Abstract
Optimization of the extraction conditions to investigate the volatile composition of papaya fruit involving headspace solid phase micro-extraction was carried out using multivariate strategies such as factorial design and response surface methodology. The performance of different combinations of time for reaching the equilibrium in the headspace and time for maximum extraction of volatiles was evaluated by GC–olfactometry of the extract (intensity of papaya characteristic aroma), number of peaks and total area in the chromatogram. Thirty-two compounds were identified by GC–MS under the optimized extraction conditions, the majority of which were aldehydes, both in number of compounds and area. Major compounds were δ-octalactone, β-citral, benzaldehyde, heptanal, benzyl isothiocyanate, isoamyl acetate, γ-octalactone, (E)-linalool oxide and benzyl alcohol. Seven aldehydes and two other compounds are reported for the first time in papaya’s volatile profile.
Keywords: Carica papaya L., Solo group, Sunrise variety, Aroma, GC–MS
Introduction
The characteristic taste of food is extremely complex and involves the interaction among volatile and non-volatile compounds. Volatile organic compounds are responsible for the characteristic aroma and flavor, while non-volatile compounds are responsible for the perception of basic tastes and chemesthesis sensations (for example, the mouth burning by capsaicinoids in chilies) (Lawless and Heymann 2010; McDonald et al. 2016).
The study of the volatile fraction of a food comprises four fundamental steps: extraction of the volatile compounds, chromatographic separation, identification and sensory analysis of the analytes (Franco and Janzantti 2004). In general, the volatiles associated with aromas and flavors are thermolabile substances subject to hydrolysis, rearrangement, cyclization and oxidation, when susceptible to any increase in temperature (Bicas et al. 2010). Therefore, the extraction stage of the volatile compounds is considered critical and should be done with a great care, in order to eliminate interferences that may alter the original aroma of the product under study.
Different techniques for extracting volatile compounds are used to analyze food products, classified in two types: Total Analysis, comprising the study of all volatile compounds present in the food matrix, and Headspace Analysis, involving the analysis of only the gas phase in equilibrium with the food (Franco and Janzantti 2004). Among headspace analysis techniques, the headspace-solid phase micro-extraction (HS–SPME) is notable for being highly sensitive, simple, fast, solvent-free and requires only minimal amounts of analytes (Merkle et al. 2015; Kataoka et al. 2000). HS–SPME has been successfully used for analysis of volatile profile of fruits (Ferreira et al. 2016; Nobre et al. 2015; Todisco et al. 2014).
According to Valente and Augusto (2000), the development of a SPME method for extraction of volatile compounds involves the definition of several parameters: type of fiber, salt addition, stirring speed, temperature, time to form the equilibrium in the headspace, time for fiber exposure (adsorption of the volatile compounds by the fiber) and desorption time in the chromatograph injector.
In the case of papaya, there is not in the literature studies about the optimization of the volatile’s extraction conditions by the SPME, but only two studies comparing the fibers. Pereira et al. (2011) observed that the CW/DVB fiber provided the highest extraction efficiency in papaya pulp, both in number of volatile compounds and normalized area. Pino (2014) found that although PDMS (non-polar coating) was more sensitive to esters than the PDMS/DVB (polar coating), and PDMS/DVB was more sensitive to alcohols than the PDMS, the best overall extraction efficiency was obtained with the DVB/CAR/PDMS coating. Other authors who studied the volatile composition in the papaya fruits by the SPME technique used temperature and extraction time conditions ranging from 30 °C/30 min to 60 °C/50 min with equilibrium time ranging from 0 to 30 min (Fuggate et al. 2010; Lee et al. 2010a; Gomes et al. 2016).
The SPME optimization studies in general have used only the number of peaks and the total area of the chromatogram as criteria for the performance evaluation of the extraction system, without worrying if the extract represents the characteristic aroma of the food to be analyzed (Pereira et al. 2011; Bogusz Junior et al. 2011; Facundo et al. 2013). Flavor chemists know that some compounds in a food do not necessarily contribute to the formation of its characteristic aroma, presenting low aroma intensity or no aroma at all (Kamadia et al. 2006). Thus, major compounds in the chromatogram can have high threshold and present low odorant power. Conversely, compounds of a low concentration in the sample, can present a high odor intensity, due to its low threshold. Therefore, it becomes necessary to know if the extracted volatile compounds are those that really contribute to the characteristic aroma of the food under analysis.
Multivariate statistics are a very useful tool in studies in which several parameters influence the response of a said system. It allows the choice of a statistical planning for investigation in the experimental region of interest and the application of analysis of variance for the regression results to obtain the most appropriate model, evidencing the lack of adjustment, when it occurs. In addition, the correlation of data matrices can be established, indicating the best conditions for performing the analysis and giving the information if there is interaction among the parameters under study.
The objective of this study was to establish the best conditions for the extraction of volatile compounds from papaya fruits by solid phase microextraction of the headspace (HS–SPME), including the sensory analysis (GC–olfactometry) of the obtained aroma as one of the criteria for performance evaluation using a multivariate statistical method, the Central Composite Rotatable Design (CCRD).
