Abstract
This study investigated the mechanism of wall rupture fermentation of the rape bee pollen by Ganoderma lucidum and Saccharomyces cerevisiae. The enzymatic activities and broken-wall ratios were determined, and the results suggested the involvement of cellulase, protease, and pectinase in the wall rupture fermentation. Among the five substrate inducers, CMCase, protease, and pectinase had the highest enzymatic activities at 23.13 ± 1.09, 63.44 ± 1.35, and 118.61 ± 2.07 U/mL, respectively. The broken-wall ratios of G. lucidum and S. cerevisiae were 85.08 ± 3.92 and 88.31 ± 2.51%, respectively. The wall rupture of rape pollen was closely related to the enzymes of the fermenting species. The wall rupture process was determined through enzyme assay, light microscopy, and scanning electron microscopy, and the broken-wall ratios provide further information on the said mechanism. The process follows three steps, namely, removal of coating on the pollen surface, continuous opening of three germinal apertures to allow enzymatic breakdown of the intine, and release of contents from the degradation of the exine.
Keywords: Rape pollen, Fermentation, Enzymes, Broken-wall ratio, Mechanism
Introduction
Bee pollen is the pollen ball of angiosperm stamens or gymnosperm pollen cells packed by worker honeybees. Previous studies reported a variety of bioactive substances, such as proteins, flavonoids, phenolics, vitamins, phospholipids, and trace elements present in bee pollen (Paola-naranjo et al. 2004; Ukiya et al. 2003; Nagata et al. 2005). In addition, bee pollen is a low-fat, high-protein food product, often referred to as a “micro-nutrient library” and a “concentrated nutrition library” (Heslop-Harrison 1975; Silva et al. 2006). Recently, bee pollen has gained increasing attention among scientists and has been widely common in healthcare products, food, cosmetics, and pharmaceuticals (Maruyama et al. 2010). In China, rape bee pollen accounts for the largest share of annual pollen production in the market, which is above 300 tons.
The structure of the pollen wall is extremely complicated and consists of the exine and the intine. The exine is made up of sporopollenin that exhibits chemical, heat, and force resistance, thereby ensuring protection of the pollen wall during pollen dispersal (Rowley et al. 1999; Brett and Waldron 1990). Intine, which is structurally similar to a plant cell wall, is composed of cellulose and pectins and has key functions in cell germination and extension in the pollen tube (Chichiriccò and Pacini 2008; Blackmore et al. 2007). The physicochemical property of mature pollen is superior, which confers protection from hostile environment and against bacteria growth (Osthoff and Wiermann 1987; Meuter-Gerhards et al. 1999). The pollen wall also has a special part called germinal aperture, where pollen tube growth occurs during germination. The germinal aperture is the hole on the pollen wall with no exine covering or has a thinner wall coverage area. Rape pollen has three germinal apertures. The pollen wall could not completely decompose in the human digestive system, and the release of its contents is only from the germinal aperture, which leads to a decrease in nutrient utilization. An unbroken pollen wall also renders the pollen useless in cosmetics, beverages, and in other fields. Thus, degradation of the pollen wall is necessary in maximizing the use of nutrients contained within the pollen itself. Studies have reported various chemical and physical methods that break the pollen wall (Chichiriccò and Pacini 2008; Duhoux 1982; Xu et al. 2009). Fermentation is an efficient method for pollen wall breakdown, but its mechanism remains unclear.
Ganoderma lucidum is rich in biologically active natural products (Liang et al. 2014) and is useful in the treatment of various diseases (Song et al. 2008). Saccharomyces cerevisiae is a unicellular organism and produces extracellular enzymes that directly and fully react with the pollen wall. In the present study, the fermentation system consists of rape bee pollen as the nitrogen source, rape honey as the carbon source, five different substrates as inducers, and G. lucidum and S. cerevisiae as fermentation species. The mechanism of wall rupture is discussed in this study, which is poorly understood previously. The findings can also provide useful insights into subsequent characterization of the rape bee pollen.
Materials and methods
Organism condition
G. lucidum was preserved in our library and stored in potato dextrose agar (PDA) at 4 °C with a preservative tube. G. lucidum was activated by reculturing thrice. The mycelium, together with the medium, was sectioned into 1.0 cm2 pieces, transferred into the culture dish containing PDA, and incubated at 28 °C for 7 days. S. cerevisiae was supplied by Angelyeast Company (Yichang, China). Approximately 0.1 g of S. cerevisiae was suspended in 100 mL of 2% sucrose solutions at 40 °C for 15 min, and incubated at 34 °C for 1 h.
