Introduction
Patients with congenital heart disease are at ongoing risk of developing both bradyarrhythmias and tachyarrhythmias decades after surgical repair. Rarely, arrhythmias can be exacerbated during pregnancy and require emergent intervention. Here, we report unique experience with nonfluoroscopic pacemaker implantation during pregnancy. Ionizing radiation, even in low doses, is associated with an increased risk of malignancy, and a fetus may be at particularly increased risk.1, 2 Over the past 2 decades, the use of fluoroscopy in cardiac ablation procedures has become nearly obsolete with the development of 3-dimensional (3D) electroanatomic mapping software such as CARTO (Biosense-Webster, Diamond Bar, CA) and NavX or EnSite (St. Jude Medical, Inc., St. Paul, MN).3 However, certain procedures, such as device implants, still commonly use fluoroscopy in most instances.2 Fluoroscopy use in patients with congenital heart disease is of utmost concern because of cumulative radiation exposure from multiple lifetime catheterization, radiographic and computed tomography imaging, and electrophysiological procedures.
Case report
The patient is a 29-year-old woman born with tetralogy of Fallot and underwent transannular patch repair at 2 years of age with no subsequent surgical or catheter-based interventions. Additional medical history includes a seizure disorder that was controlled using levetiracetam; she has been free of seizures for 3 years and discontinued levetiracetam last year. She presented to the adult congenital heart disease clinic after an episode of syncope during work in the context of her first trimester of pregnancy. Her pregnancy has been uncomplicated except for mild nausea and vomiting. Before the syncopal event, she had a history of palpitations with exertion.
An electrocardiogram demonstrated normal sinus rhythm with complete right bundle branch block (QRS duration 120 ms) and a corrected QT interval of 450 ms. An echocardiogram demonstrated free pulmonary insufficiency, a severely dilated right ventricle (RV), and normal biventricular systolic function. Cardiac magnetic resonance imaging showed an RV end-diastolic volume of 261 mL (155 mL/m2) and an RV ejection fraction of 40%, both unchanged compared to a cardiac magnetic resonance imaging 3 years ago. A ZIO Patch event monitor (iRhythm Technologies, Inc., San Francisco, CA) was placed because of complaints of dizziness. Her syncopal event was captured on the event monitor, which documented 7-second sinus arrest. She was diagnosed with sick sinus syndrome and agreed to the placement of a transvenous pacemaker.
Procedural summary
The patient was brought to the electrophysiology suite and sedated with midazolam and propofol by cardiac anesthesia; the decision to avoid intubation or general anesthesia was made by the interdisciplinary team. Her left pectoral region was prepped and draped in the usual sterile fashion. Left subclavian venous access was obtained using anatomical landmarks without the aid of fluoroscopic guidance. A 9-F tear-away sheath was inserted into the left subclavian vein using the modified Seldinger technique. A deflectable mapping catheter (Biosense Webster, Inc., Diamond Bar, CA) was then advanced and used to create 3D electroanatomic maps of the right atrium, RV, and coronary sinus (Figure 1). After detailed intracardiac anatomy was defined, the ablation catheter was removed. A transvenous pacemaker lead (CapsureFix model 4076, Medtronic, Inc., Minneapolis, MN) was connected via alligator clips to the CARTO-3 system, allowing for the visualization of the lead tip on the electroanatomic map. The lead was maneuvered toward the RV apex without the need for fluoroscopic guidance (Figure 2A and Supplemental Video 1).