Materials and methods
Samples
Fruits of the Solo group were harvested at Embrapa Mandioca e Fruticultura, in Cruz das Almas, State of Bahia, and transported to Embrapa Agroindústria Tropical, in Fortaleza, Ceará State, at maturity stage 2 (¼ maturity with surface of bark up to 25% yellow, surrounded by light green). The fruits were placed on shelves and kept at room temperature (~ 30 °C) for 2 days to continue the ripening process. At stage 5 (76–100% yellow) the fruits were stored in a cold room (9 °C at 12 °C) for a maximum of 5 days, depending on maturation, before sampling.
Experimental design
A multivariate experimental statistical design was applied in the development of a method for the extraction of papaya volatile compounds by the HS–SPME technique to evaluate the individual influence and the interrelationship between the variables. After setting the experimental design, the experiments were performed twice in random order. Initially, the chromatographic analyzes were performed and the whole design was repeated for the analysis of the aroma in the extracts (sniffing). The Central Composite Rotatable Design (CCRD) was applied and results submitted to analysis of the variance (ANOVA) to evaluate the significance of regression and lack of fit. Next, a response surface was generated to each dependent variable (Rodrigues and Iemma 2015). The selected HS–SPME conditions were later used to characterize the volatile profile in the Sunrise papaya variety. All the extractions were performed in triplicate.
Sample preparation and HS–SPME procedure
For the extraction of the volatiles, 5 g papaya pulp was placed in 20-mLvials and homogenized in deionized water (5 mL). Sodium chloride (30% w/w, preheated at 200 °C/2 h) was added to promote enzymatic inhibition and to increase vapor pressure, facilitating the removal of the compounds. The vial was sealed with a rubber septum and the sample subjected to constant moderate stirring at 30 °C for a period necessary to reach the equilibrium between the gas phase and the liquid phase, called equilibrium time (Teq). Then, the SPME fiber was manually introduced into the vial through the septum and exposed in the headspace for a period called exposure time (Texp). The fiber was DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethyl-siloxane) with 30 μm film thickness and 10 mm in length, from Supelco (Sigma-Aldrich, Bellefonte, PA, USA).
Optimization of the extraction conditions
The analytical conditions for the volatile compounds extraction were determined by Response Surface Methodology (MSR) (Khuri and Cornell 1996). The combined effect of only two independent variables, the equilibrium time (Teq) and the exposure time (Texp) were duly studied. The temperature of the system was set at 30 °C, the average ambient temperature of the city of Fortaleza, since the volatiles of the papaya are very sensitive to temperature elevation (Nongonierma et al. 2006). The dependent variables were:
number of chromatographic peaks;
total area of chromatogram;
intensity of papaya aroma.
A Central Composite Rotatable Design (CCRD) was performed for two independent variables (22), including four axial conditions and three repetitions at the central point, totaling 11 trials (Rodrigues and Iemma 2015) (Table 1).
Table 1.
The experimental planning and the CCRD responses used to optimize the extraction conditions of volatile papaya compounds by HS–SPME
| Experm | Coded | Uncoded | Response-variables | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | Teq (min) | Texp (min) | Peaks | Area (106) | Aroma | |
| 1 | 1 | − 1 | 26 | 26 | 46 | 8.53 | 6.8 |
| 2 | 1 | 1 | 26 | 54 | 61 | 11.13 | 7.4 |
| 3 | − 1 | − 1 | 4 | 26 | 39 | 7.26 | 3.1 |
| 4 | − 1 | 1 | 4 | 54 | 53 | 10.43 | 5.7 |
| 5 | 0 | 1.41 | 15 | 60 | 59 | 12.00 | 7.7 |
| 6 | 1.41 | 0 | 30 | 40 | 49 | 9.18 | 7.8 |
| 7 | 0 | − 1.41 | 15 | 20 | 34 | 6.22 | 5.2 |
| 8 | − 1.41 | 0 | 0 | 40 | 52 | 8.76 | 1.5 |
| 9 | 0 | 0 | 15 | 40 | 50 | 9.90 | 4.8 |
| 10 | 0 | 0 | 15 | 40 | 50 | 10.46 | 5.8 |
| 11 | 0 | 0 | 15 | 40 | 52 | 10.77 | 6.3 |
The experiments 9, 10 and 11 are repetitions (center point) for calculation of pure error. Teq: equilibrium time (min); Texp: fiber exposure time (min). Aroma intensity in scale of 9 cm
CCRD, Central Composite Rotatable Design
Chromatographic conditions
The first step of this work was performed using a Varian CP-3380 gas chromatograph, equipped with a flame ionization detector (GC–FID; Varian Inc., Palo Alto, CA). The analytes were thermally desorbed from the SPME fiber at 250 °C into the injector port and the fiber was maintained exposed for 10 min to eliminate carry-over effects. The injector was operated in the splitless mode with the split valve kept closed for 2 min. The separation of compounds was carried out in a fused silica capillary column DB-5 (5% phenylmethylpolysiloxane) (30 m × 0.25 mm × 0.25 μm) da Supelco (Bellefonte, PA, USA). The oven temperature program started at 30 °C for 10 min, increased to 70 °C at 20 °C/min, to 110 °C at 2 °C/min and thereafter to 250 °C at 15 °C/min. The carrier gas was hydrogen at a constant flow of 1.5 mL/min. The detector was kept at 250 °C.
