Abstract
Objective
To examine the awareness, treatment and control rates of diabetes and identify their associated risk factors among Beijing residents.
Methods
A cross-sectional survey was conducted in 2011, using a stratified multistage cluster random sampling method to select a representative sample of 20,242 residents in Beijing aged 18–79 years. Diabetes was defined as fasting blood glucose (FBG) ≥7.0 mmol/L and/or history of diabetes and/or using insulin or hypoglycemic agents. All estimates of awareness, treatment and control rates were weighted by the 2010 Beijing Population Census data and the sampling scheme. Multivariate Logistic regression was used to identify factors associated with awareness, treatment and control rates.
Results
A total of 2061 (10.3%) participants were diagnosed as diabetes. The overall awareness, treatment and control rate among patients were 60.9%, 51.3% and 22.4%, respectively, while overall control rate among treated patients was 33.8%. These rates differed across subgroups. Women were more likely to be aware of diabetes status, receive treatment and have better glucose controlled than men (69.5% vs. 54.7% for awareness, 61.0% vs. 44.3% for treatment, and 27.6% vs. 18.6% for control, respectively). In addition, only 22.2% of treated patients had both FBG and hemoglobin A1c (HbA1c) controlled well. Multivariate Logistic regression suggested that old age, women, higher education and family history of diabetes were associated with higher awareness, treatment and control rates (All P < 0.05). Treated individuals living in rural (OR = 0.67(95%CI: 0.47–0.96)) or with dyslipidemia (OR = 0.63 (95%CI: 0.44–0.91)) had a lower diabetic control rate.
Conclusions
Awareness, treatment and control rates of diabetes in Beijing were still low. A comprehensive intervention strategy on diabetes management and control is warranted.
Keywords: Diabetes, Hemoglobin A1c, Awareness, Treatment, Control
Introduction
Diabetes is a major risk factor for all-cause morbidity and mortality worldwide, and it is an important underlying cause for cardiovascular diseases, metabolic syndrome and kidney failure in adults, leading to huge burden of health care.1, 2, 3, 4 In recent decades, the prevalence of diabetes has remarkably increased and is projected to keep increasing, especially in developing countries.5, 6, 7, 8, 9 In China, the prevalence of diabetes increased from 2.5% in 1994 to 11.6% in 2010, representing about 113.9 million diabetic patients in 2010.6, 7, 8, 9, 10 Many clinical trials and epidemiologic studies have shown that improvement of glycemic control plays a crucial role in diabetes management,11, 12, 13 and it is also beneficial to reduce diabetes-related complications, such as infections, diabetic foot syndrome and cognitive decline.14, 15, 16 However, emerging data identified incredibly low awareness, treatment and control rates of diabetes in Chinese population.7, 17 Beijing, one of the most developed and populous cities in China, suffered from huge burden of health care from diabetes and its complications.2, 18 There are limited studies with respect to awareness, treatment and control rates of diabetes in Beijing and the existing studies were mostly conducted in certain population or district.19, 20, 21, 22 In 2005, the overall awareness, treatment and control rate in population from four districts of Beijing was 56.7%, 50.0% and 15.0%, respectively.19 In order to prevent and control chronic diseases, the Chinese government has increased financial investment on health care and Beijing has implemented comprehensive measurements in recent years.23 However it remains unknown if the awareness, treatment and control rates of diabetes in Beijing residents have improved.
At present, fasting blood glucose (FBG) remains the most common measurement for glycemic control in clinical practice in China. As a stable index reflecting average glucose level over several months veritably, hemoglobin A1c (HbA1c) is recommended to be a definitive and long-term indicator of control target for diabetic patients by the American Diabetes Association (ADA)24 and China Diabetes Society (CDS).25 Therefore, it also remains unknown how HbA1c are controlled among diabetic patients who achieved the glycemic control target according to FBG level (FBG < 7.0 mmol/L).
The aim of the current study was to estimate diabetes awareness, treatment and control rates and associated risk factors among residents in Beijing using a population-based cross-sectional study.
Material and methods
Study population
Beijing Chronic Diseases Survey 2011 was a cross-sectional study conducted during September to November 2011. The study was aimed to examine the prevalence and associated risk factors of chronic diseases in the resident population in Beijing. Details of the study design have been published previously.26 In brief, study participants were adults aged 18–79 years and had been living in their current residence for ≥6 months in the past 12 months in Beijing. We used a multistage stratified cluster random sampling method to select participants from 18 districts and counties in Beijing. In each district/county, two different sampling frames were designed on the basis of participants' employment status. On-post participants, accounting for three fourths of all participants, were sourced from workplace with two-stage sampling method. First, two or three worksites from each district/county were selected using probability proportional to size sampling (PPS) method. Second, 100 participants were selected using systematic sampling at each worksite. For off-post and retired participants, four-stage sampling was designed to sample the participants from households. In the first stage, towns were drawn from each district/county using PPS method. In the second stage, one residential committee/village was selected from each sampled town. In the third stage, one group was randomly sampled from each residential committee/village. In the final stage, about 100 households were randomly sampled, and one individual from each household was selected using Kish selection table. If the individual refused or was unavailable, a replacement household with similar composition was selected in the same neighborhood or village.
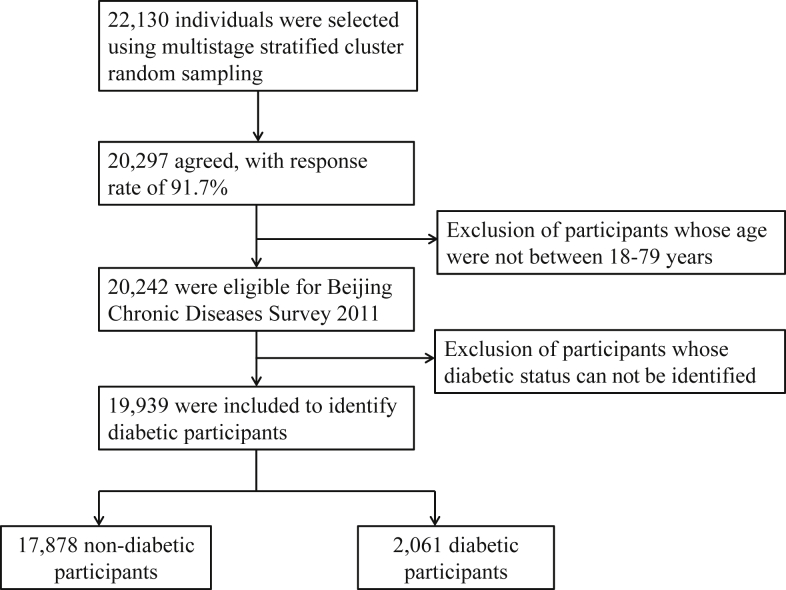
Among selected 22,130 adults, 20,297 (91.7%) agreed to participate in the study and 20,242 aged 18–79 years were eligible for the study, accounting for approximately 1/850 of the total Beijing resident population aged 18–79 years. After excluding those whose diabetic status could not be identified, who lacked of information on blood glucose level, hypoglycemic therapy and self-reported history of diabetes simultaneously, 19,939 participants were used to ascertain diabetic patients. Finally, a total of 2061 diabetic participants were included in the current analysis (Fig. 1).
