Abstract
Malaria is a major health problem that still affects numerous countries. The current study aimed to identify the role of Indigofera oblongifolia leaf extract in regulating mouse spleen macrophages during the progression of Plasmodium chabaudi infection. Three doses of the leaf extract (100, 200, and 300 mg/kg) were administered to mice inoculated with P. chabaudi infected erythrocytes. The weight of the infected mice improved after the treatment with I. oblongifolia. The infection causes disorganization of macrophage distribution in the spleen. After the mice had been treated with the leaf extract, the macrophages appeared to be reorganized in the white and red pulp areas. In addition, the I. oblongifolia leaf extract (IOLE) significantly increased the total antioxidant capacity of the mice spleens infected with P. chabaudi. The phagocytic activity of spleen macrophages was increased in the infected group as indicated by the significant decrease in the number of fluorescent particles in the spleen sections. This number increased in the mice spleens after treatment with IOLE. Based on these results, it is suggested that IOLE regulate macrophage response of the spleen during the blood stage of malaria in mice.
Keywords: Fluorescent particles, Indigofera oblongifolia leaf extract, Mice, Phagocytosis, Spleen
1. Introduction
According to a report presented by the World Health Organization (WHO), nearly half of the world's population is at risk of malaria (WHO, 2016). About 212 million malaria cases were reported including about 429,000 cases of malaria-related deaths (WHO, 2016). Known drugs that act against the parasite can affect the human health because of their side effects. Therefore, novel safe compounds with antimalarial activities are required. The extensive research targeted at discovering alternative agents to the already known drugs has succeeded in reducing the global malaria mortality rate by 29% since 2010 (WHO, 2016).
Since medicinal plants are currently essential sources of drugs, in this study, we investigated Indigofera oblongifolia, a plant traditionally used against malaria symptoms in Saudi Arabia.
In this study, we used Plasmodium chabaudi, a parasite that resembles the human pathogenic parasite Plasmodium falciparum (Wunderlich et al., 1982). Our previous studies reported the effects of I. oblongifolia leaf extract (IOLE) against lead caused toxicity in mice (Al-Quraishy et al., 2016). In addition, Dkhil et al., 2015 reported the anti-malarial role of I. oblongifolia with a parasitemia suppression rate of 68%.
The spleen is considered one of the most critical effector organs that protects against malaria symptoms induced by Plasmodium species. The spleen pulp contains several macrophages that can identify and phagocytose the parasitized erythrocytes.
In the current work, we investigated the potential effects of I. oblongifolia against P. chabaudi-induced changes in mouse spleen macrophages.
2. Materials and methods
2.1. Extract preparation
The leaves of I. Oblongifolia were collected from Jazan region, Saudi Arabia. The plant was identified by a specialist from the Department of Botany, College of Science, King Saud University. Plant leaves were air-dried, powdered then extracted with 70% methanol (Al-Quraishy et al., 2016).
2.2. Animals and infection
Adult female C57BL/6 mice, 10–12 weeks old, were obtained from the animal facility of King Saud University, Riyadh, Saudi Arabia. The mice were fed a standard diet and water ad libitum. The experiments were approved by the state authorities, and they were conducted in accordance with the Saudi Arabian rules on animal protection.
Mice were weekly inoculated with one million P. chabaudi-parasitized erythrocytes at blood-stage infection through the intraperitoneal route. Blood was drawn from the tail veins, and parasitemia was determined by examining Giemsa-stained blood smears. The cell numbers were counted using a Neubauer-chamber.
2.3. Mice groups
Animals were randomly allocated to five groups. The uninfected group contained five mice. The infected groups were intraperitoneally injected with 1 × 106 P. chabaudi-infected erythrocytes. The infected mice were allocated to four subgroups of 10 mice each. The first group was considered the positive control group. Then, after 60 min, the mice in the second, third, and fourth groups were gavaged with 100 µL IOLE at doses of 100, 200, and 300 mg/kg body weight, respectively, once daily for 7 days.
2.4. Histochemical localization of spleen macrophages
According to standard protocols, 5-µm thick spleen paraffin sections were prepared after fixation in buffered-formalin (Adam and Caihak, 1964). CD68 was detected immunohistochemically in the paraffin-embedded spleen sections as a marker of macrophages (Yu et al., 2013). In brief, after deparaffinization and rehydration, the sections were placed in a 10 mM citrate buffer solution (pH 6.0) for antigen retrieval. Sections were treated with 3% hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) for 5 min to quench the endogenous peroxidase activity before being washed in PBS. Then, the sections were blocked with 10% normal goat serum for 10 min at 37 °C and incubated overnight at 4 °C with primary antibody or an equivalent amount of normal goat IgG as a negative control. The sections were then treated with an avidin-biotin affinity system for 30 min at 25 °C room temperature, washed, stained with the 3-3/diaminobenzidine substrate, counterstained with hematoxylin, and examined under a light microscope. The activated marker of cluster of differentiation 68 (CD68) was quantified as the number of positively stained cells per high-power field.