GC–olfactometry
In a second series of experiments, the aroma intensity of the chromatographic effluent was analyzed by previously selected and trained judges (n = 5). As the transfer of compounds from the fiber to the judge’s nose needed to be instantaneous to avoid the mixture fractionation, an inactivated 1.0-m long capillary column was installed connecting the injector to the olfactory detector. The GC effluent was carried out by nitrogen (4 L/min) to the judge, using a Gerstel olfactometer, model ODP 3 (Gerstel, Mühlheim van der Ruhr, Germany). The judges responded to the intensity of the sensory stimuli using a time-intensity device with a 9-cm non-structured scale, available by SCDTI software (Time-Intensity Data Collection System, Campinas, Brazil).
Multivariate statistical analysis
The model used was an equation of second order (Eq. 1), where y represents the responses (peaks, area and aroma), with the terms β i representing the linear effects; β ii the quadratic effects and β ij the effects of the interactions between the levels of the regression variables x1, x2, …, xk.
| 1 |
The quality of the polynomial models fit was evaluated by the coefficient of determination (R2) and by the test for the lack of fit. The statistical significance (p < 0.05) was determined by the F test and the response surface was constructed from the regression equation on the response surface. The Statistica software, version 10.0 (Statsoft Inc., Tulsa, OK, USA) was used for the statistical analysis.
Volatile profile of papaya fruit
The best volatile extraction conditions previously determined were used to characterize the volatile profile of Solo papaya, variety Sunrise. The volatile compounds of the headspace of papaya fruits were identified using GC–MS (Varian, Agilent Technologies Inc., model CG-450/EM-240) using a Combipalm AC 5000 automatic injector along a stirring speed of 500 rpm. Chromatographic conditions were the same as before, however the helium was the carrier gas at 1.0 mL min−1 (8.7 psi) and 36.7 cm s−1 in constant linear velocity. Detector (Ion Trap) and transfer line temperatures (MS) were maintained at 170 °C. The electron ionization mode (+70 eV) was used with scanning from 35 to 350 m/z. The mass spectra obtained were compared to those provided by the library of the National Institute of Standards and Technology (NIST 02 MS Library, Gaithersburg, MD). Additionally, the experimental retention index (RI) was determined with a homologue mixture of n-alkanes (C8–C22) (Kovats 1965) and compared with the ones reported in the literature (Adams 2007; Nist 2015).
Results and discussion
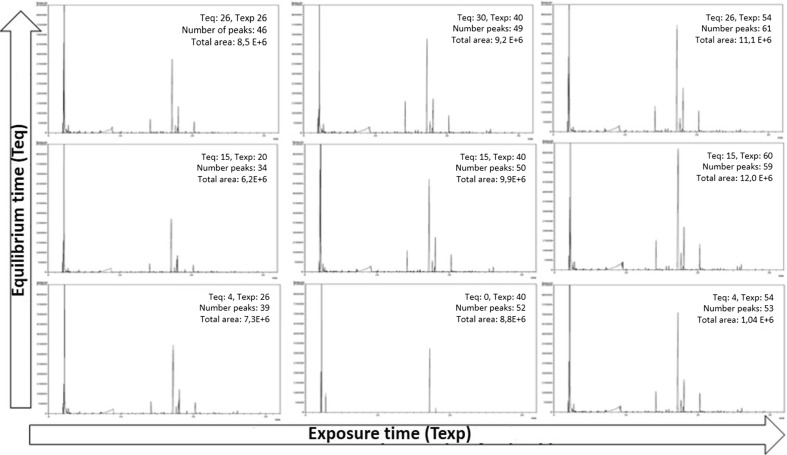
Table 1 shows the results of the eleven experiments performed by the CCRD for the response variables number of chromatographic peaks, total area in the chromatogram and intensity of papaya aroma, while Fig. 1 shows the chromatographic profiles obtained at the experimental levels of the tested factors in the CCRD. The distribution of the chromatograms followed the experimental design, with the minimum and the maximum points of each parameter at the extremities and the axial points between them. The chromatogram in the middle refers to one of the three replicates at the central point. In general, there is an increase in the number of peaks and in the total area with the increase of both, the equilibrium time and the exposure time.
Fig. 1.
Chromatograms of volatile compounds from papaya headspace obtained by HS–SPME using experimental design: the arrows indicate the direction of the increase in the levels of equilibrium time (Teq) and the exposure time (Texp) factors
In analyzing the estimated effects (Table 2), we see that the linear factor of equilibrium time (Teq L) did not have a significant effect (p > 0.05) for number of peaks and area, indicating that within the studied conditions, the increase in the time for the headspace formation did not result in an expressive enrichment of the chromatogram. However, the Teq L factor was highly significant for the aroma intensity (p < 0.01), indicating that volatiles that are important to the papaya aroma do need time to detach from the matrix. It is also clear that the increase in the equilibrium time, despite not promoting a significant increase in the quantitative parameters of the chromatogram, allowed extracting the compounds of the high odoriferous power, which may be minor compounds, with low area under the chromatographic peak.