Method of pretreatment of substrate inducers
Different substrate inducers were added in liquid media to improve the enzyme activity, and pretreatment is a key step in increasing substrate availability. (1) Pretreatment of bagasse. Bagasse is the main by-product of the sugar industry. The freshly dried bagasse from Yunnan Mosha sugar company (Yuxi, China) was pulverized and separated through a 60-mesh sieve. Acid or alkali treatment was chosen for bagasse pretreatment. Different concentrations of H2SO4 or NaOH were added to the bagasse in a tube, and the tube was immersed in boiling water for exactly 1 h. The treated bagasse was centrifuged, washed to obtain a neutral pH, and oven-dried at 60 °C for 2 h. Samples from the two pretreatment methods were tested by enzymatic hydrolysis. Dissolved 1 g of 600 FPU/g cellulase in 100 mL acetate buffer pH 4.8, then added in 1 g pretreated bagasse, the mixtures were incubated at 50 °C in a reciprocating shaker bath for 60 min. The amount of reducing sugar was estimated through the DNS (dinitrosalicylic acid) method. (2) Pretreatment of bean pulp. The bean pulp is the soybean by-product after soybean oil extraction. A bean pulp sample was pulverized and sifted through a 60-mesh sieve. A 1:3 (wt/wt) bean pulp-water was prepared by adding water, which was eventually defatted overnight by ether treatment. Finally, the ether was removed from the mixture, and the sample was dried for further use. (3) Pretreatment of CMC-Na (sodium carboxymethylcellulose). Approximately 0.1 g of CMC-Na was dissolved in distilled water, mixed thoroughly, and incubated at 60 °C until completely dissolved. (4) Pretreatment of pectin. Approximately 0.1 g of pectin was dissolved in distilled water, mixed thoroughly, and incubated at boiling water until completely dissolved. All of the above samples were analyzed in triplicate measurements.
Fermentation medium and fermentation conditions
Single factor experiment design was applied to optimize fermentation medium. The suitable formula of the medium, which consists of 0.3% w/v rape bee pollen, 5% w/v rape honey, 0.3% w/v KH2PO4, 0.15% w/v MgSO4∙7H2O, and 0.1% w/v pretreated substrate inducer, was autoclaved at 121 °C for 30 min.
An orthogonal experiment was used to optimize the fermentation conditions, and the optimal fermentation conditions for each system were as follows: (1) G. lucidum fermentation: initial pH 5.5; temperature, 30 °C; fermentation time, 8 days; medium capacity, 100 mL/250 mL; rotation speed, 160 rpm; and inoculation, six pieces of 1.0 cm2 mycelium together with PDA medium. (2) S. cerevisiae fermentation: initial pH 5.0; temperature, 36 °C; fermentation time, 8 days; medium capacity, 100 mL/250 mL; rotation speed, 140 rpm; and inoculation, 3 mL of activation liquor of S. cerevisiae.
Enzyme assay
Liquid cultures were harvested at specified time intervals (1 day), and the enzyme assay was performed on cultured supernatants. The fermentation liquor was further clarified by 3000 rpm centrifugation for 10 min at 4 °C. Enzyme assay was conducted in triplicates for all enzymes, and the data were subjected to statistical analysis.
The cellulase activity was determined based from the method of Ghose (1987). The reaction mixture for endoglucanase (CMCase, EC 3.2.1.4) consists of 0.5 mL of 5 mg/mL CMC-Na, 0.5 mL acetate buffer pH 5.0, and 0.25 mL of the enzyme source. The reaction mixture was incubated at 50 °C for 30 min, and the amount of reducing sugars released was quantified using Nelson–Somogyi method (Spiro 1966). Pectinase activity was measured according to the method of Sandhu and Kalra (1982). The amount of reducing sugar was evaluated using a DNS reagent. One unit of enzyme activity is equivalent to as 1 μmol of release glucose per min. The amount of protease was determined by the Folin phenol method described by Johnston et al. (1998). Laccase (EC 1.10.3.2) assay was conducted according to the method described by Ahlawat et al. (2010), and its activity was calculated in terms of the change in absorbance by 0.001 per min per mL of enzyme at 25 °C. Lipase (EC 3.1.1.3) was determined by a base titration method described by Brockman (1981).
Light microscopy (LM) and scanning electron microscopy (SEM)
LM images were acquired using a digital camera at 10× (eyepiece) and 40× (objective lens) magnifications. The fermentation liquor was clarified by centrifugation at 5000 rpm for 10 min, and the supernatant was removed by vacuum freeze-drying for 3 days. The powdered grains were subjected to SEM as described previously (Chichiriccò 2006).
