Key Teaching Points.
-
•
Electrical storms in Brugada syndrome (BrS) can be recurrent and life threatening.
-
•
Quinidine, the drug of choice for treatment and prevention of recurrent ventricular fibrillation (VF) in BrS, is not available in many countries around the world.
-
•
Oral quinine sulfate is effective in management of electrical storms and recurrent VF in BrS patients.
-
•
The common neurocognitive side effects of quinine is easily managed by a diet containing high levels of tryptophan.
-
•
In the present case, cilostazole was not effective in the prevention of recurrent ventricular arrhythmias.
-
•
Oral quinine sulfate is an effective alternative to quinidine for the treatment of lethal ventricular arrhythmias in BrS patients in countries where quinidine is not available.
Introduction
Brugada syndrome (BrS), an inherited ion channelopathy with an autosomal mode of inheritance, is associated with an increased risk of ventricular fibrillation (VF) and sudden death in the young. Implantable cardioverter-defibrillator (ICD) is effective in terminating VF and preventing sudden cardiac arrest in high-risk BrS patients but does not prevent recurrences of VF.1 An electrical storm occurs in 5% of asymptomatic BrS patients and in 45% of patients who have survived sudden cardiac arrest.2 Isoproterenol infusion is effective in suppressing VF episodes during electrical storms in BrS patients.3 Quinidine, a class IA drug, is effective in preventing recurrences of ventricular tachycardia (VT) and VF in a patient with BrS.4 However, quinidine is not available in most countries around the world, especially in Southeast Asia, where the disease is more prevalent.5 We present a case of BrS with an electrical storm while on therapy with oral cilostazol and discuss the management with oral quinine sulfate.
Case report
A 54-year-old man presented to the emergency room with 10 ICD shocks over a 1-hour period. Interrogation of the ICD revealed 10 episodes of VF terminated by appropriate shocks within 1 hour (Figure 1A and B). A single-chamber ICD had been implanted in 2013 for BrS and recurrent syncope. The first episode of an electrical storm with 18 shocks occurred 4 months after the ICD implantation and was managed successfully with intravenous isoprenaline. The patient was discharged on oral cilostazol 100 mg twice daily to prevent recurrent shocks because of nonavailability of quinidine. Before the present episode, while on cilostazol 100 mg twice daily, he had 1 electrical storm, 1 isolated episode of VF, 9 episodes of VT, and 17 episodes of supraventricular tachycardia (SVT) (Figure 2). At presentation, his clinical examination was unremarkable, and electrocardiogram (ECG) revealed type 1 Brugada pattern with marked ST elevation and QTc of 0.416 ms (Figure 3A) (QTc was calculated by Hodges' method as the HR is > 110 beats/min). There were no precipitating factors, and serum biochemistry was normal with serum magnesium of 1.9 meq/L, potassium 4.0 meq/L, and calcium 8.8 meq/L. He received another appropriate shock 14 hours later while on isoprenaline infusion and occasional ventricular ectopic beats were noted during this period, along with partial resolution of ST elevation on the ECG (Figure 3B). Because of nonavailability of quinidine, treatment was initiated with oral quinine sulfate 300 mg thrice daily, and he was successfully weaned off isoprenaline 48 hours later. His subsequent course was uneventful, and he was discharged 7 days later on oral quinine 300 mg thrice daily along with cilostazol 100 mg twice daily, after a pulse generator replacement for premature battery depletion. There were no further episodes for 2 months, but because the patient complained of tinnitus, episodic altered hearing, and dizziness, suggestive of cinchonism, the dose of oral quinine sulfate was reduced to 150 mg thrice daily. One month later, he presented with 1 inappropriate shock for atrial fibrillation. On device interrogation, there were more than 20 nonsustained episodes of SVT, 2 episodes of monomorphic VT terminated by antitachycardia pacing, and 5 nonsustained episodes of polymorphic VT. The dose of oral quinine sulfate was increased to 300 mg thrice daily and cilostazol discontinued, as it was ineffective. A tryptophan-rich diet was advised to avoid the side effects of quinine, based on the research in malaria patients receiving quinine.6 The dietary modification included 2–3 portions of meat (1 portion = 60 g red meat/80 g white meat), a handful of nuts (1 handful = 1.5 oz/42.5 g, or 1/3 cup), and 3 glasses (1 glass = 200 mL) of milk per day. At 4 months follow-up, the patient is asymptomatic, with no episodes of supraventricular or ventricular arrhythmias and normalization of ST segment with a QTc of 414 ms (Figure 3C) (QTc calculated by Hodges' method as the HR is > 110 beats/min), and can tolerate quinine 900 mg/day.
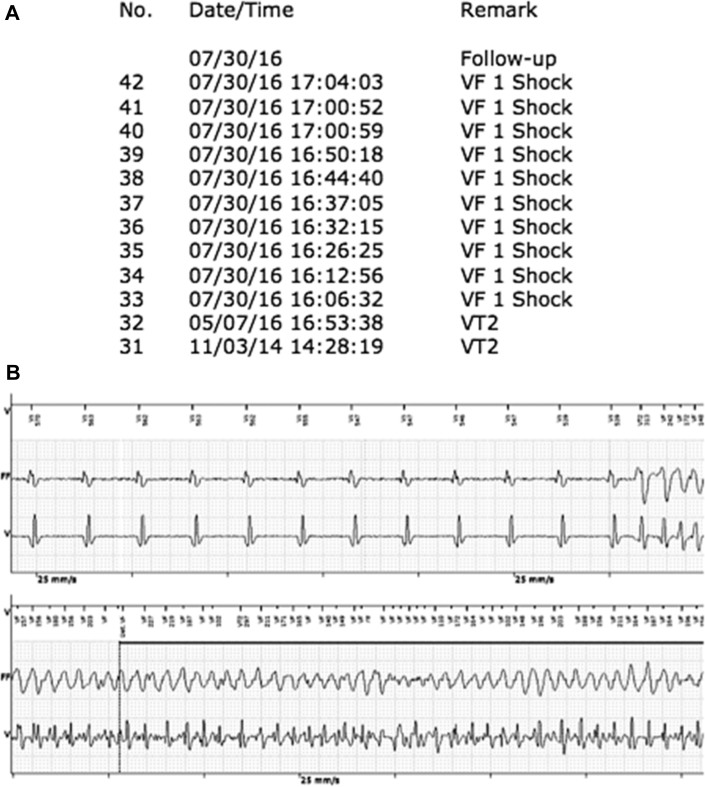
Figure 1.