Table 2.
Estimated effects for the variables number of peaks, total area of the chromatogram and intensity of papaya aroma
| Factor | Number of peaks | Total area | Papaya aroma | |||
|---|---|---|---|---|---|---|
| Effect | p | Effect | p | Effect | p | |
| (1) Teq L(min) | 2.8266 | 0.0767 | 0.6565 | 0.1576 | 36.2505 | 0.0014 |
| Teq Q (min) | 0.5264 | 0.6591 | − 1.3181 | 0.0427 | − 9.1510 | 0.2460 |
| (2) Texp L (min) | 16.0152 | 0.0026 | 3.4689 | 0.0003 | 16.7576 | 0.0288 |
| Texp Q (min) | − 3.4956 | 0.0668 | − 1.0804 | 0.0626 | 9.1905 | 0.2142 |
| 1L by 2L | 0.5000 | 0.7072 | − 0.2833 | 0.6279 | − 10.0000 | 0.2580 |
Values in bold are significant (p < 0.05)
Teq L, linear factor of equilibrium time; Teq Q, quadratic factor of equilibrium time; Texp L, linear factor of exposure time; Texp Q, quadratic factor of exposure time
The only factor that exerted significant positive effect (p < 0.05) on all dependent variables, including the aroma of the extract, was the linear factor of exposure time (Texp L). The quadratic Texp and Teq × Texp interaction factors did not show a significant effect (p > 0.05), but they influenced the adjusted equations, i.e., when these terms were removed from the equations, there were losses in the R2.
Number of peaks
The mathematical adjusted model for number of peaks was significant (p ≤ 0.05) and expressed by Eq. 2.
| 2 |
Lack of fit of this model was not significant (Table 3) and confirms the possibility of adjustment and optimization by a predictive model. The coefficient of determination (R2) in the complete model explains up to 90.9% of the variability in the responses, while the R2 of the adjusted model (R2adj) may explain up to 81.8%.
Table 3.
Analysis of variance of the regression model for the variables number of peaks, total area and intensity of papaya aroma from the optimization of extraction conditions of volatile compounds in papaya fruit by HS–SPME
| Source | DF | SS | MS | Fcal | Ftab |
|---|---|---|---|---|---|
| Number of peaks | |||||
| Regression | 5 | 552.20 | 110.44 | 9.97 | 5.05* |
| Error | 5 | 55.41 | 11.08 | ||
| Lack of fit | 3 | 52.74 | 17.58 | 13.17 | 19.16 (ns) |
| Pure error | 2 | 2.67 | 1.34 | ||
| Total | 10 | 604.54 | |||
| Total area | |||||
| Regression | 5 | 29.14 | 5.83 | 19.43 | 5.05* |
| Error | 5 | 1.5 | 0.30 | ||
| Lack of fit | 3 | 1.11 | 0.37 | 1.90 | 19.16 (ns) |
| Pure error | 2 | 0.39 | 0.20 | ||
| Total | 10 | 30.64 | |||
| Papaya aroma | |||||
| Regression | 5 | 3433.49 | 686.70 | 11.18 | 5.05* |
| Error | 5 | 307.10 | 61.42 | ||
| Lack of fit | 3 | 190.43 | 63.48 | 1.09 | 19.16 (ns) |
| Pure error | 2 | 116.67 | 58.33 | ||
| Total | 10 | 3740.59 | |||
DF, degrees of freedom; SS, sum of square; MS, mean square; Fcal, calculated F; Ftab, tabled F
*Significant (p < 0.05); ns: non significant
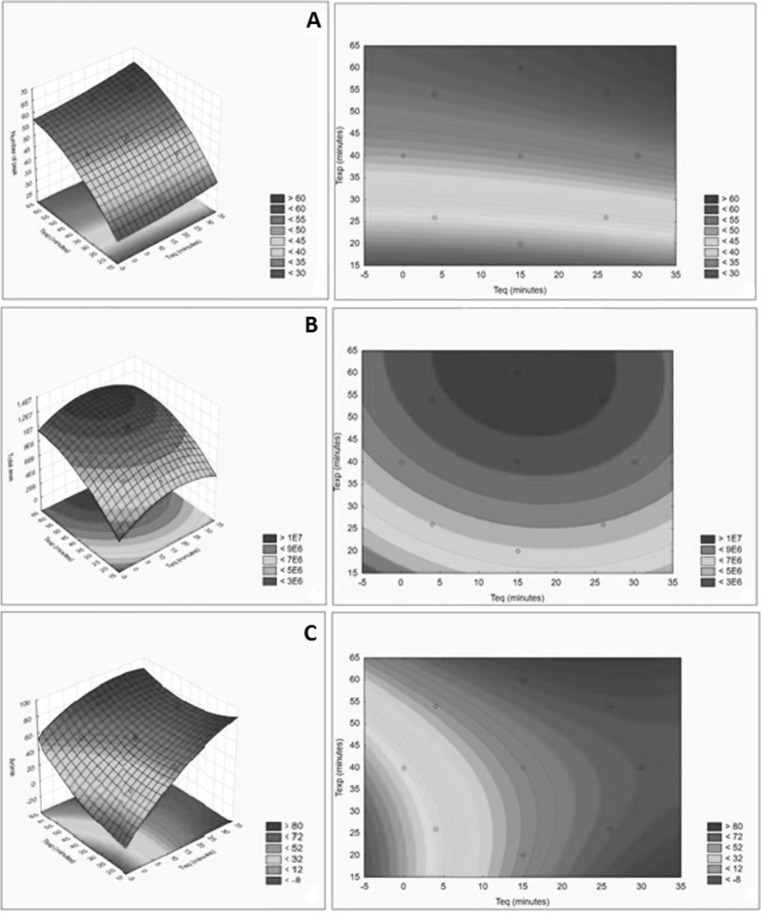
The response surface of the adjusted model for the dependent variable number of peaks is visualized in Fig. 2a. It was confirmed that the exposure time had a great influence on the number of peaks of the chromatograms of the papaya volatile compounds, as the highest response values were found with an exposure time in the order of 60 min. The equilibrium time had little influence on the number of peaks. However, the highest responses were obtained when 15-min equilibrium were applied with 60-min exposure or 26-min equilibrium with 54-min exposure.
