Introduction
The clinical benefits of implantable cardioverter-defibrillator (ICD) therapy have been widely documented in clinical studies of selected patient populations.1 Subcutaneous ICD (S-ICD) is a valid alternative to transvenous ICD in patients who do not require cardiac resynchronization or antibradycardia or antitachycardia pacing,2 and has the additional advantage of avoiding possible acute and chronic complications secondary to transvenous lead implantation. Young patients facing multiple procedures (device replacement, lead revisions) are at higher risk of these complications, which makes them optimal candidates for an S-ICD.2, 3
One of the most frequent adverse effects associated with ICD is the delivery of inappropriate shocks,4, 5, 6, 7 which, in patients implanted with an S-ICD, are mainly secondary to oversensing and are often resolved by reprogramming the device.6 However, in rare cases this noninvasive approach is unable to eliminate inappropriate therapy and the device has to be replaced by a transvenous ICD, thus leading to the risk of transvenous lead–related morbidity.3
We here describe the case of a young patient diagnosed with catecholaminergic polymorphic ventricular tachycardia (CPVT) and implanted with an S-ICD, who received an inappropriate shock secondary to the oversensing of myopotential noise, which persisted despite the changes made to the device settings. Repositioning the can, which increased the amplitude of the sensed R waves and the R wave–to-noise ratio, proved to be effective in eliminating the myopotential oversensing and avoiding the need for a transvenous ICD.
Case report
In March 2016, a 28-year-old man with a clinical and molecular diagnosis of CPVT underwent the implantation of an S-ICD (A209 Emblem S-ICD, Boston Scientific, Natick, MA) for primary prevention of sudden cardiac death. Surface electrocardiographic screening before implantation showed that 2 of the 3 sensing vectors were appropriate. Low R wave signals were found in all of the sensing configurations after various lead positions had been tested in the left and right parasternal areas, but, as the lower voltage limit of the R wave was within the acceptable range in the right parasternal position, the generator was placed in a submuscular pocket on the left side of the chest (Figure 1A) and the lead in the right parasternal area. A secondary sensing vector was automatically chosen, and the programmed gain setting was 1×. A defibrillation threshold test at 65 J was successful, and conditional (230 beats/min) and shock-only zones (250 beats/min) were programmed.
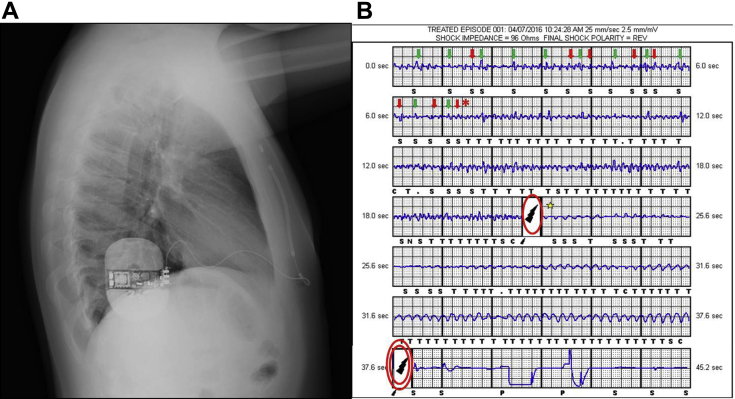
Figure 1.
A: Lateral chest radiograph after subcutaneous implantable cardioverter-defibrillator (S-ICD) implantation. B: Electrogram of S-ICD shocks. Noise from myopotential signals (red arrows) with a similar amplitude to that of QRS complexes at a heart rate of ∼90 beats/min (green arrows) leads to the incorrect detection of tachycardia (asterisk) and an inappropriate shock (single circle) that triggers ventricular fibrillation (star), which is appropriately sensed and treated by means of a second S-ICD shock (double circle).
Three weeks after implantation, the patient attended our Emergency Room because of the delivery of 2 shocks. At the time of the first shock, which was not preceded by any symptoms, he was having intercourse, supporting all of his body weight on his left arm. After the shock, he felt dizzy and quickly lost consciousness, but he soon recovered after receiving a second shock. He reported that he had not been fully compliant with beta-blocker therapy after the implantation of the device.
Interrogation of the S-ICD system (Figure 1B) showed a very low-voltage QRS complex with concomitant noise of the same voltage. This led to oversensing and the inappropriate shock, which was not synchronized with the R wave, thus causing ventricular fibrillation that was appropriately sensed and treated by the device, delivering the second shock (65 J). The patient was kept in hospital and regularly administered full-dose pharmacologic therapy and the device was reprogrammed to off-mode.