Broken-wall ratio determination
Broken-wall ratio was determined under an improved Neubauer hemocytometer method. About 30 μL of the fermentation broth was placed in a hemocytomemter, which was subjected to LM. A total of 12 fields were selected to find the pollen grains. The broken-wall ratio was determined by converting into percent values based from the equation BR (%) = (1 – unbroken-wall pollen in fermentation liquid/unbroken-wall pollen in culture before fermentation) × 100% (Ma and Liu 2016).
Statistical analyses
The mean values of the triplicate readings were determined and subjected to analysis of variance. The means were separated using Duncan’s Multiple Range Test with the aid of SPSS version 17.0 (SPSS Inc., USA).
Results
Bagasse pretreated with different concentrations of H2SO4 or NaOH
The concentrations of H2SO4/NaOH used to produce the reducing sugar are shown in Fig. 1. The maximum reducing sugar level of 0.29 ± 0.03 mg/mL occurred in 3% H2SO4, which was the most suitable and optimal acid concentration. When different NaOH concentrations were used to treat the bagasse, the amount of reducing sugar slightly decreased before rising rapidly with increasing NaOH concentration. The reducing sugar level reached a peak value of 3.32 ± 0.09 mg/mL at 2% NaOH. This concentration was the optimal NaOH concentration. Treating the bagasse with NaOH can remove hemicellulose and lignin, therefore reducing the firmness and changing the bagasse composition and structure. Thus, the cellulase has a demonstrable effect on cellulose (Sun and Cheng 2002; Pan et al. 2005; Hendriks and Zeeman 2009). Therefore, pretreatment of bagasse with 2% NaOH was used for further experiments.
Fig. 1.
Influence of H2SO4/NaOH concentration on enzymatic hydrolysis. Values represent the mean ± SD of triplicate samples
Enzyme assay
Enzymatic activity curves of G. lucidum and S. cerevisiae without substrate induction in culture are presented in Fig. 2. The CMCase activity significantly changed during the fermentation of G. lucidum and S. cerevisiae, reaching a maximum at day 3 at 11.72 ± 1.19 and 8.07 ± 2.31 U/mL, respectively, and sharply declining thereafter (Fig. 2a). In addition, the CMCase activity of G. lucidum is significantly higher than S. cerevisiae at days 3–5 (P < 0.05). As shown in Fig. 2b, the protease activities increased during the earlier period of fermentation, peaking at day 5 for G. lucidum and at day 6 for S. cerevisiae with 57.19 ± 2.93 and 52.34 ± 1.23 U/mL, respectively, before decreasing markedly thereafter. G. lucidum pectinase had the highest activity of 83.99 ± 2.81 U/mL at day 4, and S. cerevisiae pectinase had the peak activity of 90.51 ± 5.53 U/mL at day 5 (Fig. 2c). Total laccase activity for G. lucidum reached the optimal value at day 3 with 546.30 ± 8.18 U/L (Fig. 2d), while the laccase activity of S. cerevisiae was undetectable. Lipase activities for both G. lucidum and S. cerevisiae were also undetectable.
Fig. 2.
Enzymatic activity curves of G. lucidum and S. cerevisiae substrate induction inducement in culture. a CMCase, b protease, c pectinase, d laccase. Values represent the mean ± SD of triplicate samples
The enzyme had varying activities in two species (G. lucidum or S. cerevisiae) and in five substrate induction tests (Table 1). Bagasse was the most suitable substrate for CMCase induction with an activity of 23.13 ± 1.09 U/mL or 97.35% improvement for G. lucidum and 12.34 ± 0.42 U/mL or 52.91% improvement for S. cerevisiae, followed by ether-treated bean pulp and CMC-Na. CMCase activity was significantly lower in the pectin culture than without substrate (control). In the ether-treated bean pulp culture, the highest protease activity was 61.87 ± 2.71 U/mL or 8.22% improvement for G. lucidum, and 63.44 ± 1.35 U/mL or 48.40% improvement for S. cerevisiae. The other substrates did not markedly influence enzyme efficiency. Under the presence of pectin, G. lucidum and S. cerevisiae displayed higher pectinase activities of 118.61 ± 2.07 U/mL or 41.22% improvement and 111.78 ± 1.01 U/mL or 23.50% improvement, respectively. The other substrates did not significantly affect the enzyme, especially the bean pulp. The highest laccase activity for G. lucidum was recorded in the bean pulp culture at 1129.28 ± 22.50 U/L, followed by 1047.11 ± 14.32 U/L in the ether-treated bean pulp culture. The activities for the other substrates were not significant.
Table 1.
