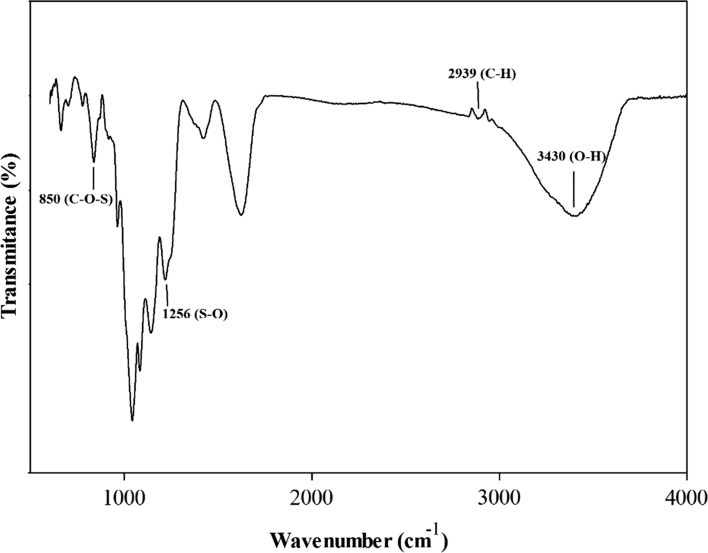
Figure 1 shows the Fourier transform infrared spectra of fucoidan from S. angustifolium between 500 and 4000 cm−1. Two signals were appeared in the region of 2500–3600 cm−1; a broad signal at 3430 cm−1 attributing the stretching vibrations of O–H and a small signal at 2939 cm−1 attributing the stretching vibration of C–H (Sari-Chmayassem et al. 2016). There were signals at 1660 cm−1, related to asymmetric stretching vibration of COO− of uronic acids and at 1446 cm−1, related to symmetric stretching vibration of COO− (Zhao et al. 2010). The peaks at 850 and 1256 cm−1 derived from the bending vibration of C–O–S of sulfate in axial position and stretching vibration of S–O of sulfate, respectively (Gan et al. 2011). The signals in the region of 1200–1500 cm−1 were mainly due to coupling of the deformation vibrations of groups containing hydrogen atoms, including HCH, CCH, HCO, and COH (Vanloot et al. 2012).
Fig. 1.
FT-IR spectra of a native fucoidan from S. angustifolium
Chemical composition of fucoidans
Table 1 shows the proximate composition of native and partially hydrolyzed fucoidans. The native fucoidan consisted mainly of carbohydrate (49.45%) which did not significantly change after acid hydrolysis (p > 0.05). Likewise, there was minor amount of protein content (4.13%) in the native fucoidan that stayed intact after degradation (p > 0.05). The extracted polysaccharide was an acidic polymer with high amount of uronic acid (10.32%). The hydrolyzed fucoidans also possessed a similar level of uronic acid with their native form (p > 0.05). As an important constituent of sulfated polysaccharides, the native fucoidan was rich in sulfate esters (22.98%) and it did not vary after hydrolysis (p > 0.05).
Table 1.