Fig. 1.
Flow chart of the study sample showing inclusion and exclusion of participants.
The study was approved by both of the Ethical Review Board of Fuwai Hospital and Ethical Review Board of Beijing Center for Disease Control and Prevention. Written informed consent was obtained from each participant before the survey.
Data collection
A standard questionnaire was administered by trained staff to obtain information on demographic characteristics, personal and family medical history, and lifestyle risk factors. Family history of diabetes was identified if at least one parent or sibling had ever been diagnosed as diabetes. Smoking was defined as having smoked at least 100 cigarettes through one's lifetime. Alcohol drinking was defined as drinking at least 12 times in the past year. Physical activity was recorded as total of metabolic equivalents-hours (METs·hours) per week, collected by the modified International Physical Activity Questionnaire.27 Anthropometric measurements were performed by trained staff according to a standard protocol. Body weight and height was measured in light indoor clothing to the nearest 0.1 kg and 0.1 cm, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Overweight and obesity was defined as BMI of 24.0–27.9 kg/m2, and 28.0 kg/m2 or greater, respectively.28 Abdominal obesity was defined as waist circumference (WC) of 90 cm or more in men and 80 cm or more in women. Three blood pressure measurements were collected in sitting position after resting for 5 minutes. Hypertension was defined as systolic blood pressure/diastolic blood pressure ≥140/90 mmHg and/or use of antihypertensive medication. Overnight fasting blood samples were taken at the field center and serum samples were frozen at −20 °C after centrifuged within 3 hours at the field center. Dyslipidemia was defined as total cholesterol ≥240 mg/dl and/or low-density lipoprotein cholesterol ≥160 mg/dl and/or high-density lipoprotein cholesterol <40 mg/dl and/or triglycerides ≥200 mg/dl and/or use of lipid-lowering agents.
Blood glucose measurement and diagnostic criteria
FBG was measured using glucose oxidase method within 20 days (HITACHI 7600 automatic biochemical analyzer, Hitachi High-Technologies, Corp., Japan). Serum HbA1c level was measured by high-performance liquid chromatography method (VARIANT II Hemoglobin Testing System, Bio-Rad Laboratories, Inc., USA) with up to 3 days storage at 2°C–8°C. All measurements were conducted according to a standard protocol by Beijing IPE Center for Clinical Laboratory, which completed a standardization and certification program.
Diabetes was defined as FBG ≥ 7.0 mmol/L and/or self-reported history of diabetes and/or using insulin or oral hypoglycemic agents. Awareness rate was defined as the proportion of persons with self-reported physician-diagnosed diabetes among those having diabetes. Treatment rate was defined as the proportion of persons taking hypoglycemic therapy among those having diabetes. Control was defined as FBG level of less than 7.0 mmol/L. We further evaluated long-term glycemic control, which was defined as HbA1c < 7.0%.
Statistical analysis
Awareness, treatment and control rates were weighted to represent the total Beijing population aged 18–79 years. The weights were calculated on the basis of the 2010 Beijing Population Census data29 and the sampling scheme. Proportions and standard errors (SEs) for awareness, treatment and control rates were estimated in total participants and subgroups. Data were analyzed by SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina, USA). PROC SURVEYMEANS and SURVEYFREQ were used for calculation of means and proportions, as well as corresponding standard errors, Rao-Scott chi square was used to test proportion difference among binary variables (sex, district, employment status, smoke, alcohol drinking, hypertension, dyslipidemia etc.) and SURVEYREG was used for linear trend test within age, education, income, physical activity and BMI. A multivariate Logistic regression was used to identify factors associated with diabetic awareness, treatment and control rates. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
Results
Baseline characteristics of sampled participants
A total of 2061 (10.3%) participants were diagnosed as diabetes among 19,939 included residents in Beijing (Table 1). Diabetic patients were older (54.1 years) and more likely to be men (55.8%) than non-diabetic participants. The average level of FBG among diabetic and non-diabetic participants was 8.8 mmol/L and 5.1 mmol/L, while the average level of HbA1c among diabetic and non-diabetic participants was 7.9% and 5.7%, respectively. Besides, characteristics of diabetic participants and excluded participants, and characteristics of HbA1c status among those treated diabetic patients with FBG < 7.0 mmol/L are presented in Table 2 and Table 3, respectively.
Table 1.