Enzymatic activities of G. lucidum or S. cerevisiae in five substrate induction cultures
| Substrate inducement | Ganoderma lucidum | Saccharomyces cerevisiae | ||||||
|---|---|---|---|---|---|---|---|---|
| CMCase | Pectinase | Protease | Laccase | CMCase | Pectinase | Protease | Laccase | |
| Control | 11.72 ± 1.22b | 83.99 ± 0.08d | 57.19 ± 1.02c | 546.30 ± 5.45b | 8.07 ± 0.68b | 90.51 ± 1.36d | 42.75 ± 1.32a | ND |
| Bagasse | 23.13 ± 1.09e | 76.54 ± 1.10c | 49.54 ± 1.84b | 792.82 ± 9.64d | 12.34 ± 0.42d | 83.11 ± 1.61c | 54.34 ± 0.79c | ND |
| CMC-Na | 14.62 ± 0.31c | 70.48 ± 1.11b | 47.30 ± 0.87ab | 715.36 ± 6.83c | 10.12 ± 0.92c | 79.95 ± 1.04b | 49.34 ± 0.85b | ND |
| Pectin | 10.21 ± 0.04a | 118.61 ± 2.07e | 46.37 ± 0.54ab | 427.46 ± 5.62a | 6.44 ± 1.10a | 111.78 ± 1.01f | 50.40 ± 1.52b | ND |
| Ether-bean pulp | 17.47 ± 0.05d | 86.16 ± 2.94d | 61.87 ± 2.71d | 1047.11 ± 14.32e | 11.56 ± 0.74d | 101.07 ± 2.33e | 63.44 ± 1.35d | ND |
| Bean pulp | 13.40 ± 0.83c | 44.56 ± 0.61a | 44.82 ± 1.97a | 1129.28 ± 22.50f | 9.33 ± 0.50bc | 52.28 ± 2.16a | 47.34 ± 3.00b | ND |
Values are expressed as mean ± SD of triplicate measurements. Significant P values are presented (P < 0.05). Vertically, significantly different values are represented as letters
CMCase, pectinase, protease: (U/mL), laccase: (U/L), ND: not detected
Broken-wall ratio analysis
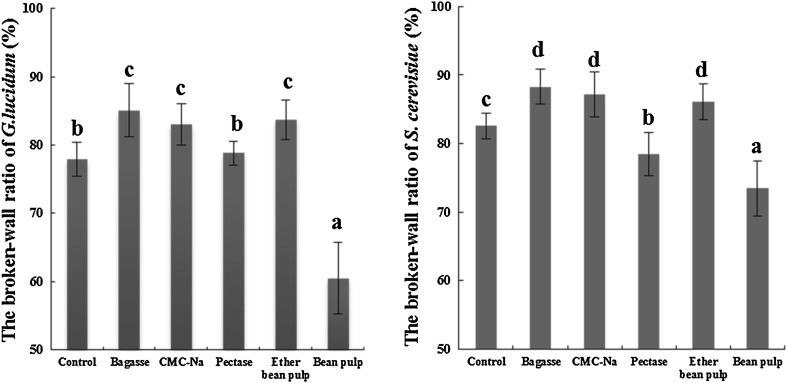
The broken-wall ratios of the two species (G. lucidum or S. cerevisiae) in different substrates inducement are shown in Fig. 3. In the bagasse culture, the broken-wall ratio (P < 0.05) shows a significant improvement at 85.08 ± 3.92 and 88.31 ± 2.51% for G. lucidum and S. cerevisiae, respectively. A significant downward trend occurs in the bean pulp culture in comparison with the control. The broken-wall ratios of G. lucidum and S. cerevisiae in the bean pulp culture were 60.49 ± 5.24 and 73.46 ± 4.01%, respectively.
Fig. 3.
Comparison of broken-wall ratios of G. lucidum and S. cerevisiae in different substrates. Significantly different values are represented as letters. Significant P values are presented (P < 0.05). Values represent the mean ± SD of triplicate measurements
Pollen wall rupturing process under LM and SEM
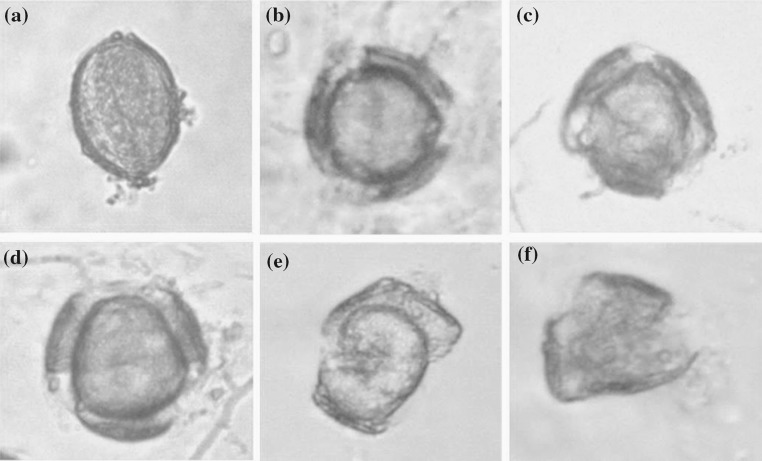
The wall rupturing process during fermentation by G. lucidum is shown in Fig. 4a–f. The complete rape pollen is oval-shaped, with the exine and intine bound to each other (Fig. 4a). Three germinal apertures began to open while the contents remained intact (Fig. 4b). These apertures opened continuously throughout fermentation time (Fig. 4c, d) before the contents exited through the hole of the pollen surface (Fig. 4e) and dispersed in the broth, as the pollen wall remained intact (Fig. 4f). At the end of the fermentation, no residue was observable in the fermentation broth (Personal observation, results not shown).