2.5. Phagocytic activity
Mice were anesthetized with diethyl ether before 100 µL PBS containing 2.9 × 108 green fluorescent beads (Duke Scientific, Palo Alto, CA, USA) was injected intravenously (i.v.). After 5 min, the mice were euthanized, and the spleen tissue was processed for immunofluorescence microscopy using the cryostat (CM1950; Leica, Wetzlar, Germany). Sections without counterstaining were used for quantification of trapping (Nolte et al., 2003). Signal intensity was evaluated using the ImageJ software (Krücken et al., 2005).
2.6. Total antioxidant capacity
Parts of the spleen were weighed and homogenized immediately to obtain a 50% (w/v) homogenate in ice-cold medium containing 50 mM Tris-hydrochloride (HCl) and 300 mM sucrose (Tsakiris et al., 2004). The homogenate was centrifuged at 500g for 10 min at 4 °C. The supernatant (10%) was used to determine the total antioxidant capacity (Koracevic et al., 2001). In brief, the ability of antioxidants in the test sample to hydrolyze exogenous H2O2 after the prespecified reaction time is determined based on the remaining H2O2 analyzed colorimetrically using an enzymatic reaction. The reaction involves the conversion of 3,5,di-2-hydroxy benzene sulfate substrate into a colored product, and the color intensity is inversely proportional to the total antioxidant amount in the original sample.
2.7. Statistical analysis
A one-way analysis of variance (ANOVA) was carried out, and the statistical comparisons among the groups were performed with the Duncan's t-test using the statistical package for the social sciences (SPSS) program (version 17.0). A P value <0.01 was considered significant for all statistical analysis in this study.
3. Results
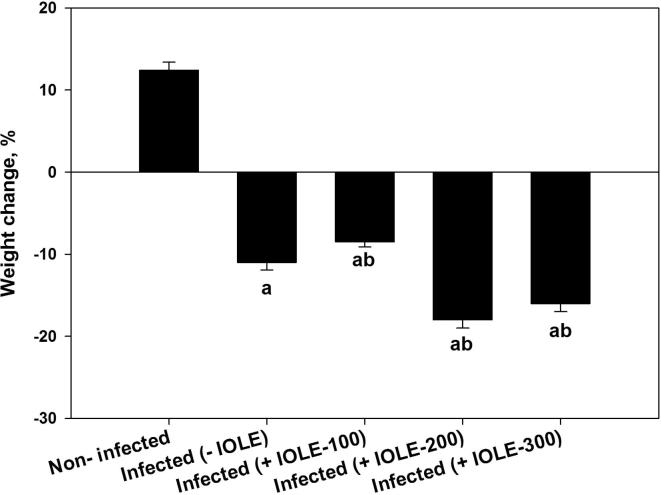
The IOLE inhibited the weight loss induced by P. chabaudi infection significantly (P ≤ 0.01) only when administered at a dose of 100 mg/kg (Fig. 1). The mice in the other two groups treated with 200 or 300 mg/kg showed a significant reduction in weight (Fig. 1). Our previous studies indicated that IOLE could significantly reduce the increased parasitemia during infection (Lubbad et al., 2015) from about 40% to 10%.
Fig. 1.
I. oblongifolia-induced weight changes in mice due to infection with P. chabaudi on day 7 postinfection (p.i.). Values are means ± standard deviation (SD). a P < 0.05 and b P < 0.01 compared with non-infected and infected (-IOLE) group, respectively.
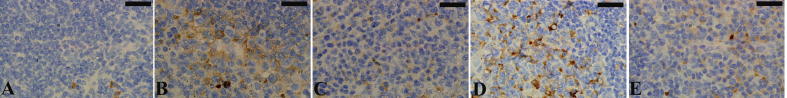
The results of the anti-CD68 antibody method revealed that the spleen of mouse injected with P. chabaudi infected erythrocytes showed a positive affinity to the reaction. The number of CD68-positive cells increased considerably in the infected spleens (Fig. 2, Fig. 3) compared with the normal non-infected spleens (Fig. 2, Fig. 3).
Fig. 2.