Characteristics of the sampled participants by diabetic status.a
| Variables | Diabetesb (n = 2061) | Non-diabetes (n = 17,878) | Overall (n = 19,939) | Pc |
|---|---|---|---|---|
| Age, years | 54.1 ± 11.3 | 43.3 ± 13.1 | 44.4 ± 13.3 | <0.001 |
| Women | 910 (44.2) | 10,002 (56.0) | 10,912 (54.7) | <0.001 |
| Rural | 658 (31.9) | 6280 (35.1) | 6938 (34.8) | 0.004 |
| Employed | 1178 (57.2) | 14,281 (79.9) | 15,459 (77.5) | <0.001 |
| Education, years | ||||
| <6 | 286 (13.9) | 1249 (7.0) | 1535 (7.7) | <0.001e |
| 7–12 | 1341 (65.1) | 10,047 (56.2) | 11,388 (57.2) | |
| ≥13 | 432 (21.0) | 6571 (36.8) | 7003 (35.2) | |
| Income, 10,000 yuan/year | ||||
| ≤1.9 | 621 (31.9) | 4467 (26.5) | 5088 (27.1) | <0.001e |
| 2.0–3.4 | 829 (42.5) | 6862 (40.7) | 7691 (40.9) | |
| ≥3.5 | 499 (25.6) | 5534 (32.8) | 6033 (32.1) | |
| Smoke | 849 (41.2) | 5345 (29.9) | 6194 (31.1) | <0.001 |
| Alcohol drinking | 778 (37.8) | 5857 (32.8) | 6635 (33.3) | <0.001 |
| Physical activity, Mets·hour/weekd | 55.7 (28.0–98.0) | 51.1 (24.3–91.7) | 51.1 (24.9–92.0) | <0.001f |
| Family history of diabetes | 808 (39.2) | 3400 (19.0) | 4208 (21.1) | <0.001 |
| BMI, kg/m2 | 26.8 ± 3.6 | 25.0 ± 3.8 | 25.2 ± 3.8 | <0.001 |
| WC, cm | 90.3 ± 10.2 | 83.0 ± 10.9 | 83.7 ± 11.1 | <0.001 |
| HbA1c, % | 7.9 ± 1.9 | 5.7 ± 0.5 | 6.0 ± 1.0 | <0.001 |
| FBG, mmol/L | 8.8 ± 3.0 | 5.1 ± 0.6 | 5.5 ± 1.6 | <0.001 |
| SBP, mmHg | 141.2 ± 19.6 | 128.5 ± 18.4 | 129.8 ± 19.0 | <0.001 |
| DBP, mmHg | 86.3 ± 11.8 | 81.6 ± 11 | 82.1 ± 11.2 | <0.001 |
| TC, mg/dL | 200.6 ± 45.2 | 184.4 ± 37.2 | 186.0 ± 38.4 | <0.001 |
| HDL-C, mg/dl | 42.2 ± 10.3 | 45.1 ± 10.7 | 44.8 ± 10.7 | <0.001 |
| LDL-C, mg/dl | 111.0 ± 33.5 | 101.4 ± 29.4 | 102.4 ± 30.0 | <0.001 |
| TG, mg/dl | 154.1 ± 1.9 | 105.8 ± 1.8 | 110.0 ± 1.9 | <0.001 |
BMI: body mass index; WC: waist circumference; Mets: metabolic equivalents; HbA1c: hemoglobin A1c; FBG: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides.
Values are expressed as means ± SD for continuous variables or n (%) for categorical variables.
Diabetes was defined as fasting blood glucose ≥7.0 mmol/L and/or history of diagnosed diabetes and/or using anti-diabetic agents.
P was for diabetes and non-diabetes.
Physical activity is described as median (interquartile range).
P values were calculated using chi-square test.
P value was calculated using non-parametric test.
Table 2.
Characteristics of diabetic participants and excluded participants.a
| Variables | Diabetic patients | Excluded participantsb | P |
|---|---|---|---|
| n | 2061 | 303 | |
| Age, years | 54.1 ± 11.3 | 41.7 ± 12.5 | <0.001 |
| Women | 910 (44.2) | 151 (49.8) | 0.063 |
| Rural | 658 (31.9) | 201 (66.3) | <0.001 |
| Employed | 1178 (57.2) | 256 (84.5) | <0.001 |
| Education, years | |||
| <6 | 286 (13.9) | 25 (8.3) | <0.001d |
| 7–12 | 1341 (65.1) | 129 (42.7) | |
| ≥13 | 432 (21.0) | 148 (49.0) | |
| Income, 10,000 yuan/ year | |||
| ≤1.9 | 621 (31.9) | 60 (21.8) | <0.001d |
| 2.0–3.4 | 829 (42.5) | 116 (42.2) | |
| ≥3.5 | 499 (25.6) | 99 (36.0) | |
| Physical activity, Mets-hour/weekc | 55.7 (28.0–98.0) | 53.4 (24.0–100.0) | 0.518e |
| Family history of diabetes | 808 (39.2) | 45 (14.9) | <0.001 |
| Smoke | 849 (41.2) | 101 (33.3) | 0.009 |
| Alcohol drinking | 778 (37.8) | 121 (39.9) | 0.464 |
| BMI, kg/m2 | 26.8 ± 3.6 | 25.1 ± 4.0 | <0.001 |
| WC, cm | 90.3 ± 10.2 | 81.5 ± 12.3 | <0.001 |
| HbA1c, % | 7.9 ± 1.9 | 5.9 ± 0.68 | <0.001 |
| FBG, mmol/L | 8.8 ± 3.0 | – | |
| SBP, mmHg | 141.2 ± 19.6 | 128.3 ± 18.5 | <0.001 |
| DBP, mmHg | 86.3 ± 11.8 | 82.9 ± 11.6 | <0.001 |
| TC, mg/dl | 200.6 ± 45.2 | 198.5 ± 29.6 | 0.917 |
| HDL-C, mg/dl | 42.2 ± 10.3 | 40.5 ± 26.9 | 0.721 |
| LDL-C, mg/dl | 111.0 ± 33.5 | 61.1 ± 51.7 | <0.001 |
| TG, mg/dl | 154.1 ± 1.9 | 101.8 ± 1.4 | 0.157 |
BMI: body mass index; WC: waist circumference; Mets: metabolic equivalents; HbA1c: hemoglobin A1c; FBG: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides.
Values are expressed as means ± SD for continuous variables or n (%) for categorical variables.
Excluded patients mean patients whose diabetic status can't be identified.
Physical activity is described as median (interquartile range).
P values were calculated using chi-square test.
P value was calculated using non-parametric test.
Table 3.