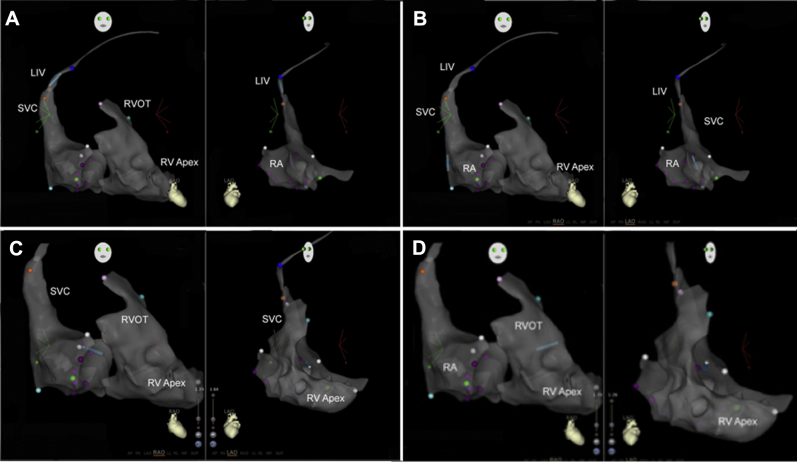
Figure 1.
Series of catheter manipulations using the CARTO-3 3-dimensional electroanatomic mapping software in the right anterior oblique and left anterior oblique views. A: The lead begins in the innominate vein near the superior vena cava–right atrium (SVC-RA) junction. B: The lead is advanced into the RA, and the soft stylet is exchanged for a curved stylet to facilitate crossing the tricuspid valve. C: The lead is visualized crossing the tricuspid valve. D: The lead advances to the right ventricular outflow tract (RVOT), which is confirmed on CARTO-3 imaging in addition to the induction of premature ventricular complexes. LIV = left innominate vein; RV = right ventricle.
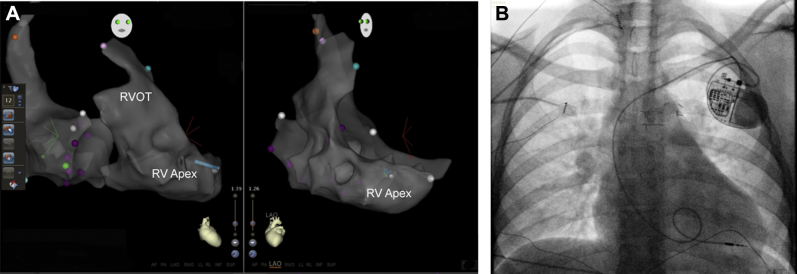
Figure 2.
A: Right anterior oblique and left anterior oblique images obtained using the CARTO-3 3-dimensional electroanatomic mapping software, demonstrating final catheter placement for lead placement. B: Fluoroscopic flash image 0.5 seconds, confirming lead position and adequate slack. A loop is seen, and the tip appears to be at the right ventricular apex. RV = right ventricle; RVOT = right ventricular outflow tract.
After proper positioning, the lead was fixated to the ventricular endocardium and tension placed on the lead to ensure that it was properly engaged while monitoring the lead tip on CARTO. A deep breath was given to confirm adequate lead tip stability. Sensing and pacing thresholds were excellent, with normal impedance and lack of diaphragmatic stimulation with high-output testing. The lead was connected to a generator and placed in a left prepectoral pocket; device-based testing confirmed normal electrical parameters and lead stability. With lead aprons under and over the patient’s abdomen, <1 second of fluoroscopy was used to document proper location of the pacemaker lead with sufficient slack for patient movement (Figure 2B). In retrospect, this was probably unnecessary but an extra precaution since it is a novel technique for lead implantation. The patient had an uncomplicated procedure and recovery.
Discussion
Fluoroscopy typically is used for the placement of a transvenous pacing system; we were able to perform nonfluoroscopic mapping to avoid patient and fetal exposure to ionizing radiation. There are previous reports of device implantation without fluoroscopy, using either transesophageal echocardiography or 3D electroanatomic mapping.4, 5, 6, 7 However, none of these were performed on a pregnant patient with repaired congenital heart disease, although this has been suggested.8 This successful and uncomplicated procedure is encouraging.
A single-chamber device was chosen to decrease procedure time, simplify the procedure in a pregnant patient with infrequent symptomatic events and predominantly intact atrioventricular conduction, and reduce the risk of complications from the implantation and fixation of an additional lead.