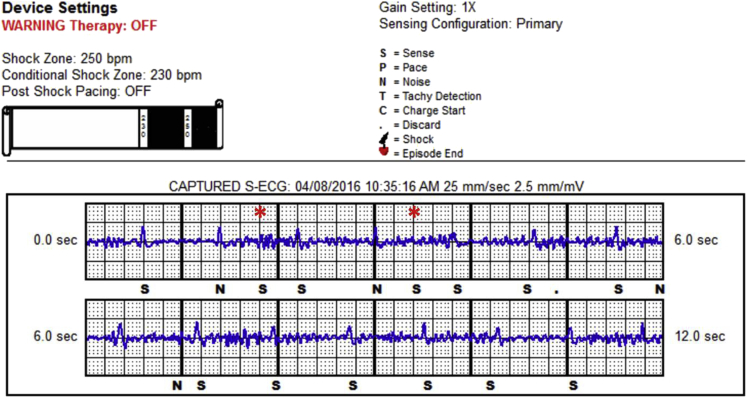
When the patient was asked to perform maneuvers resembling those that had led to the oversensing, the myopotential noise was identical to that occurring at the time of the inappropriate shock (Figure 2). Changing all of the programming vectors and the gain did not increase the magnitude of the R wave signal and so, after a careful signal analysis by the manufacturer’s technical service, it was decided to change the device pocket. Guided by the amplitude of the R wave, various attempts were made a few centimeters below and above the initial position until a final constant amplitude of >1 mV led to an acceptable R wave–to-noise ratio.
Figure 2.
Device interrogation before system repositioning. The primary vector (1× gain) showed less oversensing but an unstable R-wave amplitude owing to postural movements (intermittently <1 mV). Maneuvers resembling the exercise that caused the inappropriate shock lead to intermittent oversensing (asterisks). Small R-wave amplitudes were also observed in alternate and secondary vectors.
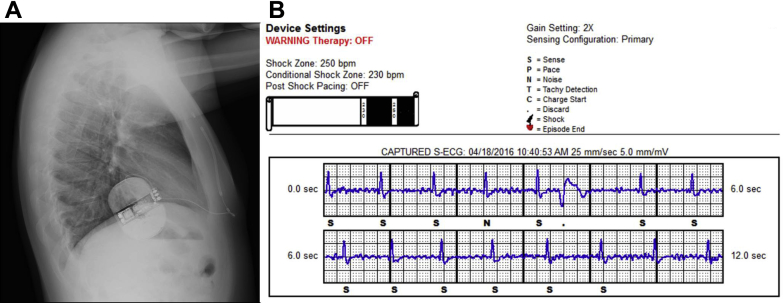
After the repositioning of the can (Figure 3A), an additional defibrillation threshold test proved to be successful (65 J). The myopotential noise related to provocative maneuvers during muscular activity was not oversensed and was appropriately classified by the device as noise in the primary vector using a programmed 2× gain so as to provide a better signal and improve noise identification (Figure 3B).
Figure 3.
A: Lateral chest radiograph after system repositioning (can relocation only). B: Device interrogation repeating the maneuvers in the primary vector (gain setting 2×) did not show oversensing; R-wave amplitude remained constantly >1 mV even with postural changes; and there was better 1:1 beat classification (a premature ventricular contraction was also correctly discarded; see the “•” marker).
Three weeks after repositioning of the device, SMART Pass technology became available as a result of a software upgrade and was activated in the patient’s S-ICD. The patient has remained free of inappropriate shocks and without any local complication or device displacement for the last 15 months.
Discussion
An S-ICD is an effective alternative to a transvenous ICD in patients at high risk of sudden cardiac death who do not require antitachycardia or antibradycardia pacing or resynchronization therapy because it avoids lead-related comorbidities such as pneumothorax, bleeding, hematomas, cardiac tamponade, pericarditis, or endocarditis. This is particularly important in the case of young patients, who will require numerous device replacements and are also at higher risk of chronic lead complications.2, 3
Both transvenous ICDs and S-ICDs can lead to similar rates of inappropriate shocks (4%–18% vs 7%),3, 6, 7, 8 but the main reasons for them are different: supraventricular arrhythmias in the case of transvenous ICDs, and a low signal amplitude leading to T-wave or myopotential oversensing in the case of S-ICDs. Although S-ICDs have a number of algorithms for accurately discriminating cardiac signals from external noise or noncardiac signals, this case highlights the fact that a very low ratio between R waves and noise signals may give rise to inappropriate shocks.
According to the literature, most cases of inappropriate shocks delivered by S-ICDs can be easily resolved by reprogramming the device settings: that is, programming a dual-therapy zone with supraventricular tachycardia–discriminating algorithms, programming the conditional shock zone for heart rates of >220 beats/min, or changing the sensing vector.3, 9 Unfortunately, in our case a noninvasive option was not feasible because of the limited range of the starting signal owing to the low R wave–to-noise ratio, despite the successful preimplant screening evaluation. Repositioning the device increased the R wave signal, which generally improves the ratio between the signal and myopotential noise and reduces the risk of a rapidly decaying detection algorithm that leads to the detection of noise floor potential oversensing and inappropriate shocks. Although invasive, this approach allowed us to solve the problem of inappropriate shocks and avoid the need to implant a transvenous ICD in a very young patient.