Spleen sections labeled with macrophage detecting anti-cluster of differentiation 68 (CD68) antibody. CD68-positive cells appeared brown. (A) Uninfected spleen. (B) Infected spleen. Infected I. oblongifolia-treated (C) 100, (D) 200, and (E) 300 mg/kg spleens. Scale bar = 25 µm.
Fig. 3.
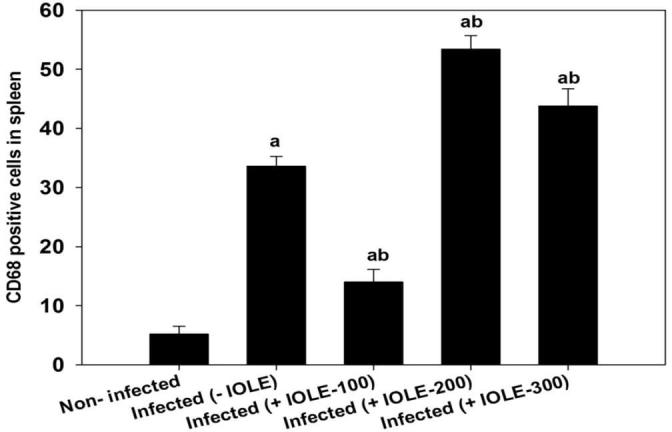
I. oblongifolia-induced changes in number of cluster of differentiation 68 (CD68)-positive cells in the spleen of mouse infected with P. chabaudi on day 7 postinfection (p.i.). Results are total positive numbers from 10 random and continuous fields from each section. Values are means ± standard deviation (SD). a P < 0.01 and b P < 0.01 compared with non-infected and infected (-IOLE) groups, respectively.
The IOLE reduced the number of CD68-positive cells in the spleens of mice treated with a 100 mg/kg dose (Fig. 2, Fig. 3). However, the spleen of mice treated with 200 or 300 mg/kg IOLE showed no clear difference in affinity for labeling with the macrophage marker in the anti-CD68 antibody detection method compared to the infected group. Fig. 3 shows the statistical evaluation of the CD68-positive cells in the spleen tissues from all the experimental groups. This analysis clearly shows that IOLE (100 mg/kg) significantly reduced the number of macrophages to about 50% compared to that of the infected mice spleens (Fig. 3). Treatment of the infected mice with IOLE with the two doses, 200 and 300 mg/kg, induced a significant increase in the number of CD68-positive cells (Fig. 3).
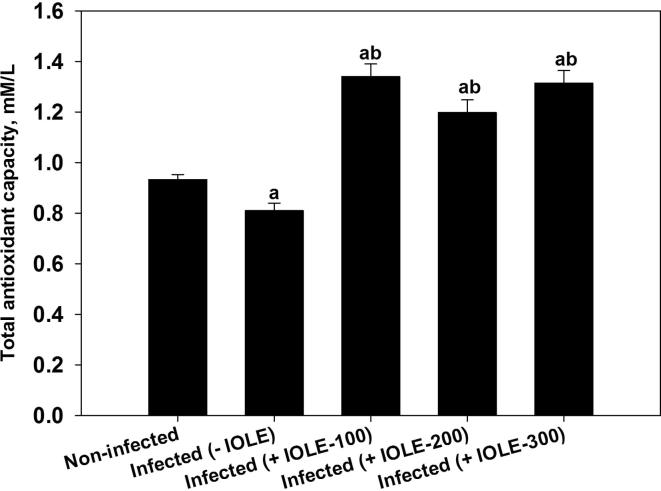
Fig. 4 shows the antioxidant capacity of IOLE in spleens of mice infected with P. chabaudi. The 100 mg/kg dose showed the most effective antioxidant effect.
Fig. 4.
Changes in total antioxidant capacity of the spleens of mice infected with P. chabaudi and after treatment with I. oblongifolia leaf extracts. Values are means ± standard deviation (SD). a P < 0.01 and b P < 0.01 compared with non-infected and infected (-IOLE) groups, respectively.