Fig. 2.
Response surfaces and the contour curves for the number of chromatographic peaks (a), total area of chromatogram (b) and intensity of papaya aroma (c) obtained by HS–SPME extraction of volatile compounds in papaya fruit as a function of the equilibrium time and exposure time
Total area
The analysis of the variance (Table 3) showed that the adjusted model for total area (Eq. 3) was significant at (p < 0.05) and did not present lack of fit.
| 3 |
The coefficient of determination (R2) indicated that the complete model can explain up to 94.9% of this variability, as the linear factor of equilibrium time had no significant effect (p > 0.05). A new statistical model was constructed using the adjusted regression coefficients. However, there were losses in the adjusted coefficient of determination that explained only 88.4% of the variability in this particular response, suggesting that we cannot exclude the terms from the model.
In the response surface for total area (Fig. 2b), a similar behavior to that observed for number of peaks was found, evidencing that with an increased exposure time, there is also a considerable increase in the total area of the chromatogram. With reference to this variable, the region of maximum values was reached along the inflection point in the 15-min equilibrium condition with the 60-min exposure.
Intensity of papaya aroma
The mathematical model for the response variable intensity of papaya aroma (Eq. 4), presented statistical significance (p ≤ 0.05) without presenting lack of fit (Table 3).
| 4 |
The coefficient of determination (R2) indicates that this model can explain up to 91.9% of the variability of the aroma intensity response and the adjusted model can explain up to 89.8%. The response surface in Fig. 2c shows the behavior of interaction of different equilibrium times versus different exposure times and their influence on the intensity of the papaya aroma. It is observed that the conditions with the greatest response were those with higher equilibrium times, with the experiments 2 (Teq 26 min, Texp 54 min), 5 (Teq 15 min, Texp 60 min) and 6 (Teq 30 min, Texp 40 min) remaining in the same region of the intensity, between 7.2 and 8.0. As all of these extraction conditions resulted in very close analysis times (70–80 min), anyone could be used for the extraction of papaya volatiles. However, the conditions of the experiment 5 showed to be more efficient allowing obtaining a richer chromatogram in terms of area. The optimal conditions selected for the extraction of volatile compounds from the papaya by the HS–SPME technique with DVB/CAR/PDMS fiber were 15 min of equilibrium time and 60 min of fiber exposure at 30 °C.
Volatile composition of papaya, var. Sunrise using the optimized conditions
Thirty-two compounds were identified in the headspace of papaya var. Sunrise (Solo group) using the optimal extraction conditions (Table 4). The same number of compounds were detected in papaya juice prepared from Sekaky cultivar (Malaysia) by Lee et al. (2010a) who used CAR/PDMS fiber, no equilibrium time, 50 min of extraction and heating at 60 °C. Although we cannot directly compare the results of different fibers and different papaya varieties, we can see that the use of a drastic temperature condition was not able to extract a high number of volatile compounds. Pereira et al. (2011), using the same fiber (CAR/PDMS) but much milder extraction conditions (30 min at 30 °C, considered no heating), detected only 5 volatile compounds in papaya pulp var. Golden, indicating that maybe heating the system could favor the extraction. However, the authors were able to extract 22 compounds in the same sample, at the same parameters of time and temperature, with a different fiber (CW/DVB). Gomes et al. (2016) studied the cold storage effect on the volatile profile of this same Golden papaya variety using the fiber indicated by Pino (2014), a long equilibration period (30 min) and 30 min of fiber exposure at a relatively high temperature (50 °C), but could not detected more than 19 compounds.
Table 4.