Chemical composition of native and hydrolyzed fucoidans from S. angustifolium
| Total sugar (%) | Protein (%) | Uronic acid (%) | Sulfate (%) | |
|---|---|---|---|---|
| Sa-Native | 49.45 ± 0.25a | 4.13 ± 0.28a | 10.32 ± 0.03a | 22.98 ± 0.71a |
| Sa-HD10 | 48.90 ± 0.20a | 3.93 ± 0.30a | 10.02 ± 0.07a | 21.31 ± 0.36a |
| Sa-HD15 | 48.30 ± 0.30a | 4.00 ± 0.20a | 9.96 ± 0.04a | 21.26 ± 0.51a |
a,b,c Indicate a significant difference (p < 0.05) between the samples
Molecular properties of native and hydrolyzed fucoidans
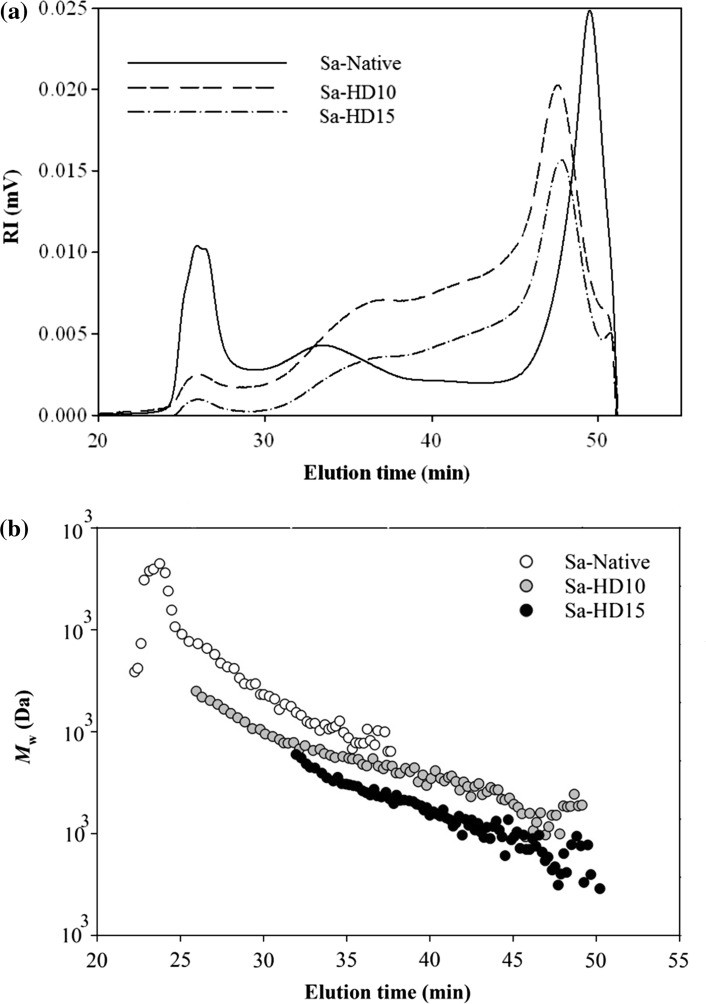
Figure 2a and b show the HPSEC chromatograms of native and partially hydrolyzed fucoidans obtained after heating in boiling water with 0.01 N HCl for 10 and 15 min. The polymer molecules of native fucoidan with no heat treatment (0 min) were eluted from the SEC columns between elution times of 24 and 40 min. After hydrolysis for 10 and 15 min, the shape of IR spectrums remarkably changed and moved toward higher elution times (25–45 min) indicating the successful degradation of fucoidan. As shown in Table 1, the weight average molecular weight (M w) of native fucoidan was found to be 421 × 103 g/mol which was in the wide range of 47–987 × 103 g/mol reported for the fucoidans from S. thunbergii, S. binderi, S. fusiforme and S. pallidum (Luo et al. 2016; Lim et al. 2016; Chen et al. 2016; Li et al. 2017). The significant variation in the molecular weight of fucoidans could be driven by differences in species, growth environment and extraction methods (Tabarsa et al. 2015).
Fig. 2.
The HPSEC chromatograms of partially hydrolyzed fucoidan polymers obtained by heating in boiling water (a) with 0.01 N HCl at different times (0, 10, 15 min)
The M w of fucoidan polymers notably decreased to 104.1 × 103 g/mol when 10 min of hydrolysis was applied to native fucoidan (p < 0.05). A further reduction to 63.9 × 103 g/mol was achieved for M w of fucoidans treated under 15 min heating (p < 0.05). Likewise, degraded fucoidans showed to have lower radius of gyration (R g) compared to its native form decreasing from 52.1 nm to 43.8 nm (p < 0.05). The M w and R g values were used to calculate the specific volume of gyration for different fucoidans (SV g). The SV g provide the theoretical gyration volume per unit of molar mass and is inversely proportional to the degree of molecular compactness (You and Lim, 2000). A significant variation was obtained in the SV g values of native and hydrolyzed fucoidans ranging from 0.84 to 3.32 cm3/g (Table 2). These results indicated that hydrolysis process produced fucoidan polymers more expanded structure and loosed conformation.