As in any case of CVPT, it is important to stress the essential role of beta-blockers as first-line therapy and the fact that noncompliance with pharmacologic treatment puts patients at risk of developing severe arrhythmias or arrhythmic storm, especially after an appropriate or inappropriate ICD shock.10
Conclusions
This case underlines the importance of thorough preoperative electrocardiographic screening to test different lead or device positions in candidates for an S-ICD whose measured sensing values are at the limits of acceptability, including a maximized R wave. We found that increasing R-wave amplitude by repositioning the device can be a valuable option in the case of oversensing, even in the continued presence of noise. Finally, every effort should be made to help CPVT patients understand the importance of complying with beta-blocker therapy so as to avoid severe arrhythmias and ICD shocks.
Key Teaching Points.
-
•
Although subcutaneous implantable cardioverter-defibrillators (S-ICDs) have various algorithms for accurately discriminating cardiac signals from external noise or noncardiac signals, a very low ratio between R waves and noise signals may expose patients to the potential risk of inappropriate shocks.
-
•
Thorough preoperative electrocardiographic screening before implanting an S-ICD device (including the maximization of R waves and testing different lead or device positions) is of the utmost importance, particularly in patients whose measured sensing values are at the limits of acceptability.
-
•
Increasing R-wave amplitude by repositioning the device can be a valuable option in the case of oversensing, even in the continued presence of noise.
Acknowledgments
The authors thank Stefano Accinelli, BSc, and Maurizio Malacrida, MSc, for their helpful discussions of this manuscript.
Footnotes
This study was supported by the project “Epidemiologia e genetica della morte improvvisa cardiaca in Sardegna e correlazione con le canalopatie. Codice CRP- 6175. Regione Autonoma della Sardegna.”
References
- 1.Hohnloser S.H., Israel C.W. Current evidence base for use of the implantable cardioverter-defibrillator. Circulation. 2013;128:172. doi: 10.1161/CIRCULATIONAHA.112.000547. [DOI] [PubMed] [Google Scholar]
- 2.Priori S.G., Blomström-Lundqvist C., Mazzanti A. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2796–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 3.Lambiase P.D., Barr C., Theuns D.A., EFFORTLESS Investigators Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Cisnal A., Arce-León A., Arana-Rueda E. Analyses of inappropriate shocks in a Spanish ICD primary prevention population: predictors and prognoses. Int J Cardiol. 2015;195:188–194. doi: 10.1016/j.ijcard.2015.05.146. [DOI] [PubMed] [Google Scholar]
- 5.Gilliam F.R., Hayes D.L., Boehmer J.P., Day J., Heidenreich P.A., Seth M., Jones P.W., Stein K.M., Saxon L.A. Real world evaluation of dual-zone ICD and CRT-D programming compared to single-zone programming: the ALTITUDE REDUCES study. J Cardiovasc Electrophysiol. 2011;22:1023–1029. doi: 10.1111/j.1540-8167.2011.02086.x. [DOI] [PubMed] [Google Scholar]
- 6.Powell B.D., Asirvatham S.J., Perschbacher D.L., Jones P.W., Cha Y.M., Cesario D.A., Cao M., Gilliam F.R., 3rd, Saxon L.A. Noise, artifact, and oversensing related inappropriate ICD shock evaluation: ALTITUDE noise study. Pacing Clin Electrophysiol. 2012;35:863–869. doi: 10.1111/j.1540-8159.2012.03407.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarkozy A., Boussy T., Kourgiannides G., Chierchia G.B., Richter S., De Potter T., Geelen P., Wellens F., Spreeuwenberg M.D., Brugada P. Long-term follow-up of primary prophylactic implantable cardioverter-defibrillator therapy in Brugada syndrome. Eur Heart J. 2007;28:334–344. doi: 10.1093/eurheartj/ehl450. [DOI] [PubMed] [Google Scholar]
- 8.Olde Nordkamp L.R., Brower T.F., Barr C., Theuns D.A., Boersma L.V., Johansen J.B., Neuzil P., Wilde A.A., Carter N., Husby M., Lambiase P.D., Knops R.E. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135. [DOI] [PubMed] [Google Scholar]
- 9.Corzani A., Ziacchi M., Biffi M., Diemberger I., Martignani C., Boriani G. Inappropriate shock for myopotential over-sensing in a patient with subcutaneous ICD. Indian Heart J. 2015;67:56–59. doi: 10.1016/j.ihj.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roses-Noguer F., Jarman J.W., Claque J.R., Till J. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2014;11:58–66. doi: 10.1016/j.hrthm.2013.10.027. [DOI] [PubMed] [Google Scholar]