Volatile compounds identified in Carica papaya L. var. Sunrise (group Solo), extracted by HS–SPME
| Peak | RIa | Compoundsb | Area % | Compound reported in literature |
|---|---|---|---|---|
| 1 | <800 | 2-methyl-3-buten-2-ol | 0.77 | |
| 2 | <800 | isobutanol | 0.08 | 3, 12, 14, 15 |
| 3 | <800 | 1-penten-3-ol | 0.20 | 2, 3, 7, 14 |
| 4 | <800 | 2-pentanone | 0.40 | 3, 10 |
| 5 | <800 | pentanal | 3.13 | |
| 6 | <800 | 2-pentanol | 0.28 | 3, 10, 12, 14 |
| 7 | <800 | methyl butanoate | 0.43 | 2, 3, 8, 9, 10, 10, 14 |
| 8 | <800 | 2-methyl-3-pentanone | 0.15 | |
| 9 | <800 | (E)-2-pentenal | 0.03 | |
| 10 | <800 | 1-pentanol | 0.85 | 12, 14 |
| 11 | <800 | (Z)-2-penten-1-ol | 0.33 | 3, 6 |
| 12 | <800 | isobutyl acetate | 1.43 | 14 |
| 13 | <800 | 3-methyl-2-butenal | 0.44 | |
| 14 | <800 | ethyl butanoate | 0.16 | 3, 10, 14, 15 |
| 15 | 833 | propyl butanoate | 0.31 | 8, 14 |
| 16 | 856 | ρ-xylene | 0.40 | 10 |
| 17 | 870 | isoamyl acetate | 6.54 | 14, 15 |
| 18 | 887 | heptanal | 7.52 | 11 |
| 19 | 917 | (E,E)-2.4-hexadienal | 0.42 | |
| 20 | 950 | (Z)-2-heptenal | 1.83 | |
| 21 | 956 | benzaldehyde | 8.72 | 3, 4, 6, 7, 10, 11, 12, 13, 14, 15 |
| 22 | 997 | octanal | 3.90 | 11 |
| 23 | 1001 | (E,E)-2.4-heptadienal | 4.95 | |
| 24 | 1040 | benzyl alcohol | 5.10 | 1, 2, 3, 4, 6, 7, 8, 9, 10, 12, 13, 14 |
| 25 | 1045 | (Z)-β-ocimene | 1.85 | 11 |
| 26 | 1125 | benzyl isothiocyanate | 6.66 | 1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 14, 15 |
| 27 | 1162 | (E)-2-nonenal | 3.48 | |
| 28 | 1169 | (E)-linalool oxide (piranoid) | 5.50 | 1, 3, 6, 9 |
| 29 | 1246 | γ-octalactone | 5.91 | 1, 2, 3, 6, 10, 11 |
| 30 | 1269 | β-citral (geranial) | 13.31 | 4 |
| 31 | 1287 | δ-octalactone | 13.33 | 1, 6, 13 |
| 32 | 1497 | (E)-β-ionone | 1.60 | 3, 10 |
a Retention index in DB-5 column
b Compounds identified by MS and RI
1: Gomes et al. (2016); 2: Kelebek et al. (2015); 3: Pino (2014); 4: Pereira et al. (2011); 5: Fuggate et al. (2010); 6: Ulrich and Wijaya (2010); 7: Lee et al. (2010a); 8: Voon et al. (2007); 9: Almora et al. (2004); 10: Pino et al. (2003); 11: Flath et al. (1990); 12: Schwab et al. (1989); 13: MacLeod and Pieris (1983); 14: Flath and Forrey (1977); Lee et al. (2010b)
Pino (2014) compared four fiber coatings for the extraction of papaya cv. Red Maradol (Cuba). Authors concluded that DVB/CAR/PDMS coating (the same used in the present work) allowed the best extraction efficiency, also generating the most representative odor. Using 10 min equilibrium time, 30 min of extraction and heating at 40 °C they detected 93 peaks, but when classical GC–O was applied (AEDA), only 21 compounds were reported as contributing to the overall papaya aroma. Our findings suggest that important odor-contributing compounds of papaya var. Sunrise were also adsorbed by this coating, but using lower temperature and longer time for volatile’s extraction.
The volatile profile of Solo papaya var. Sunrise comprised compounds belonging to the following chemical classes: aldehydes (10), alcohols (7), esters (6), terpenes (5), lactones (2) and ketones (2). The most abundant classes were aldehydes (34.4% of total area) and terpenes (22.7%), followed by lactones (19.2%) and esters (15.5%). Major compounds were δ-octalactone, β-citral, benzaldehyde, heptanal, benzyl isothiocyanate, isoamyl acetate, γ-octalactone, (E)-linalool oxide and benzyl alcohol. Most of volatile compounds identified in Sunrise variety are also reported in other literature (Table 1), while several aldehydes found in the present study, namely pentanal, 3-methyl-2-butenal, (E)-2-pentenal, (Z)-2-heptenal, (E)-2-nonenal, (E,E)-2,4-hexadienal and (E,E)-2,4-heptadienal have not been reported elsewhere for papaya fruits. The minor compounds 2-methyl-3-buten-2-ol and 2-methyl-3-pentanone are also reported for the first time among papaya’s volatile constituents. The differences in the volatile profiles among the cited literature might be due mainly to the different studied cultivars and techniques.