Table 2.
Weight average molecular weight (M w), number average molecular weight (M n), radius of giration (R g) and specific volume of gyration (SV g) of native and partially hydrolyzed fucoidans
| Alginates | M w × 103 (g/mol) | M n × 103 (g/mol) | R g (nm) | SV g (cm3/g) |
|---|---|---|---|---|
| Sa-Native | 421.0 ± 2.82a | 184.6 ± 0.84a | 52.1 ± 0.28a | 0.84 |
| Sa-HD10 | 104.1 ± 1.34b | 27.2 ± 0.42b | 49.8 ± 0.49b | 2.99 |
| Sa-HD15 | 63.9 ± 2.40c | 11.3 ± 0.56c | 43.8 ± 0.49c | 3.32 |
a,b,c Indicate a significant difference (p < 0.05) between the samples
Effect of Mw on proliferation and stimulation of RAW264.7 cells
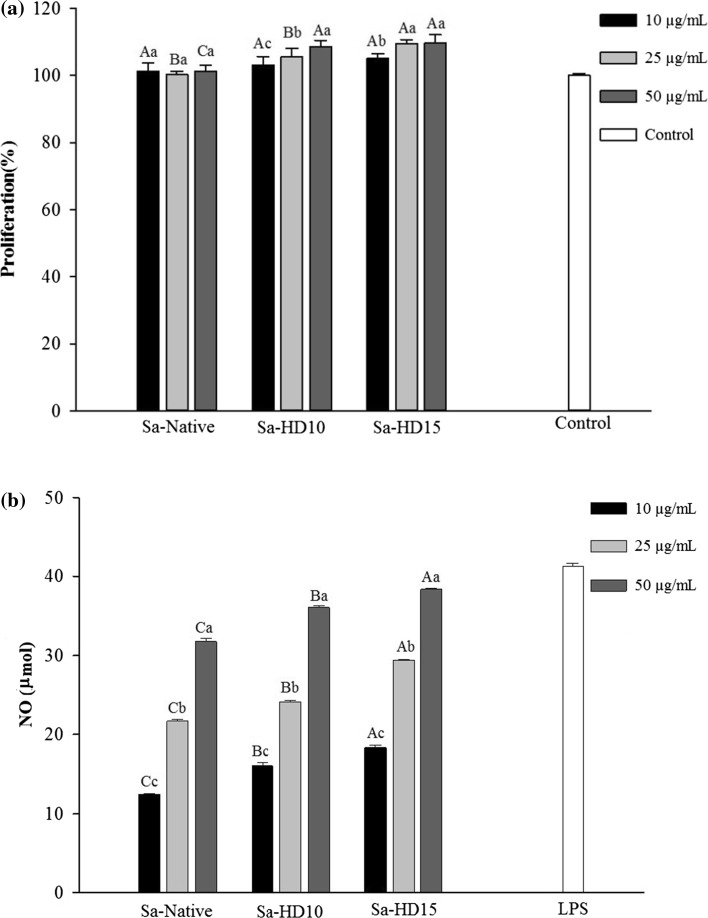
The effect of native and hydrolyzed fucoidans from S. angustifolium was examined on the proliferation of RAW264.7 cells at concentrations of 10, 25 and 50 μg/mL (Fig. 3a). After the addition of fucoidans into the culture medium of macrophage cells and incubation for 72 h, none of the native fucoidans or its low molecular weight polymers exerted toxic effects. In fact, conversely, degradation of fucoidan led to an increase in their potential in proliferating RAW264.7 cells in a dose dependent manner (p < 0.05).
Fig. 3.