Characteristics of HbA1c status among those treated diabetic patients with FBG <7.0 mmol/L.a
| Variables | FBG < 7.0 mmol/L and HbA1c < 7.0% | FBG < 7.0 mmol/L and HbA1c ≥ 7.0% | P |
|---|---|---|---|
| n | 247 | 120 | |
| Age, years | 58.0 ± 10.6 | 58.5 ± 9.9 | 0.649 |
| Women | 147 (59.5) | 73 (60.8) | 0.809 |
| Rural | 59 (23.9) | 41 (34.2) | 0.038 |
| Employed | 99 (40.1) | 48 (40.0) | 0.988 |
| Education, years | |||
| <6 | 38 (15.4) | 18 (15.0) | 0.031c |
| 7–12 | 150 (60.7) | 87 (72.5) | |
| ≥13 | 59 (23.9) | 15 (12.5) | |
| Income, 10,000 yuan/ year | |||
| ≤1.9 | 62 (27.1) | 38 (33.3) | 0.475c |
| 2.0–3.4 | 110 (48.0) | 49 (43.0) | |
| ≥3.5 | 57 (24.9) | 27 (23.7) | |
| Physical activity, Mets-hour/weekb | 55.5 (28.8–93.6) | 63.1 (36.6.0–117.4) | 0.057d |
| Family history of diabetes | 111 (44.9) | 45 (37.5) | 0.176 |
| Smoke | 68 (27.5) | 35 (29.2) | 0.744 |
| Alcohol drinking | 59 (23.9) | 29 (24.2) | 0.953 |
| BMI, kg/m2 | 25.8 ± 3.8 | 26.7 ± 3.8 | 0.027 |
| WC, cm | 86.9 ± 10.2 | 89.2 ± 9.0 | 0.041 |
| HbA1c, % | 6.19 ± 0.48 | 7.66 ± 0.84 | <0.001 |
| FBG, mmol/L | 5.81 ± 0.7 | 6.31 ± 0.6 | <0.001 |
| SBP, mmHg | 137.5 ± 18.6 | 144.4 ± 18.4 | 0.001 |
| DBP, mmHg | 81.8 ± 11.1 | 83.9 ± 11.6 | 0.094 |
| TC, mg/dl | 180.9 ± 39.7 | 191.3 ± 41.0 | 0.021 |
| HDL-C, mg/dl | 43.2 ± 10.7 | 43.2 ± 10.2 | 0.988 |
| LDL-C, mg/dl | 99.7 ± 30.7 | 105.8 ± 31.1 | 0.075 |
| TG, mg/dl | 115.4 ± 1.7 | 133.4 ± 1.7 | 0.016 |
BMI: body mass index; WC: waist circumference; Mets: metabolic equivalents; HbA1c: hemoglobin A1c; FBG: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides.
Values are expressed as means ± SD for continuous variables or n (%) for categorical variables.
Physical activity is described as median (interquartile range).
P values were calculated using chi-square test.
P value was calculated using non-parametric test.
Awareness, treatment and control rates of diabetes
Table 4 presents the awareness, treatment and control rates of diabetes by characteristics. Among all the diabetic patients in Beijing, 60.9% were aware of their condition, with 54.7% in men and 69.5% in women. As for treatment rate, the estimated proportion was 51.3% in Beijing adults, 44.3% in men and 61.0% in women. The control rate among treated patients was substantially higher than the control rate among all patients (33.8% vs. 22.4% for overall, 30.7% vs. 18.6% for men and 36.8% vs. 27.6% for women). In general, women had higher awareness, treatment and control rates than men among all diabetic patients, and women also tended to have a marginally significant higher control rate among treated patients than that in men. Both awareness rate and treatment rate increased with ages. Similar trend was also observed for the control rate among all patients (13.0% in 18–44 years, 22.9% in 45–59 years, and 27.5% in 60–79 years), however, no significant difference was observed for control rate among treated patients, with 25.5%, 35.3% and 34.1% in the 18–44, 45–69 and 60–79 years age groups, respectively. Compared with urban residents, rural residents had similar awareness and treatment rates but a relatively lower control rate among all patients and treated patients (61.7% vs. 60.3% for awareness, 52.2% vs. 50.7% for treatment, 19.2% vs. 24.4% for control among patients, and 27.8% vs. 37.7% for control among treated patients, respectively). Besides, the control rates among treated patients were lower among diabetic patients with dyslipidemia and smoking habits, and tended to be lower among overweight/obese or abdominal obese patients.
Table 4.
Estimated awareness, treatment and control rates of diabetes in Beijing.
| Variables | n | Awareness ratea |
Treatment ratea |
Control rate among all patientsa |
Control rate among treated patientsa |
||||
|---|---|---|---|---|---|---|---|---|---|
| % (SE)b | P | % (SE)b | P | % (SE)b | P | % (SE)b | P | ||
| Total | 2061 | 60.9 (1.3) | 51.3 (1.3) | 22.4 (1.0) | 33.8 (1.7) | ||||
| Sex | |||||||||
| Men | 1151 | 54.7 (1.7) | <0.001 | 44.3 (1.7) | <0.001 | 18.6 (1.3) | <0.001 | 30.7 (2.4) | 0.061 |
| Women | 910 | 69.5 (1.8) | 61.0 (2.0) | 27.6 (1.7) | 36.8 (2.5) | ||||
| Age, yearsc | |||||||||
| 18–44 | 378 | 39.0 (2.9) | <0.001 | 26.3 (2.6) | <0.001 | 13.0 (2.0) | <0.001 | 25.5 (4.8) | 0.162 |
| 45–59 | 1131 | 60.6 (1.6) | 50.5 (1.6) | 22.9 (1.4) | 35.3 (2.2) | ||||