Gomes et al. (2016) studied the volatile profile of papaya fruits from a different variety of the Solo group (Carica papaya cv. Golden) by SPME. Linalool constituted 61.6% of the volatile compounds, followed by linalool oxide derivatives. Other studies about the volatile profile of different varieties of papayas revealed linalool as one of the most aroma-active compounds in papaya fruit by using olfactometry (GC–O) (Pino 2014; Jirovetz et al. 2003). In the present work, we did not detect linalool, but only (E)-linalool oxide. However, several other detected compounds was considered as odoriferous by Pino (2014), contributing to the typical aroma of papaya cv. Red Maradol, like δ-octalactone (sweet, herbal), benzyl isothiocyanate (papaya), methyl butanoate (fruity) and ethyl butanoate (fruity). According to author, linalool was not the major compound even in the ripe fruit, and esters were the most powerful odorants.
Kelebek et al. (2015), determining the volatile constituents of two papaya varieties (Sel-42 and Tainung) grown in Turkey, observed that of all aroma compounds detected, acids were present in the highest levels, followed by esters. In Madeira island papaya fruits, Pereira et al. (2011) observed that the volatile composition was dominated by two esters, benzyl isothiocyanate (32.1%) and methyl pyruvate (12.1%).
Conclusion
The solid-phase microextraction methodology is efficient for extracting important volatiles from papaya’s headspace. The multivariate optimization of the extraction conditions assisted by olfactometry is powerful in setting the equilibrium time and extraction time. These optimized conditions allowed the extraction of already known potent odorants of papaya’s aroma, together with nine new volatiles.
Acknowledgements
We thanks Empresa Brasileira de Pesquisa Agropecuária (Embrapa), by financial support for the research project. We are also grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), by financial support for the master’s degree of first author.
Contributor Information
Renier Felinto Julião da Rocha, Email: hfelinto@gmail.com.
Ídila Maria da Silva Araújo, Email: idila.araujo@embrapa.br.
Sílvia Maria de Freitas, Email: silvia@dema.ufc.br.
Deborah dos Santos Garruti, Email: deborah.garruti@embrapa.br.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Illinois: Allured books; 2007. [Google Scholar]
- Almora K, Pino JA, Hernández M, Duarte C, González J, Roncal E. Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja) Food Chem. 2004;86:127–130. doi: 10.1016/j.foodchem.2003.09.039. [DOI] [Google Scholar]
- Bicas JL, Silva JC, Dionísio AP, Pastore GM. Biotechnological production of bioflavors and functional sugars. Ciência e Tecnol Aliment. 2010;30:7–18. doi: 10.1590/S0101-20612010000100002. [DOI] [Google Scholar]
- Bogusz Junior S, Melo AMT, Zini CA, Godoy HT. Optimization of the extraction conditions of the volatile compounds from chilI peppers by solid phase micro-extraction. J Chromatogr A. 2011;1218:3345–3350. doi: 10.1016/j.chroma.2010.12.060. [DOI] [PubMed] [Google Scholar]
- Facundo HVV, Garruti DS, Cordenunsi BR, Lajolo FM (2013) Isolation of volatiles compounds in banana by HS–SPME: optimization for the whole fruit and pulp. Intern. J Biosci Biochem Bioinform 3:110–115. http://www.ijbbb.org/papers/176-F10009.pdf
- Ferreira DDF, Garruti DS, Barin JS, Cichoski AJ, Wagner R. Characterization of odor-active compounds in gabiroba fruits (Campomanesia xanthocarpa O. Berg) J Food Qual. 2016;39:90–97. doi: 10.1111/jfq.12177. [DOI] [Google Scholar]
- Flath RA, Forrey RR. Volatile components of papaya (Carica papaya L., Solo Variety) J Agric Food Chem. 1977;25:103–109. doi: 10.1021/jf60209a051. [DOI] [Google Scholar]
- Flath RA, Light DM, Jang EB, Mon TR, John JO. Headspace examination of volatile emissions from ripening papaya (Carica papaya L., Solo variety) J Agric Food Chem. 1990;38:1060–1063. doi: 10.1021/jf00094a032. [DOI] [Google Scholar]
- Franco MRB, Janzantti NS. Avanços na metodologia instrumental da pesquisa do sabor. In: Franco MRB, editor. Aroma e sabor de alimentos: temas atuais. 1. São Paulo: Varela; 2004. pp. 1–32. [Google Scholar]
- Fuggate P, Wongs-Areea C, Noichindac S, Kanlayanarata S. Quality and volatile attributes of attached and detached ‘Pluk Mai Lie’ papaya during fruit ripening. Sci Hortic. 2010;126:120–129. doi: 10.1016/j.scienta.2010.06.019. [DOI] [Google Scholar]
- Gomes BL, Fabi JP, Purgatto E. Cold storage affects the volatile profile and expression of a putative linalool synthase of papaya fruit. Food Res Int. 2016;89:654–660. doi: 10.1016/j.foodres.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbaue G, Shahabi M (2003) Aroma compounds of mango and papaya from Cameroon. Perfum Flavor 28:40–52. http://www.perfumerflavorist.com/fragrance/rawmaterials/natural/2759006.html