Proliferation activity (a) and nitric oxide production of the RAW264.7 cells following treatment with native and partially hydrolyzed fucoidans. The RAW264.7 cells were plated in an RPMI-1640 medium containing 10% FBS in a 96-well microplate (1 × 104 cells/well) and incubated with 100 μL of 10, 25 and 50 μg/mL of different fucoidans or 1 μg/mL LPS (positive control). The letters a, b, c, d indicate a significant difference (p < 0.05) between the concentrations of the fucoidans, with A, B, C, D indicating a significant difference (p < 0.05) between the fucoidans at each concentration
In the current study, the immunostimulatory effects of the native and partially hydrolyzed fucoidans were evaluated using the ability of RAW264.7 macrophage cells to release nitric oxide (NO). Macrophages are important immunomodulatory effector cells that play a pivotal role in the immune system by maintaining homeostasis and providing defense against pathogens and cancer cells through the secretion of NO and different cytokines (Gamal-Eldeen et al. 2007). The NO release-inducing capacities of the native and partially hydrolyzed fucoidans at the concentrations of 10, 25 and 50 μg/mL are shown in Fig. 3b. The level of NO released by native fucoidan increased by increasing concentration up to 32 μmol at 50 μg/mL. The ability of fucoidans to stimulate macrophage cells to release NO has been previously reported for polysaccharides isolated from Fucus vesiculosus and Agarum cribrosum (Cho et al. 2014; Choi et al. 2005). Interestingly, a completely opposite result was observed for fucoidan from Agarum cribrosum where strong anti-inflammatory activity of the extracted polymer suppressed NF-kB activation and down-regulated the MAPK pathways (Park et al. 2011). These variations in the type and magnitude of bioactivities of sulfated polysaccharides have been attributed to their differences in sulfate content, molecular weight, linkage pattern, uronic acid content and etc. (Qi et al. 2005; Tabarsa et al. 2015).
The fucoidan polymers having the M w of 104.1 × 103 g/mol obtained by 10 min hydrolysis exhibited a slight increase in NO (37.0 μmol) at the same concentration (p < 0.05). Further improvement in the amount of NO released was observed with the fucoidans having the M w of 63.9 × 103 g/mol produced after 15 min hydrolysis (p < 0.05). The NO-releasing capacity of native and degraded fucoidans clearly showed that fucoidans with lower molecular weights are more effective in stimulating macrophage cells. The determinant effect of molecular weight of fucoidans on their bioactivity was also reported for polysaccharides extracted from Ecklonia kurome where fucans with molecular weights ranging from 10 × 103 to 300 × 103 g/mol exhibited the most potent anticoagulant activities (Nishino et al. 1991). Yang et al. (2008) reported that lower molecular weight fucoidans had higher suppression effect on the growth inhibition of lung cancer cells.