Fig. 4.
a–f Behaviors of rape pollen during fermentation by G. lucidum as observed via LM. a A pollen grain without additional processing. b Three germinal apertures on pollen surface began to open at days 1–2. c Three germinal apertures further opened up at days 3–4. d A crevasse was observable in the germinal apertures and led to the formation of three exine petals at day 5. e The content was released together with exine at days 6–7. f The entire contents were released, leaving an empty husk at day 8
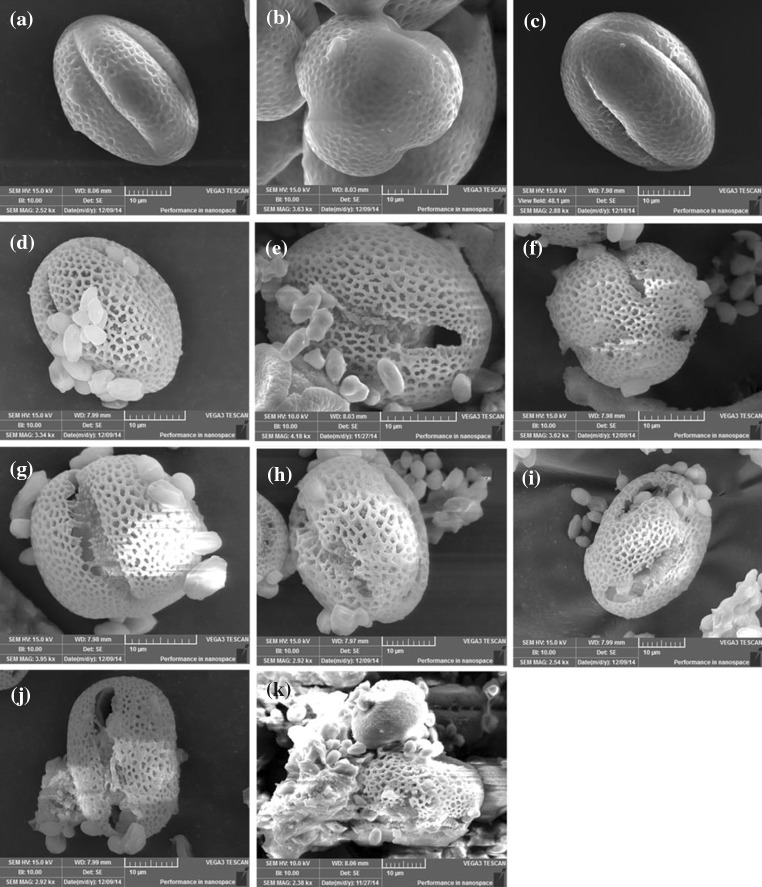
The process of wall rupture during fermentation by S. cerevisiae is shown Fig. 5d–k. The pollen grain, which is almost elliptically shaped, has a coating on the pollen surface (Fig. 5a–c). This coating is called the pollenkitt, which consists of proteins, lipids, and carotenoids (Knox et al. 2011). Three germinal apertures were observed on the rape pollen surface (Fig. 5a, b), and the absence of difference between Fig. 5a and c indicates the feasibility of vacuum-freeze drying of the pollen. The coating of the rape pollen disappeared and exposed the germinal apertures and porous exine at day 1 (Fig. 5d). The content was intact while the germinal apertures remained open at days 2–3 (Fig. 5e, f). Along with fermentation time, the germinal apertures continued to expand, and the bond structure was observable at the interior of the pollen at days 4–5 (Fig. 5g, h). As shown in Fig. 5i, the partial exuviae of exine was independently observed. The pollen grain content overflowed onto the exine hole at days 6–7 (Fig. 5j). The content was released while the structures of the wall were destroyed at day 8 (Fig. 5k). At the end of the fermentation, no residue was found in the fermentation broth (Personal observation, results not shown).