Conclusion
We present a unique report of a nonfluoroscopic transvenous pacemaker placement in a pregnant patient with repaired congenital heart disease. The use of electroanatomic mapping allowed for the visualization of the pacemaker lead to ensure proper location in the RV apex before and after endocardial fixation. While further experience is required before widespread use in patients with congenital heart disease, fluoroscopy-free pacemaker implantation using electroanatomic mapping may follow the course of catheter ablation procedures and transition from a safe and effective case-reportable option to eventually becoming the standard of care.
Key Teaching Points.
-
•
Three-dimensional electroanatomic mapping software can be used to place transvenous pacing systems in patients with complex congenital heart disease, negating the need for ionizing radiation.
-
•
Ionizing radiation should be avoided during pregnancy because of the increased risk to the developing fetus.
-
•
Three-dimensional electroanatomic mapping software clearly has benefits beyond electrophysiology studies and ablation.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.hrcr.2017.07.020
Appendix. Supplementary data
The video loop demonstrates the visualization of the pacing electrode on the electroanatomic map. The bipolar lead is attached to the recording system using alligator clips. The pacing lead can be seen traversing the subclavian vein to superior vena cava and then entering the right atrium, coursing across the tricuspid valve into the right ventricular outflow tract before a straight stylet is placed to reposition the tip closer to the right ventricular apical septum.
References
- 1.Doll R., Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 2.Perisinakis K., Damilakis J., Theocharopoulos N., Manios E., Vardas P., Gourtsoyiannis N. Accurate assessment of patient effective radiation dose and associated detriment risk from radiofrequency catheter ablation procedures. Circulation. 2001;104:58–62. doi: 10.1161/hc2601.091710. [DOI] [PubMed] [Google Scholar]
- 3.Clark B.C., Sumihara K., McCarter R., Berul C.I., Moak J.P. Getting to zero: impact of electroanatomical mapping on fluoroscopy use in pediatric catheter ablation. J Interv Card Electrophysiol. 2016;46:183–189. doi: 10.1007/s10840-016-0099-4. [DOI] [PubMed] [Google Scholar]
- 4.Castrejon-Castrejon S., Perez-Silva A., Gonzalez-Villegas E., Al-Razzo O., Silvestre J., Doiny D., Estrada-Mucci A., Filgueiras-Rama D., Ortega-Molina M., Lopez-Sendon J.L., Merino J.L. Implantation of cardioverter defibrillators with minimal fluoroscopy using a three-dimensional navigation system: a feasibility study. Europace. 2013;15:1763–1770. doi: 10.1093/europace/eut127. [DOI] [PubMed] [Google Scholar]
- 5.Choudhuri I., Krum D., Hare J., Kelley J., Becker D., Mortada M.E., Akhtar M., Sra J. Pacing lead implantation without live fluoroscopy: feasibility of acute success in the live canine model. J Cardiovasc Electrophysiol. 2009;20:916–922. doi: 10.1111/j.1540-8167.2009.01451.x. [DOI] [PubMed] [Google Scholar]
- 6.Merino J.L., Peinado R., Silvestre J. Dual-chamber implantable cardioverter defibrillator implantation guided by non-fluoroscopic electro-anatomical navigation. Europace. 2008;10:1124–1125. doi: 10.1093/europace/eun175. [DOI] [PubMed] [Google Scholar]
- 7.Colella A., Giaccardi M., Colella T., Modesti P.A. Zero x-ray cardiac resynchronization therapy device implantation guided by a nonfluoroscopic mapping system: a pilot study. Heart Rhythm. 2016;13:1481–1488. doi: 10.1016/j.hrthm.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Ito S., Stout K.K., Robinson M.R. Non-sustained ventricular tachycardia in a pregnant woman with repaired tetralogy of Fallot: a challenging case. Int J Cardiol. 2016;203:1133–1134. doi: 10.1016/j.ijcard.2015.09.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video loop demonstrates the visualization of the pacing electrode on the electroanatomic map. The bipolar lead is attached to the recording system using alligator clips. The pacing lead can be seen traversing the subclavian vein to superior vena cava and then entering the right atrium, coursing across the tricuspid valve into the right ventricular outflow tract before a straight stylet is placed to reposition the tip closer to the right ventricular apical septum.