| 60–79 | 552 | 75.0 (2.3) | 68.3 (2.4) | 27.5 (2.1) | 34.1 (2.9) | ||||
| District | |||||||||
| Urban | 1403 | 60.3 (1.5) | 0.638 | 50.7 (1.5) | 0.611 | 24.4 (1.3) | 0.028 | 37.7 (2.0) | 0.011 |
| Rural | 658 | 61.7 (2.3) | 52.2 (2.4) | 19.2 (1.7) | 27.8 (2.8) | ||||
| Employed | |||||||||
| No | 883 | 71.7 (1.8) | <0.001 | 64.5 (1.9) | <0.001 | 26.7 (1.7) | <0.001 | 35.3 (2.3) | 0.215 |
| Yes | 1178 | 50.7 (1.7) | 38.9 (1.6) | 18.3 (1.3) | 31.4 (2.4) | ||||
| Education, yearsc | |||||||||
| ≤6 | 286 | 63.5 (3.7) | 0.471 | 59.3 (3.8) | 0.049 | 20.9 (2.8) | 0.532 | 31.3 (4.6) | 0.908 |
| 7–12 | 1341 | 59.5 (1.6) | 50.0 (1.6) | 21.9 (1.2) | 34.2 (2.0) | ||||
| ≥13 | 432 | 62.4 (2.9) | 48.7 (2.8) | 24.7 (2.4) | 35.0 (3.7) | ||||
| Income, 10,000 yuan/yearc | |||||||||
| ≤1.9 | 621 | 58.2 (2.6) | 0.190 | 50.4 (2.6) | 0.894 | 18.3 (1.8) | 0.023 | 29.4 (3.1) | 0.198 |
| 2.0–3.4 | 829 | 61.0 (2.0) | 51.9 (2.0) | 24.1 (1.6) | 36.7 (2.6) | ||||
| ≥3.5 | 499 | 64.7 (2.6) | 51.4 (2.6) | 24.6 (2.2) | 33.8 (3.3) | ||||
| Smoke | |||||||||
| No | 1211 | 65.9 (1.6) | <0.001 | 57.0 (1.7) | <0.001 | 26.0 (1.4) | <0.001 | 36.7 (2.2) | 0.016 |
| Yes | 849 | 54.0 (2.1) | 43.5 (2.0) | 17.3 (1.5) | 28.5 (2.7) | ||||
| Alcohol drinking | |||||||||
| No | 1283 | 66.6 (1.6) | <0.001 | 58.3 (1.7) | <0.001 | 25.5 (1.4) | <0.001 | 35.5 (2.8) | 0.094 |
| Yes | 778 | 51.5 (2.1) | 39.9 (2.1) | 17.2 (1.5) | 29.7 (2.9) | ||||
| Physical activity, Mets·hour/weekc | |||||||||
| Low | 575 | 57.7 (2.6) | 0.278 | 50.5 (2.7) | 0.789 | 20.5 (2.0) | 0.541 | 32.2 (3.5) | 0.877 |
| Moderate | 689 | 62.1 (2.1) | 52.8 (2.2) | 22.8 (1.8) | 33 (2.8) | ||||
| High | 690 | 63.0 (2.2) | 51.3 (2.2) | 23.7 (1.8) | 35.2 (2.8) | ||||
| Family history of diabetes | |||||||||
| No | 1253 | 54.4 (1.7) | <0.001 | 46.7 (1.8) | <0.001 | 20.3 (1.3) | 0.010 | 34.5 (2.4) | 0.883 |
| Yes | 808 | 72.3 (1.9) | 59.4 (2) | 25.9 (1.8) | 32.7 (2.4) | ||||
| BMI, kg/m2c | |||||||||
| ≤23.9 | 428 | 66.2 (2.8) | <0.001 | 55.2 (2.9) | <0.001 | 30.6 (2.6) | <0.001 | 41.5 (3.8) | 0.058 |
| 24.0–27.9 | 907 | 64.4 (2.0) | 54.4 (2.0) | 23.8 (1.6) | 33.5 (2.5) | ||||
| ≥28.0 | 694 | 52.0 (2.2) | 43.7 (2.1) | 16.1 (1.5) | 29.9 (2.9) | ||||
| Abdominal obesity | |||||||||
| No | 583 | 63.3 (2.4) | 0.170 | 51.4 (2.5) | 0.805 | 27.8 (2.2) | 0.002 | 39.5 (3.3) | 0.053 |
| Yes | 1437 | 59.3 (1.6) | 50.7 (1.6) | 20.4 (1.2) | 32.2 (2.0) | ||||
| Hypertension | |||||||||
| No | 655 | 58.7 (2.3) | 0.281 | 47.3 (2.3) | 0.043 | 23.1 (1.9) | 0.768 | 34.2 (3.0) | 0.946 |
| Yes | 1388 | 61.7 (1.6) | 53.0 (1.6) | 22.2 (1.3) | 34.0 (2.1) | ||||
| Dyslipidemia | |||||||||
| No | 629 | 65.3 (2.2) | 0.022 | 53.3 (2.3) | 0.309 | 29.8 (2.1) | <0.001 | 41.9 (3.1) | 0.002 |
| Yes | 1432 | 59.0 (1.6) | 50.4 (1.6) | 19.3 (1.2) | 30.2 (2.0) | ||||
SE: standard error; BMI: body mass index; Mets: metabolic equivalents; FBG: fasting blood glucose.
Awareness was defined as participants' self-reporting a history of physician-diagnosed diabetes among all patients with diabetes. Treatment was defined as use of a prescription medication among all patients with diabetes. Control of diabetes was defined as an FBG level of less than 7.0 mmol/L among patients with diabetes who were treated.
Data are weighted proportions.
P was statistic value for trend.
Long-term glycemic control among diabetic patients
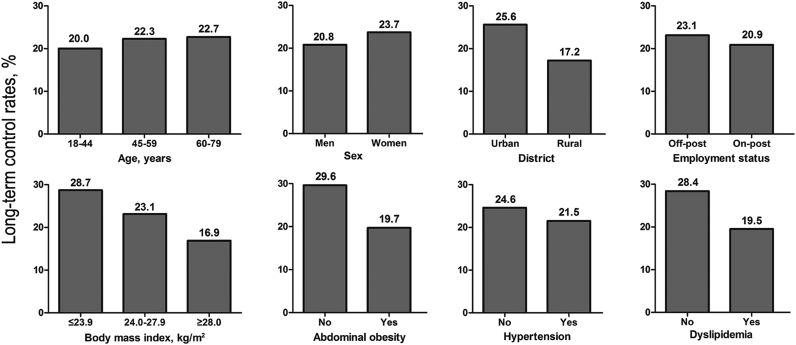
Among FBG-controlled diabetic patients, an estimated 65.9% patients further had their HbA1c adequately controlled (<7.0%). However, only 22.2% of all treated diabetic patients both had their FBG and HbA1c well controlled. Among treated diabetic patients, participants who were resident in rural, obese/abdominal obese, with dyslipidemia had significantly lower control rates (FBG < 7.0 mmol/L and HbA1c < 7.0%); while no significant differences were observed among subgroup populations by sex, age, employment status and blood pressure levels (Fig. 2).
Fig. 2.
Proportions of patients whose FBG was <7.0 mmol/L and HbA1c was <7.0% among treated diabetic patients. Proportions were weighted according to the 2010 Beijing Population Census data and the sampling scheme. Differences between/among subgroup populations by district, body mass index, waist circumference and dyslipidemia status were statistically significant (P < 0.05). FBG: fasting blood glucose; HbA1c: hemoglobin A1c.