Fig. 5.
a–k Behaviors of rape pollen during fermentation by S. cerevisiae as observed via SEM. a, b A pollen grain without additional processing. a The parallel view and b is the vertical view of the rape pollen. c Vacuum-freeze dried pollen without fermentation. d–k Behaviors of rape pollen fermented by S. cerevisiae at days 1–8. d The coating on the pollen surface was removed, exposing the germinal apertures and porous exine. e The germinal aperture opened up while the content remained intact. f Similar to e, where three germinal apertures further opened up. g, h Some bond structures were observed in the interior of the pollen. i The exuviae separated itself from structure was destroyed
Hypothesis of rape pollen wall rupture
The flowcharts of the wall rupture and enzymatic regularities were similar for both G. lucidum and S. cerevisiae (Figs. 4, 5). This study proposes a hypothesis to explain the mechanism of the pollen wall rupture. (1) In fermented infancy (days 0–1), the fermentation species could secrete protease and other enzymes to remove the coating of pollen and to expose the germinal apertures and the porous exine; (2) In the earlier stage of fermentation (days 2–5), cellulase, pectinase, and other enzymes from the species could easily permeate through the inner pollen to destroy the intine. At this time, some bond structures form at the pollen interior, and these structures could completely immobilize the pollen content; (3) In the middle of the fermentation process (days 6–7), cellulase and pectinase could abscise the bonds, leading to the separation of the exuviae. The content then overflowed at the exine hole; (4) At the late stage of fermentation (day 8), all of the bonds were abscised, absolutely releasing the entire contents. The content and fragments of the pollen wall remained scattered in the fermentation broth.
Discussion
Influential factors on broken-wall ratio in fermentation process
Along with the fermentation of G. lucidum or S. cerevisiae and the improvement of enzymatic activity, the pollen broken-wall ratio increases gradually. After 8 days of fermentation, the enzymatic activities decreased, and the broken-wall ratios also had no significant increase in this study, indicating the close relation of the former with the latter. However, the relationship is not linear because of variances and morphological variants of species. The CMCase and protease produced by G. lucidum had higher activities than those produced by S. cerevisiae (Fig. 2a), and G. lucidum secreted laccase whereas S. cerevisiae did not secrete laccase during fermentation (Fig. 2). However, the culture of S. cerevisiae had a significantly higher broken-wall ratio than G. lucidum (Fig. 3) because the former is a single-celled organism, which secretes extracellular enzymes that directly and fully react with the pollen wall. In contrast, G. lucidum can grow a mycelium pellet that can cover the pollen to prevent the enzymatic action (Kelly et al. 2004). From another point of view, the broth of G. lucidum might contain more nutrients than S. cerevisiae.
Effect of substrate inducement on enzyme production and broken-wall ratio
Addition of the substrate can improve the efficiency of enzymatic activity (Guigón-López et al. 2014). In this study, five substrates were used to determine which one could improve the enzymatic activity and the broken-wall ratio. Several intriguing results were obtained from a detailed analysis of Table 1 and Fig. 3. The use of bagasse and CMC-Na substrates as the cellulose origin can induce CMCase excretion (Table 1) and improve the broken-wall ratio (Fig. 3). Bean pulp is a complex substrate that contains protein and a small amount dietary fiber. The protease activities of G. lucidum and S. cerevisiae improved in ether-treated bean pulp culture, and the CMCase activity also increased (Table 1). In ether-treated bean pulp culture, the broken-wall ratio improved remarkably (Fig. 3). In the bean pulp culture, the CMCase, protease, and pectinase activities of G. lucidum and S. cerevisiae decreased significantly (Table 1) probably because the bean pulp did not undergo degreasing with ether, which contained lipids (Onyeike et al. 1995). The thick broths were produced with low oxygen content, thereby resulting in a non-conducive environment for microbial growth and low enzymatic activities for G. lucidum and S. cerevisiae. Lipase was not detected during the fermentation; which probably indicated the absence of its expression in the fermentation broth. Thus, the broken-wall ratio was the lowest in the bean pulp culture (Fig. 3). In the bean pulp or ether-treated bean pulp culture, the laccase activity of G. lucidum increased significantly (Table 1), which might have come from the components in the bean pulp, such as phenols and their derivatives, aromatic amines and their derivatives, steroid hormones, biological pigments, and other non-phenolic substrates (Shuttleworth and Bollag 1986; Kersten et al. 1990; Sterjiades et al. 1993; Fahraeus and Ljunggren 1961). These substances are useful laccase substrates. However, the association between laccase and broken-wall ratio is non-existent.