Antioxidant properties of fucoidans
DPPH radical scavenging activity
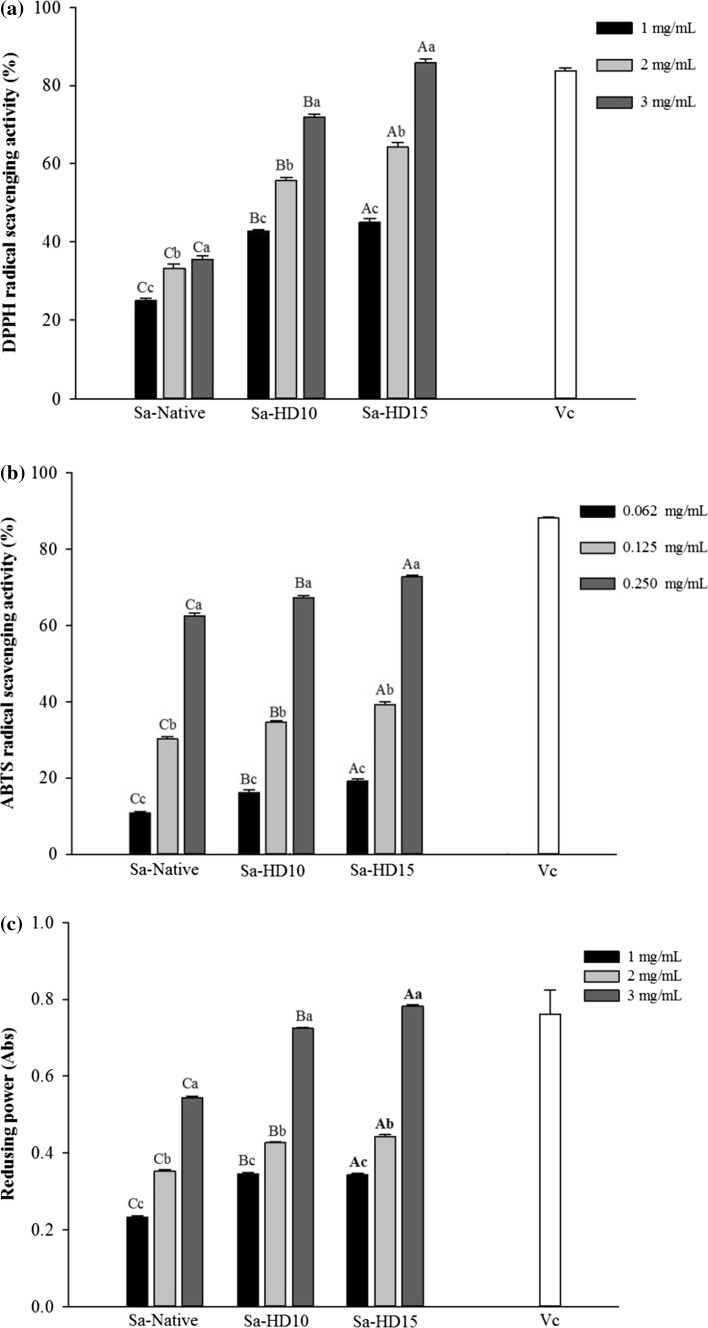
The DPPH radical scavenging is a widely used model to examine antioxidant activities in a relatively short time compared with other methods. The antiradical capacity of an antioxidant is conducted by measurement of the decrease in the absorbance of DPPH radical at 517 nm (Wang et al. 2010). The reduction in the absorbance of DPPH radical is caused by hydrogen atom transferred from an H-donor leading to the disappearance of the visible band in DPPH (Foti 2015). The DPPH radical scavenging of native and partially hydrolyzed fucoidans obtained by 0.01 N HCl and heat treatment in boiling water for different times from 0, 10 and 15 min, is shown in Fig. 4a. The radical scavenging effect of native fucoidan was measured from 25 to 35% over the concentration range of 1–3 mg/mL. After hydrolysis, fucoidan with M w of 104.1 × 103 g/mol exhibited an incredibly high radical scavenging effect up to 72% (p < 0.05). Increased hydrolysis time to 15 min and molecular weight reduction to 63.9 104.1 × 103 g/mol led to higher scavenging activity (86%) which was comparable with that of ascorbic acid (p < 0.05). The results indicated that DPPH radical scavenging activity of fucoidan improves with reduced molecular weights.
Fig. 4.
DPPH radical scavenging (a), ABTS radical scavenging (b) and reducing power (c) of native and partially hydrolyzed fucoidans. The letters a, b, c, d indicate a significant difference (p < 0.05) between the concentrations of the fucoidans, with A, B, C, D indicating a significant difference (p < 0.05) between the fucoidans at each concentration
ABTS radical scavenging activity
ABTS assay is a quick method to determine the antioxidant activity of hydrogen-donating compounds to aqueous phase radicals and chain-breaking antioxidants of lipid peroxyl radicals (Nalinanon et al. 2011). Figure 4b shows the ABTS radical scavenging activity of native and partially hydrolyzed fucoidans in concentrations ranging from 0.062 to 0.250 mg/mL. The native fucoidan reduced the ABTS radicals dose-dependently and reached up to 64% at the highest concentration. Lowering the molecular weight of fucoidans significantly increased their ABTS radical scavenging where the highest activity (75%) was observed for fucoidan degraded for 15 min (p < 0.05). Overall, lower molecular weight fucoidans showed to be stronger radical scavengers compared with their native form.