- Kamadia VV, Yoon Y, Schilling MW, Marshall DL. Relationship between odorant concentration and aroma intensity. J Food Sci. 2006;71:193–197. doi: 10.1111/j.1365-2621.2006.tb15640.x. [DOI] [Google Scholar]
- Kataoka H, Lord HL, Pawliszyn J (2000). Applications of solid-phase microextraction in food analysis. J Chromatogr A 880:35–62. http://www.sciencedirect.com/science/article/pii/S0021967300003095 [DOI] [PubMed]
- Kelebek H, Selli S, Gubbuk H, Gunes E. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem. 2015;173:912–919. doi: 10.1016/j.foodchem.2014.10.116. [DOI] [PubMed] [Google Scholar]
- Khuri A, Cornell J. Response surface: designs and analyses. 2. New York: Marcel Dekker; 1996. [Google Scholar]
- Kovats E. Advances in chromatography. In: Giddings JC, Keler RA, editors. Retetion index system. New York: Marcel Dekker; 1965. pp. 229–247. [Google Scholar]
- Lawless HT, Heymann H. Sensory evaluation of food. New York: Springer; 2010. [Google Scholar]
- Lee PR, Yu B, Curran P. Kinetics of volatile organic compounds during papaya juice fermentation by three commercial wine yeasts. Nutr Food Sci. 2010;40:566–580. doi: 10.1108/00346651011090374. [DOI] [Google Scholar]
- Lee PR, Ong YL, Yu B, Curran P, Liu SQ (2010b) Profile of volatile compounds during papaya juice fermentation by a mixed culture of Saccharomyces cereviseae and Williopsis saturnus. Food Microb 27:853–861. https://www.ncbi.nlm.nih.gov/pubmed/20688226 [DOI] [PubMed]
- MacLeod AJ, Pieris NM. Volatile components of papaya (Carica papaya L.) with particular reference to glucosinolate products. J Agric Food Chem. 1983;31:1005–1008. doi: 10.1021/jf00119a021. [DOI] [Google Scholar]
- McDonald ST, Bolliet DA, Hayes JE. Chemesthesis: chemical touch in food and eating. West Sussex: Wiley; 2016. [Google Scholar]
- Merkle S, Kleeberg K, Fritsche J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—a review. Chromatography. 2015;2:293–381. doi: 10.3390/chromatography2030293. [DOI] [Google Scholar]
- NIST (2015) Índice de Kovats. http://webbook.nist.gov/chemistry/. Accessed 15 Dec 2015
- Nobre ACO, Almeida ASSS, Lemos APS, Magalhães HCR, Garruti DS. Volatile profile of cashew apple juice fibers from different production steps. Molecules. 2015;20:9803–9815. doi: 10.3390/molecules20069803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma A, Voilley A, Cayot P, Le Quéré JL, Springett M. Mechanisms of extraction of aroma compounds from foods, using adsorbents. Effect of various parameters. Food Rev Int. 2006;22:51–94. doi: 10.1080/87559120500379951. [DOI] [Google Scholar]
- Pereira J, Pereira J, Câmara JS. Effectiveness of different solid-phase microextraction fibers from differentiation of selected Madeira island fruits based on their volatile metabolite profile-Identification of novel compounds. Talanta. 2011;83:899–906. doi: 10.1016/j.talanta.2010.10.064. [DOI] [PubMed] [Google Scholar]
- Pino J. Odour-active compounds in papaya fruit cv Red Maradol. Food Chem. 2014;146:120–126. doi: 10.1016/j.foodchem.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Pino AJ, Almora K, Marbot R. Volatile compounds of papaya (Carica papaya L., Maradol variety) fruit. Flavour Fragance J. 2003;18:492–496. doi: 10.1002/ffj.1248. [DOI] [Google Scholar]
- Rodrigues MI, Iemma AF. Experimental design and process optimization. 3. Boca Raton: CRC Press; 2015. [Google Scholar]
- Schwab W, Mahr C, Schreier P (1989) Studies on the enzymic hydrolysis of bound aroma components from Carica papaya fruit. J Agric Food Chem 37:1009–1012. http://agris.fao.org/agris-search/search.do?recordID=US201302672218
- Todisco KM, Castro-Alves VC, Garruti DS, Costa JMC, Clemente E. The use of headspace solid-phase microextraction (HS–SPME) to assess the quality and stability of fruit products: an example using red mombin pulp (Spondias purpurea L.) Molecules. 2014;19:16851–16860. doi: 10.3390/molecules191016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Wijaya CH (2010) Volatile patterns of different papaya (Carica papaya L.) varieties. J Appl Bot Food Qual 83:128–132. https://ojs.openagrar.de/index.php/JABFQ/article/view/2139
- Valente ALP, Augusto F (2000) Microextração por fase sólida. Química Nova 23:523–530. http://www.scielo.br/pdf/qn/v23n4/2653
- Voon YY, Hamida NSA, Rusul G, Osman A, Quek SY. Volatile flavour compounds and sensory properties of minimally processed durian (Durio zibethinus cv. D24) fruit during storage at 4°C. Postharvest Biol Technol. 2007;46:76–85. doi: 10.1016/j.postharvbio.2007.04.004. [DOI] [Google Scholar]