Break wall of rape pollen by fermentation
The flowcharts of broken-wall for both G. lucidum and S. cerevisiae are shown in Figs. 4 and 5, respectively. The mycelium pellets of G. lucidum covered the pollen grains, which prevented separation before and after vacuum freeze-drying. Therefore, the behaviors of the pollen grains were only detectable in LM (Fig. 4).
As shown in Fig. 5, the white granules that adhered to the pollen surface were S. cerevisiae, which could produce enzymes that degrade the pollen wall. At the end of the fermentation process, the complete release of contents enhances the utilization of nutrition. Nutritious components (e.g. polysaccharide, ganoderic acid, and so on) may be present in the fermentation broth of G. lucidum, and the broth of S. cerevisiae had a distinct sweet smell of alcohol. Consequently, the fermentation method is suitable in breaking the pollen wall, which is critical in subsequent methods for pollens.
This study explored the mechanism of wall rupture in fermented rape pollens by G. lucidum and S. cerevisiae. We determined the enzymatic activities, enzyme variety laws, and the relationship of the enzyme with the broken-wall ratios. Notably, the broken-wall mechanism, which has been scarcely investigated until now, was discussed.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (Grant No. 31560576).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Zheng Zhang and Honggang Cao have contributed equally to this work.
References
- Ahlawat OP, Gupta P, Kamal S, Dhar BL. Variability in intra-specific and monosporous isolates of Volvariella volvacea based on enzyme activity, ITS and RAPD. Indian J Microbiol. 2010;50:192–198. doi: 10.1007/s12088-010-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore S, Wortley AH, Skvarla JJ, Rowley JR. Pollen wall development in flowering plants. New Phytol. 2007;174:483–498. doi: 10.1111/j.1469-8137.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- Brett CT, Waldron KW. Physiology and biochemistry of plant cell walls. Dordrecht: Springer; 1990. p. 98. [Google Scholar]
- Brockman HL. Triglyceride lipase from porcine pancreas. Methods Enzymol. 1981;71:619–627. doi: 10.1016/0076-6879(81)71074-7. [DOI] [PubMed] [Google Scholar]
- Chichiriccò G. Calcium in the micropylar secretion and receptivity of explanted Crocus ovules to intra- and interspecific pollen. Plant Syst Evol. 2006;262:89–96. doi: 10.1007/s00606-006-0450-9. [DOI] [Google Scholar]
- Chichiriccò G, Pacini E. Cupressus arizonica pollen wall zonation and in vitro hydration. Plant Syst Evol. 2008;270:231–242. doi: 10.1007/s00606-007-0610-6. [DOI] [Google Scholar]
- Duhoux E. Mechanism of exine rupture in hydrated taxoid type of pollen. Grana. 1982;21:1–7. doi: 10.1080/00173138209427673. [DOI] [Google Scholar]
- Fahraeus G, Ljunggren H. Substrate specificity of a purified fungal laccase. Biochem Biophys Acta. 1961;46:22–32. doi: 10.1016/0006-3002(61)90641-2. [DOI] [PubMed] [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. [Google Scholar]
- Guigón-López C, Guerrero-Prieto V, Lanzuise S, Lorito M. Enzyme activity of extracellular protein induced in Trichoderma asperellum and T. longibrachiatum by substrates based on Agaricus bisporus and Phymatotrichopsis omnivora. Fungal Biol. 2014;118:211–221. doi: 10.1016/j.funbio.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Biores Technol. 2009;100:10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. Incompatibility and the pollen-stigma interaction. Ann Rev Plant Physiol. 1975;26:403–425. doi: 10.1146/annurev.pp.26.060175.002155. [DOI] [Google Scholar]
- Johnston DB, Shoemaker SP, Smith GM, Whitaker JR. Kinetic measurements of cellulase activity on insoluble substrates using disodium 2,2′ bicinchoninate. J Food Biochem. 1998;22:301–319. doi: 10.1111/j.1745-4514.1998.tb00246.x. [DOI] [Google Scholar]
- Kelly S, Grimm LH, Hengstler J. Agitation effects on submerged growth and product formation of Aspergillus niger. Bioprocess Biosyst Eng. 2004;26:315–323. doi: 10.1007/s00449-004-0368-y. [DOI] [PubMed] [Google Scholar]