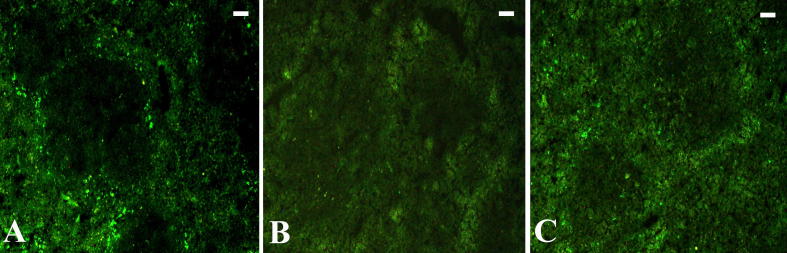
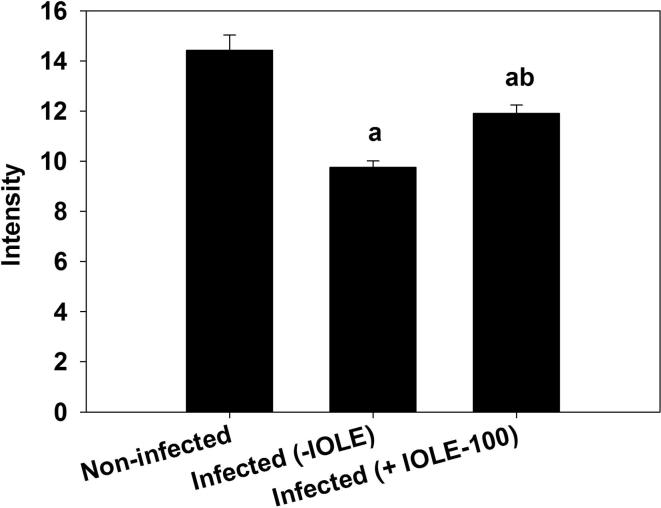
The phagocytic activity of the spleen macrophages was upregulated during the infection as deduced from the reduced amount of fluorescent particles detected in the spleen sections (Fig. 5). Treatment of mice with I. oblongifolia resulted in an increase in the fluorescent intensity (Fig. 5, Fig. 6) of the spleen sections.
Fig. 5.
I. oblongifolia regulates spleen phagocytosis during P. chabaudi infection (A) Uninfected spleen with more fluorescent particles. (B) Infected spleen with reduced fluorescent particles. (C) Infected I. oblongifolia-treated (100 mg/kg) spleens with increased fluorescent particles. Scale bar = 100 µm.
Fig. 6.
Semiquantitative evaluation of fluorescence intensity for 5 cryosections per mouse. Values are means ± standard deviation (SD). a P < 0.01 and b P < 0.01 compared with non-infected and infected (-IOLE) groups, respectively.
4. Discussion
The suppression of P. chabaudi parasitemia by I. oblongifolia is attributed to the presence of active components such as quinines that are known to be effective against malaria (Shahjahan et al., 2005).
The spleen red pulp macrophages are the first line defense against parasitized erythrocytes in the blood stage of malaria when they can phagocytose and remove infected erythrocytes (Engwerda et al., 2005).
The increased intensity of fluorescent particles in spleen white and red pulp of the mice indicates a reduction of the phagocytic activity of the spleen macrophages (Krücken et al., 2009). I. oblongifolia significantly maintained the number of spleen macrophage compared to that of the control group. Dkhil et al. (2015) investigated the effect of I. oblongifolia in mice infected with P. chabaudi and concluded that it regulated the immune responses of mice during the infection.
The total antioxidant activity of mouse spleens was increased after treatment of the infected mice with I. oblongifolia. This was due to the ameliorative effect of the extract on the induced oxidative damage in the spleen cells (Dkhil et al., 2015). IOLE was found to be effective in maintaining the spleen index at the normal value (Dkhil et al., 2015).
Recent studies have investigated the oxidative stress-related pathology in mice suffering from malaria (Guha et al., 2006, Lubbad et al., 2015, Hafiz et al., 2016, Mubaraki et al., 2017). Reactive oxygen species (ROS) are generated because of oxidative stress, and the infection induces the generation of free radicles (Guha et al., 2006). In this study, the antioxidant activity of I. oblongifolia-treated group was higher than that of the infected and the non-infected groups. Dkhil et al. (2016) investigated the antioxidant activity of I. oblongifolia on the damage induced by lead in both the kidneys and testes of mice. In addition, Lubbad et al. (2015) reported the antioxidant activity of I. oblongifolia in mouse spleens infected with P. chabaudi by studying the regulatory role of nitric oxide, malondialdehyde, and catalase.
The immunohistochemical localization of spleen macrophages after treatment of the P. chabaudi-infected mice with I. oblongifolia was altered. Regulation of the spleen immune response resulted in this alteration (Krücken et al., 2005). During the infection, a disorganization in distribution of spleen macrophages was observed due to the disorganization of the red and white pulps in mouse spleen (Dkhil, 2009). In addition, the number of spleen macrophages was reported to be decreased on day 8 postinfection with P. chabaudi (Krücken et al., 2005).
Collectively, the macrophage response in the mice inoculated with P. chabaudi-infected erythrocytes could be regulated by administration of IOLE. Further studies are required to investigate the role of other cell types during infection.