Related factors associated with diabetic awareness, treatment and control
Several factors were observed to influence awareness, treatment and control rates of diabetes to some extent (Table 5). Among all patients, older age and family history of diabetes were significantly associated with higher diabetic awareness, treatment and control rates, while men and patients with low education level were substantially less aware of diabetes status, less likely to be treated and less to be FBG-controlled. For example, compared with men, women had about more than 50% increased proportions of diabetic awareness, treatment and control rates. The multivariate-adjusted odds ratio (OR) was 1.73 (95% confidence interval (CI): 1.22–2.46) for awareness, 1.52 (95%CI: 1.10–2.11) for treatment, and 1.81 (95%CI: 1.28–2.57) for control, respectively. In addition, among treated patients rural patients and patients with dyslipidemia were less likely to have controlled FBG levels, with the multivariate-adjusted ORs of 0.67 (95%CI: 0.47–0.96) and 0.63 (95%CI: 0.44–0.91), respectively.
Table 5.
Multivariate-adjusted odds ratio for risk factors associated diabetic awareness, treatment and control rates.a
| Variables | Awareness rate |
Treatment rate |
Control rate among all patients |
Control rate among treated patients |
||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age, per 10-year increment | 1.87 (1.64–2.14) | <0.001 | 1.93 (1.70–2.19) | <0.001 | 1.29 (1.13–1.47) | <0.001 | 0.95 (0.79–1.14) | 0.599 |
| Women | 1.73 (1.22–2.46) | 0.002 | 1.52 (1.10–2.11) | 0.012 | 1.81 (1.28–2.57) | 0.001 | 1.43 (0.92–2.21) | 0.112 |
| Rural residency | 1.13 (0.87–1.47) | 0.347 | 1.20 (0.94–1.55) | 0.144 | 0.80 (0.60–1.06) | 0.119 | 0.67 (0.47–0.96) | 0.030 |
| Education | ||||||||
| Moderate education vs. low education | 1.96 (1.32–2.91) | 0.001 | 1.71 (1.17–2.51) | 0.006 | 1.72 (1.12–2.66) | 0.014 | 1.45 (0.88–2.41) | 0.146 |
| High education vs. low education | 2.87 (1.69–4.87) | <0.001 | 2.16 (1.33–3.51) | 0.002 | 2.36 (1.35–4.12) | 0.003 | 1.81 (0.91–3.60) | 0.094 |
| Income | ||||||||
| Moderate income vs. low income | 1.16 (0.87–1.55) | 0.308 | 1.09 (0.83–1.43) | 0.540 | 1.38 (1.00–1.90) | 0.052 | 1.32 (0.89–1.95) | 0.170 |
| High income vs. low income | 1.49 (1.01–2.20) | 0.046 | 1.20 (0.84–1.71) | 0.319 | 1.52 (0.98–2.36) | 0.062 | 1.25 (0.71–2.21) | 0.438 |
| Smoke | 1.12 (0.82–1.52) | 0.479 | 1.07 (0.79–1.44) | 0.678 | 1.09 (0.77–1.54) | 0.615 | 1.00 (0.64–1.55) | 0.985 |
| Alcohol drinking | 0.72 (0.54–0.97) | 0.030 | 0.66 (0.49–0.87) | 0.004 | 0.77 (0.55–1.09) | 0.137 | 0.80 (0.51–1.25) | 0.331 |
| Physical activity | ||||||||
| Moderate PAL vs. low PAL | 1.03 (0.76–1.39) | 0.874 | 0.93 (0.69–1.25) | 0.623 | 0.99 (0.71–1.39) | 0.956 | 0.94 (0.62–1.44) | 0.786 |
| High PAL vs. low PAL | 1.06 (0.77–1.45) | 0.731 | 0.83 (0.61–1.12) | 0.229 | 1.00 (0.72–1.39) | 0.986 | 0.97 (0.64–1.47) | 0.879 |
| Abdominal obesity | 0.78 (0.58–1.04) | 0.095 | 0.90 (0.69–1.18) | 0.444 | 0.72 (0.53–0.97) | 0.029 | 0.80 (0.55–1.16) | 0.233 |
| Hypertension | 0.77 (0.59–1.01) | 0.058 | 0.80 (0.62–1.04) | 0.096 | 0.97 (0.73–1.29) | 0.831 | 1.27 (0.88–1.84) | 0.202 |
| Dyslipidemia | 0.97 (0.74–1.27) | 0.805 | 1.17 (0.91–1.51) | 0.212 | 0.67 (0.50–0.88) | 0.005 | 0.63 (0.44–0.91) | 0.013 |
| Family history of diabetes | 2.56 (2.00–3.27) | <0.001 | 2.00 (1.59–2.51) | <0.001 | 1.32 (1.02–1.72) | 0.038 | 0.86 (0.62–1.20) | 0.385 |
OR: odds ratio; PAL: physical activity level. Mets: metabolic equivalents.
Tertile cutoff value was 1954.0 and 4890.0 for Mets·hour/wk, respectively; low education, ≤6 years; moderate education, 7–12 years; high education, ≥13 years; low income, ≤19,000 yuan per year; moderate income, 20,000–34,000 yuan per year; high income, ≥35,000 yuan per year; abdominal obesity, ≥90 cm in men/≥80 cm in women; OR > 1, indicated a higher proportions of diabetic awareness, treatment or control rate; OR < 1, indicated a lower proportions of diabetic awareness, treatment or control rate.
Data are weighted ORs and P values.
Discussion
The overall awareness, treatment and control rate of diabetes among Beijing residents in 2011 was 60.9%, 51.3% and 22.4%, respectively. Diabetic patients who were male, younger, lower educated tended to have low awareness, treatment and control rates. Control rate among treated patients was 33.8%, and treated patients who were living in rural, smokers and had dyslipidemia were more difficult to achieve the target of FBG <7.0 mmol/L. Only 22.2% had their FBG- and HbA1c-controlled meanwhile. These data might raise concern of diabetes-related mortality and disease burden in upcoming years.