Reducing power
The reducing power of a molecule may serve as a significant indicator of its potential antioxidant activity. In reducing power assay, the presence of reductants in the tested samples results in reducing Fe3+/ferricyanide complex to the Fe2+ which subsequently changes the yellow color of test solution into blue colors depending on the reducing power of antioxidant samples (Zhao et al. 2006). The reducing power of native and partially hydrolyzed fucoidans was evaluated at concentrations ranging from 1 to 3 mg/mL. As it is shown in Fig. 4c, a dose-dependent increase in reducing power of fucoidan polymers was observed in all samples and there was an adverse relationship between the absorbance and sample M w. Furthermore, the fucoidan polymers having the M w of 104.1 × 103 and 63.9 × 103 g/mol showed a significantly enhanced reducing power (0.724 and 0.781 Abs, respectively) compared with native fucoidan (0.544 Abs) at the highest concentration. Therefore, the assay showed the higher reducing power of fucoidan from S. angustifolium at lower molecular weights.
Conclusion
The current study presents the relationship of molecular weight of fucoidan from S. angustifolium on its immunomodulatory and antioxidant properties. The results suggested that by acid hydrolysis using 0.01 N HCl in boiling water can produced hydrolyzed polymers with various molecular weight and conformation properties. Besides, the employment of mild acid hydrolysis did not significantly change the chemical composition of fucoidan especially sulfate content. RAW264.7 cells were incubated with fucoidans, lower molecular weight polymers induced more proliferation and NO release indicating the higher effectiveness of hydrolyzed fucoidans as immunomodulatory agents. The antioxidant properties of low molecular weight fucoidans in scavenging free radicals and reducing ferric ion was significantly improved compared to native form.
Acknowledgements
The authors would like to thank the Iran National Science Foundation (INSF) for their financial support of this research (Project No. 95818095).
References
- Ale MA, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. 2011;9:2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Chen BJ, Shi MJ, Cui S, Hao SX, Hider RC, Zhou T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from Sargassum fusiforme by degradation. Int J Biol Macromol. 2016;92:715–722. doi: 10.1016/j.ijbiomac.2016.07.082. [DOI] [PubMed] [Google Scholar]
- Cho ML, Lee DJ, Kim JK, You SG. Molecular characterization and immunomodulatory activity of sulfated fucans from Agarum cribrosum. Carbohydr Polymer. 2014;113:507–514. doi: 10.1016/j.carbpol.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Choi EM, Kim AJ, Kim YO, Hwang JK. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food. 2005;8:446–453. doi: 10.1089/jmf.2005.8.446. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Filisetti-Cozzi TMCC, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-Z. [DOI] [PubMed] [Google Scholar]
- Foti MC. Use and abuse of the DPPH· radical. J Agr Food Chem. 2015;63:8765–8776. doi: 10.1021/acs.jafc.5b03839. [DOI] [PubMed] [Google Scholar]
- Gamal-Eldeen AM, Amer H, Helmy WA, Talaat RM, Ragab H. Chemically-modified polysaccharide extract derived from Leucaena leucocephala alters Raw 264.7 murine macrophage functions. Int Immunopharmacol. 2007;7:871–878. doi: 10.1016/j.intimp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Gan D, Ma L, Jiang C, Xu R, Zeng X. Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis Bi. Carbohydr Polymer. 2011;84:997–1003. doi: 10.1016/j.carbpol.2010.12.058. [DOI] [Google Scholar]
- Jia Y, Sun Y, Weng L, Li Y, Zhang Q, Zhou H, Yang B. Low molecular weight fucoidan protects renal tubular cells from injury induced by albumin overload. Sci Report. 2016;6:1–10. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KT, Rioux LE, Turgeon SL. Molecular weight and sulfate content modulate the inhibition of a-amylase by fucoidan relevant for type 2 diabetes management. PharmaNutr. 2015;3:108–114. doi: 10.1016/j.phanu.2015.02.001. [DOI] [Google Scholar]