Acknowledgment
The authors would like to appreciate the Deanship of Scientific Research at King Saud University for funding this study through the research group project No. RG-198.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adam H., Caihak G. Fischer Verlag Stuttgart; Grosses zoologisches parktikum tell: 1964. Arbeitsmethoden der makroskopischen und mikroskopischen anatomic Mit 283 Abbildungen Gustav. [Google Scholar]
- Al-Quraishy S., Dkhil M.A., Ibrahim S.R., Moneim A.E.A. Neuroprotective potential of Indigofera oblongifolia leaf methanolic extract against lead acetate-induced neurotoxicity. Neural Regen. Res. 2016;11(11):1797–1803. doi: 10.4103/1673-5374.194749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A., Al-Khalifa M.S., Al-Quraishy S., Zrieq R., Moneim A.E.A. Indigofera oblongifolia mitigates lead-acetate-induced kidney damage and apoptosis in a rat model. Drug Des Devel. Ther. 2016;10:1847–1856. doi: 10.2147/DDDT.S105511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A., Lubbad M.Y., Al-Shaebi E.M., Delic D., Al-Quraishy S. The antiplasmodial and spleen protective role of crude Indigofera oblongifolia leaf extract traditionally used in the treatment of malaria in Saudi Arabia. Drug Des Devel Ther. 2015;9:6235–6246. doi: 10.2147/DDDT.S94673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A.E. Apoptotic changes induced in mice splenic tissue due to malaria infection. J. Microbiol. Immunol. Infect. 2009;42(1):13–18. [PubMed] [Google Scholar]
- Engwerda C.R., Beattie L., Amante F.H. The importance of the spleen in malaria. Trends Parasitol. 2005;21(2):75–80. doi: 10.1016/j.pt.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Guha M., Kumar S., Choubey V., Maity P., Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20(8):1224–1226. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- Hafiz T.A., Mubaraki M.A., Al-Quraishy S., Dkhil M.A. The potential role of Punica granatum treatment on murine malaria-induced hepatic injury and oxidative stress. Parasitol. Res. 2016;115(4):1427–1433. doi: 10.1007/s00436-015-4876-2. [DOI] [PubMed] [Google Scholar]
- Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54(5):356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krücken J., Delic D., Pauen H., Wojtalla A., El-Khadragy M., Dkhil M.A., Mossmann H., Wunderlich F. Augmented particle trapping and attenuated inflammation in the liver by protective vaccination against Plasmodium chabaudi malaria. Malaria J. 2009;8:54. doi: 10.1186/1475-2875-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krücken J., Mehnert L.I., Dkhil M.A., El-Khadragy M., Benten W.P.M., Mossmann H., Wunderlich F. Massive destruction of malaria-parasitized red blood cells despite spleen closure. Infect. Immun. 2005;73(10):6390–6398. doi: 10.1128/IAI.73.10.6390-6398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbad M.Y., Al-Quraishy S., Dkhil M.A. Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitol. Res. 2015;114(9):3431–3438. doi: 10.1007/s00436-015-4568-y. [DOI] [PubMed] [Google Scholar]
- Mubaraki M.A., Hafiz T.A., Al-Quraishy S., Dkhil M.A. Oxidative stress and genes regulation of cerebral malaria upon Zizyphus spina-christi treatment in a murine model. Microb. Pathog. 2017;107:69–74. doi: 10.1016/j.micpath.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Nolte C., Amores A., Kovacs E.N., Postlethwait J., Featherstone M. The role of a retinoic acid response element in establishing the anterior neural expression border of Hoxd4 transgenes. Mech. Dev. 2003;120(3):325–335. doi: 10.1016/s0925-4773(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Shahjahan M., Vani G., Devi C.S. Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J. Med. Food. 2005;8(2):261–265. doi: 10.1089/jmf.2005.8.261. [DOI] [PubMed] [Google Scholar]
- Tsakiris S., Schulpis K.H., Marinou K., Behrakis P. Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol. Res. 2004;49(5):475–479. doi: 10.1016/j.phrs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization. WHO Document Production Services; Geneva, Switzerland: 2016. World Malaria Report.<http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/> [Google Scholar]
- Wunderlich F., Stubig H., Konigk E. Development of Plasmodium chabaudi in mouse red blood cells: structural properties of the host and parasite membranes. J. Protozool. 1982;29(1):60–66. doi: 10.1111/j.1550-7408.1982.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Yu Y., Li Y., Wang W., Jin M.H., Du Z.J., Li Y.B., Duan J.C., Yu Y.B., Sun Z.W. Acute toxicity of amorphous silica nanoparticles in intravenously exposed ICR mice. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061346. [DOI] [PMC free article] [PubMed] [Google Scholar]