Diabetes has become a major public health problem in Beijing and China. Many clinical trials and epidemiologic studies showed that glycemic control is fundamental to diabetes management.12, 30, 31 Compared with nationwide data, Beijing had higher awareness and treatment rates,7, 17, 32, 33 while lower contemporaneous control rate. Control rate among all diabetic patients was 22.4% in Beijing and 30.6% nationwide on the basis of FBG < 7.0 mmol/L, and the corresponding data among treated diabetic patients were 33.8% in Beijing and 34.7% nationwide on the basis of FBG < 7.0 mmol/L.32, 33 One plausible explanation is the aging in Beijing, which accounted for ever-increasing numbers of people with diabetes.34 In addition, elderly diabetic patients were more likely to suffer longer duration, develop concomitant diseases (hypertension, dyslipidemia, abdominal obesity etc.) and progressive impairment of insulin secretion, all of which may result in lower control rate in Beijing.35, 36, 37, 38 Compared with historical data in Beijing in 2005, we observed a slightly increasing of awareness, treatment and control rates of diabetes. From 2005 to 2011, awareness rate increased from 56.7% to 60.9%, and treatment rate increased from 50.0% to 51.3%, respectively.19 In 2005, one sixth (15.0%) of all diabetic patients controlled their FBG well. Although the control rate among all patients was still very low, it was nearly one quarter (22.4%) among all diabetic patients in 2011.19 It might result from economic development, urbanization, policy support, improvement of public health system and basic medical care. However, when compared with developed countries, though Beijing was a metropolitan city, the awareness, treatment and control rates were still much lower.39, 40, 41
In our study, among all diabetic patients the elderly tended to have higher awareness, treatment and control rates, which may be attributable to more access to physical examination and being more conscious of health. Besides, rapid growth in economy and modernization leads to heavier work stress among men in Beijing, thus men were less aware of their diabetes status and receive treatment.42 Our study also showed that better educated patients had higher awareness, treatment and control rates, which probably was resulted from higher likelihood of obtaining disease-related knowledge, having better socioeconomic status and being more convenient access to medical care.41, 43 Treated patients living in rural district or having dyslipidemia had poor control situation, which were consistent with previous studies.7, 17, 37 For rural patients, the low control rate may be due to their poor medication adherence and lack of disease-related knowledge. In addition, accumulating evidence has demonstrated the comorbidity of dyslipidemia and poor glycemic control in patients with diabetes in China and other countries,7, 37 and association of high free fatty acid with insulin resistance and lipoapoptosis of pancreatic beta-cells.44 This might be the possible explanation for lower control rate among treated patients with dyslipidemia.
Compared with FBG, HbA1c is a more stable and less day-to-day perturbations index. It could reflect average glucose level over several months veritably. It is demonstrated that intensive therapy among diabetic patients lowered HbA1c levels, further leading to a significant decreased risk of first major cardiovascular event than standard therapy.31 Studies also showed HbA1c reduction was associated with obvious reduction in microvascular and neuropathic complications.11, 12 Every percentage point drop in HbA1c was estimated to associate with about 40% reduced risk of microvascular complications.45 Therefore, ADA and CDS recommended HbA1c as an index of long-term glycemic control for treated diabetic patients.24, 25 However, as an indirect index, HbA1c does not reflect true glucose levels, especially in individuals with anemia and hemoglobinopathies.24 Considering the high prevalence of anemia and hemoglobinopathies in Chinese population,46 it should be cautious when using HbA1c.
The present study estimated the average HbA1c among patients was 7.9% in Beijing, indicating considerable health benefits if it is controlled adequately. Besides, 120 patients (32.1% of treated diabetic patients) who were considered as well glycemic controlled (FBG < 7.0 mmol/L) still had uncontrolled HbA1c levels (HbA1c ≥ 7.0%), indicating they suffered poor long-term glycemic control. These patients tended to have increased risk of cardiovascular event or microvascular complications since poor HbA1c-control was substantially associated with unexpected prognosis of diabetes.13, 14, 16, 31 In the current study, patients with only FBG-controlled tended to have higher BMI, WC, TC, TG and SBP levels compared with patients whose FBG and HbA1c were both well controlled. Although ADA has suggested patients with HbA1c ≥6.5% should be diagnosed as diabetes in American standards of medical care, evidence on cutoff value of HbA1c for diagnosis of diabetes is still insufficient for Chinese population.47 Therefore, we did not identify diabetic patients integrating HbA1c levels in the present study.
The present study provided reliable estimates of diabetes awareness, treatment and control rates in a most recent cross-sectional survey that was conducted among a large representative sample of general population of Beijing residents. Data collection and samples processing were under strict quality control. However, some limitations should be addressed. First, 2-hour oral glucose tolerance test was not conducted, which may introduce misclassification bias. Second, diabetic status cannot be determined among 303 participants. Compared with diabetic patients, these 303 participants tended to be younger, and lower BMI, WC, blood pressure and blood lipid level, which may result in overestimation of diabetes prevalence and underestimation of awareness and treatment rates. Third, we did not further distinguish between type 1 and type 2 diabetes, though type 2 diabetes is the majority of diabetes.
In summary, although the awareness and treatment rates in Beijing were relatively higher than data nationwide, but compared with developed countries the awareness, treatment and control rates of diabetes in Beijing residents were still lower. A comprehensive and intensive intervention strategy on diabetes management, especially on diabetes control, is warranted.
Conflicts of interest
None.