- Li C, Li X, You L, Fu X, Liu RH. Fractionation, preliminary structural characterization and bioactivities of polysaccharides from Sargassum pallidum. Carbohydr Polymer. 2017;155:261–270. doi: 10.1016/j.carbpol.2016.08.075. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Aida WMW, Maskat MY, Latip J, Badri KH, Hassan O, Yamin BM. Characterization of fucoidan extracted from Malaysian Sargassum binderi. Food Chem. 2016;209:267–273. doi: 10.1016/j.foodchem.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Luo D, Yuan X, Zeng Y, Nie K, Li Z, Wang Z. Structure elucidation of a major fucopyranose-rich heteropolysaccharide (STP-II) from Sargassum thunbergii. Carbohydr Polymer. 2016;143:1–8. doi: 10.1016/j.carbpol.2016.01.049. [DOI] [PubMed] [Google Scholar]
- Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–1362. doi: 10.1016/j.foodchem.2010.07.089. [DOI] [Google Scholar]
- Nishino T, Kiyohara H, Yamada H, Nagumo T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochem. 1991;30:535–539. doi: 10.1016/0031-9422(91)83722-W. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction–antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;6:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW, Kim ND, Nam TJ, Kwon TK, Choi YH. Anti-inflammatory effects of fucoidan through inhibition of NF-(B, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol. 2011;49:1745–1752. doi: 10.1016/j.fct.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rhein-Knudsen N, Ale MT, Meyer AS. Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar Drugs. 2015;13:3340–3359. doi: 10.3390/md13063340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Camargo ADP, Montero L, Stiger-Pouvreau V, Tanniou A, Cifuentes A, Herrero M, Ibáñez E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016;192:67–74. doi: 10.1016/j.foodchem.2015.06.098. [DOI] [PubMed] [Google Scholar]
- Sari-Chmayassem N, Taha S, Mawlawi H, Guegan JP, Jeftic J, Benvegnu T. Extracted and depolymerized alginates from brown algae Sargassum vulgare of Lebanese origin: chemical, rheological, and antioxidant properties. J App Phycol. 2016;28:1915–1929. doi: 10.1007/s10811-015-0676-4. [DOI] [Google Scholar]
- Skriptsova AV. Fucoidans of brown algae: biosynthesis, localization, and physiological role in thallus. Russ J Mar Biol. 2015;41:145–156. doi: 10.1134/S1063074015030098. [DOI] [Google Scholar]
- Tabarsa M, Park GM, Shin IS, Lee EJ, Kim JK, You SG. Structure-activity relationships of sulfated glycoproteins from Codium fragile on nitric oxide releasing capacity from RAW264.7 Cells. Marine Biotechnol. 2015;17:266–276. doi: 10.1007/s10126-015-9615-2. [DOI] [PubMed] [Google Scholar]
- Vanloot P, Dupuy N, Guiliano M, Artaud J. Characterization and authentication of A. Senegal and A. seyalexudates by infrared spectroscopy and chemometrics. Food Chem. 2012;135:2554–2560. doi: 10.1016/j.foodchem.2012.06.125. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Zhang Z, Song H, Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol. 2010;46:6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Wijesekara I, Pangestuti R, Kim SK. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polymer. 2011;84:14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- Yang C, Chung DH, You SG. Determination of physicochemical properties of sulphated fucans from sporophyll of Undaria pinnatifida using light scattering technique. Food Chem. 2008;111:503–507. doi: 10.1016/j.foodchem.2008.03.085. [DOI] [PubMed] [Google Scholar]
- You SG, Lim ST. Molecular characterization of corn starch using an aqueous HPSEC-MALLS-RI system under various dissolution and analytical conditions. Cereal Chem. 2000;77:303–308. doi: 10.1094/CCHEM.2000.77.3.303. [DOI] [Google Scholar]
- Zhao T, Zhang Q, Qi H, Zhang H, Niu X, Xu Z, Li Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int J Biol Macromol. 2006;38:45–50. doi: 10.1016/j.ijbiomac.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Zhao L, Dong Y, Chen G, Hu Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr Polymer. 2010;80:783–789. doi: 10.1016/j.carbpol.2009.12.029. [DOI] [Google Scholar]