- Kersten PJ, Kalyanaraman B, Hanmel KE, Reinhammar B, Kirk TK. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990;268:475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RB, Williams EG, Dumas C. Pollen, pistil, and reproductive function in crop plants. Plant Breed. 2011;4:9–79. [Google Scholar]
- Liang Z, Yi Y, Guo Y, Wang R, Hu Q, Xiong X. Chemical characterization and antitumor activities of polysaccharide extracted from Ganoderma lucidum. Int J Mol Sci. 2014;15:9103–9116. doi: 10.3390/ijms15059103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Liu Y. Influence of combined wall-breaking method on wall-breaking rate and functional components of bee pollen. Food Ferment Ind. 2016;42:184–186. [Google Scholar]
- Maruyama H, Sakamoto T, Araki Y, Hara H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement Altern Med. 2010;10:30. doi: 10.1186/1472-6882-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter-Gerhards A, Riegert S, Wiermann R. Studies on sporopollenin biosynthesis in Cucurbita maxima (DUCH.)—II. The involvement of Aliphatic Metabolism. J Plant Physiol. 1999;154:431–436. doi: 10.1016/S0176-1617(99)80279-X. [DOI] [Google Scholar]
- Nagata H, Takei T, Kojima R, Kodera Y, Nishimura H, Inada Y, Matsushima A. Characteristics of an aminopeptidase from Japanese cedar (Cryptomeria japonica) pollen. J Agric Food Chem. 2005;53:5445–5448. doi: 10.1021/jf047794b. [DOI] [PubMed] [Google Scholar]
- Onyeike EN, Olungwe T, Uwakwe AA. Effect of heat-treatment and defatting on the proximate composition of some Nigerian local soup thickeners. Food Chem. 1995;53:173–175. doi: 10.1016/0308-8146(95)90784-5. [DOI] [Google Scholar]
- Osthoff KS, Wiermann R. Phenols as integrated compounds of sporopollenin from pinus pollen. J Plant Physiol. 1987;131:5–15. doi: 10.1016/S0176-1617(87)80262-6. [DOI] [Google Scholar]
- Pan X, Xie D, Gilkes N, Greqq DJ, Saddler JN. Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual lignin content. Appl Biochem Biotechnol. 2005;124:1069–1079. doi: 10.1385/ABAB:124:1-3:1069. [DOI] [PubMed] [Google Scholar]
- Paola-naranjo D, Sánchez-Sánchez J, González-Paramás AM, Rivas-Gonzalo JC. Liquid chromatographic-mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J Chromatogr A. 2004;1054:205–210. doi: 10.1016/j.chroma.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Rowley JR, Claugher D, Skvarla JJ. Structure of the exine in Artemisia vulgaris (Asteraceae): a review. Taiwania. 1999;44:1–21. [Google Scholar]
- Sandhu DK, Kalra MK. Production of cellulase, xylanase and pectinase by Trichoderma longibrachiatum on different substrates. Trans Br Mycol Soc. 1982;79:409–413. doi: 10.1016/S0007-1536(82)80034-X. [DOI] [Google Scholar]
- Shuttleworth KL, Bollag J. Soluble and immobilized laccase as catalysts for the transformation of substituted phenols. Enzyme Microb Technol. 1986;8:171–177. doi: 10.1016/0141-0229(86)90108-0. [DOI] [Google Scholar]
- Silva TMS, Camara CA, Lins ACS, Barbosa-Filho JM, Silva EMSD, Freitas BM, Santos FDAR. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J Food Compos Anal. 2006;19:507–511. doi: 10.1016/j.jfca.2005.12.011. [DOI] [Google Scholar]
- Song Y, Hui J, Kou W, Xin R, Jia F, Wang N, Hu F, Zhang H, Liu H. Identification of inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr Microbiol. 2008;57:454–462. doi: 10.1007/s00284-008-9233-6. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Analysis of sugars found in glycoproteins. Methods Enzymol. 1966;8:3–26. doi: 10.1016/0076-6879(66)08005-4. [DOI] [Google Scholar]
- Sterjiades R, Dean JFD, Gamble G, Himmelsbach DS, Eriksson KL. Extracellular laccases and peroxidases from sycamore maple (Acer pseudoplatanus) cell-suspension cultures. Planta. 1993;190:75–87. doi: 10.1007/BF00195678. [DOI] [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Ukiya M, Akihisa T, Tokuda H, Koike K, Takayase J, Okuda H, Kimura Y, Nikado T, Nishino H. Isolation, structural elucidation, and inhibitory effects of terpenoid and lipid constituents from sunflower pollen on Epstein-barr virus early antigen induced by tumor promoter, TPA. J Agric Food Chem. 2003;51:2949–2957. doi: 10.1021/jf0211231. [DOI] [PubMed] [Google Scholar]
- Xu X, Sun L, Dong J, Zhang H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov Food Sci Emerg. 2009;10:42–46. doi: 10.1016/j.ifset.2008.08.004. [DOI] [Google Scholar]