Acknowledgments
The current study is supported by Beijing Municipal Science and Technology planning project (D9050703650901) and Chinese Academy of Medical Sciences Initiative for Innovative Medicine (2016-12M-3-018). The sponsor has no role in designing, conducting, data analyzing, and manuscript writing of this study. We acknowledge the dedicated participants and medical, nursing, technical and administrative staff that have contributed to this project.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Danaei G., Lawes C.M., Vander Hoorn S., Murray C.J., Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 2.King H., Aubert R.E., Herman W.H. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Lin X., Zhang Z. The economic burden of inpatients with type 2 diabetes: a case study in a Chinese hospital. Asia Pac J Public Health. 2015;27:49S–54S. doi: 10.1177/1010539515572220. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W., Lu J., Weng J. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y., Wang L., He J. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 7.Hu D., Fu P., Xie J. Increasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA study. Diabetes Res Clin Pract. 2008;81:250–257. doi: 10.1016/j.diabres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2014. Global Status Report on Noncommunicable Diseases.http://www.who.int/nmh/publications/ncd-status-report-2014/en/ [EB/OL]. Available: Accessed 26 December 2015. [Google Scholar]
- 9.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Pan X.R., Yang W.Y., Li G.W., Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care. 1997;20:1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 11.UK Prospecitve Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 12.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 13.Valensi P., Benroubi M., Borzi V. The IMPROVE study–a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008;62:1809–1819. doi: 10.1111/j.1742-1241.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 14.Tuligenga R.H., Dugravot A., Tabák A.G. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2:228–235. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson-Stuttard J., Blundell S., Harris T., Cook D.G., Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 16.Hasan R., Firwana B., Elraiyah T. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(2 suppl):22S–28S.e1–2. doi: 10.1016/j.jvs.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Li M.Z., Su L., Liang B.Y. Trends in prevalence, awareness, treatment, and control of diabetes mellitus in mainland China from 1979 to 2012. Int J Endocrinol. 2013;2013:753150. doi: 10.1155/2013/753150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao D., Zhao F., Li Y., Zheng Z. Projected and observed diabetes epidemics in China and beyond. Curr Cardiol Rep. 2012;14:106–111. doi: 10.1007/s11886-011-0227-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P.H., Jiao S.J., Zhou Y. Studies on prevalence and control of several common chronic diseases among Beijing adults in 2005. Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28:625–630. [in Chinese] [PubMed] [Google Scholar]
- 20.Zhan Y.Q., Yu J.M., Hu D.Y. Prevalence and related knowledge of diabetes mellitus among residents of Beijing. Chin J Public Health. 2012;28:19–21. [in Chinese] [Google Scholar]
- 21.Ye W.L., Ma J., Fan X.H. Chronic disease control among rural residents in Beijing Pinggu district. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:528–533. doi: 10.3881/j.issn.1000-503X.2015.05.007. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 22.Liu M., Wang J., He Y. Awareness, treatment and control of type 2 diabetes among Chinese elderly and its changing trend for past decade. BMC Public Health. 2016;18:278. doi: 10.1186/s12889-016-2874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health of the People's Republic of China . 2012-05-08. Work Plan for Chronic Disease Prevention and Control in China (2012–2015) [Google Scholar]
- 24.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.China Disease Society China guideline for type 2 diabetes (2010) Chin J Diabetes. 2012;1:81–117. [in Chinese] [Google Scholar]
- 26.Li G., Hu H., Dong Z., Xie J., Zhou Y. Urban and suburban differences in hypertension trends and self-care: three population-based cross-sectional studies from 2005-2011. PLoS One. 2015;10:e0117999. doi: 10.1371/journal.pone.0117999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IPAQ Group. International physical activity questionnaire [EB/OL]. Available: http://www.sdp.univ.fvg.it/sites/default/files/IPAQ_English_self-admin_long.pdf(Octorber2002). Accessed 15 December 2015.
- 28.Division of Disease control, Minstry of Health of the Pelple's Republic of China . People's Medical Publishing House; Beijing: 2006. The Guideline for Prevention and Control of Overweight and Obesity in Chineses Adults. [Google Scholar]
- 29.National Brueau of Statistics of China. 2010 Population Census. Available: http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm. Accessed 28 November 2015.
- 30.Ismail-Beigi F., Craven T., Banerji M.A. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;7:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayward R.A., Reaven P.D., Emanuele N.V., VADT Investigators Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:978. doi: 10.1056/NEJMc1508386. [DOI] [PubMed] [Google Scholar]
- 32.Bureau of Disease Prevention and Control, National Health and Family Planning Commission of PRC . People's Medical Publishing House; Beijing: 2015. 2015 Report on Chinese Nutrition and Chronic Disease. [Google Scholar]
- 33.Chinese Center for Disease Control and Prevention . Military Medical Science Press; Beijing: 2012. Report on Chronic Disease Risk Factor Surveillance in China 2010. [Google Scholar]
- 34.Bloomgarden Z., Ning G. Diabetes and aging. J Diabetes. 2013;5:369–371. doi: 10.1111/1753-0407.12086. [DOI] [PubMed] [Google Scholar]
- 35.Benoit S.R., Fleming R., Philis-Tsimikas A., Ji M. Predictors of glycemic control among patients with Type 2 diabetes: a longitudinal study. BMC Public Health. 2005;5:36. doi: 10.1186/1471-2458-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valle T., Koivisto V.A., Reunanen A., Kangas T., Rissanen A. Glycemic control in patients with diabetes in Finland. Diabetes Care. 1999;22:575–579. doi: 10.2337/diacare.22.4.575. [DOI] [PubMed] [Google Scholar]
- 37.Khattab M., Khader Y.S., Al-Khawaldeh A., Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complicat. 2010;24:84–89. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Gao L., Ji L., Lu J. Current status of blood glucose control and treatment of type 2 diabetes in China 2009-2012. Chin J Diabetes. 2014;22:594–598. [in Chinese] [Google Scholar]
- 39.Selvin E., Parrinello C.M., Sacks D.B., Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser A., Vollenweider P., Waeber G., Marques-Vidal P. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: the CoLaus study. Diabet Med. 2012;29:190–197. doi: 10.1111/j.1464-5491.2011.03422.x. [DOI] [PubMed] [Google Scholar]
- 41.Sims M., Diez Roux A.V., Boykin S. The socioeconomic gradient of diabetes prevalence, awareness, treatment, and control among African Americans in the Jackson Heart Study. Ann Epidemiol. 2011;21:892–898. doi: 10.1016/j.annepidem.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin J., Yeung R., Luk A. Gender, diabetes education, and psychosocial factors are associated with persistent poor glycemic control in patients with type 2 diabetes in the Joint Asia Diabetes Evaluation (JADE) program. J Diabetes. 2016;8:109–119. doi: 10.1111/1753-0407.12262. [DOI] [PubMed] [Google Scholar]
- 43.Park M.J., Yun K.E., Lee G.E., Cho H.J., Park H.S. A cross-sectional study of socioeconomic status and the metabolic syndrome in Korean adults. Ann Epidemiol. 2007;17:320–326. doi: 10.1016/j.annepidem.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Teng X.Y., Yang Y.N. Lipoapoptosis of beta-cells. Sect Endocrinol Foreign Med Sci. 2005;25:15–18. [in Chinese] [Google Scholar]
- 45.USA Department of Health and Human Services Centers for Disease Control and Prevention. National Diabetes Fact Sheet, 2011. Available: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 3 February 2016.
- 46.Li L., Luo R., Medina A., Rozelle S. The prevalence of anemia in central and eastern China: evidence from the China Health and Nutrition Survey. Southeast Asian J Trop Med Public Health. 2015;46:306–321. [PubMed] [Google Scholar]
- 47.Xu L., Chan W.M., Hui Y.F., Lam T.H. Association between HbA1c and cardiovascular disease mortality in older Hong Kong Chinese with diabetes. Diabet Med. 2012;29:393–398. doi: 10.1111/j.1464-5491.2011.03456.x. [DOI] [PubMed] [Google Scholar]