Details of implantable cardioverter-defibrillator (ICD) interrogation at the time of presentation. A: The log of therapies delivered by the ICD reveals that from 16.06 to 17.07 hours the patient has 10 shocks delivered for ventricular fibrillation (VF). B: Intracardiac electrogram of 1 of the above episodes from the device shows a ventricular ectopic beat falling on the T wave, initiating a polymorphic ventricular tachycardia (VT) degenerating into VF correctly identified by the device. The patient received an appropriate therapy with a 35-joule shock terminating the VF (not in the picture).
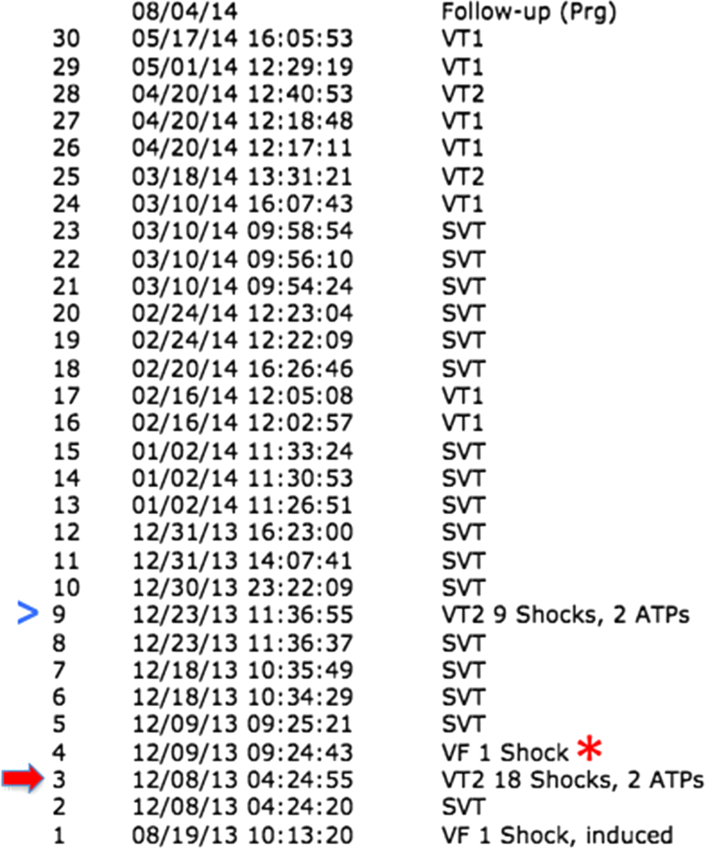
Figure 2.
Interrogation data from the implantable cardioverter-defibrillator before the present episode while on oral cilostazol therapy after the first electrical storm (red block arrow). While on cilostazol, the patient had 1 electrical storm (blue arrowhead), 1 episode of ventricular fibrillation (VF, red star), 9 episodes of ventricular tachycardia (VT) not requiring therapy, and 17 episodes of superventricular tachycardia (SVT).
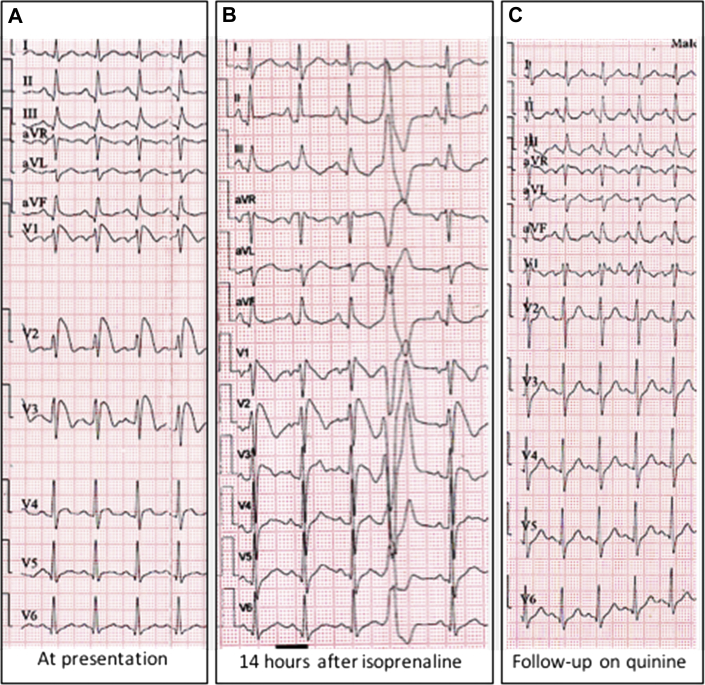
Figure 3.
Twelve-lead electrocardiograms (ECGs) of the patient. A: ECG at presentation has type 1 Brugada pattern with marked ST elevation seen in V1 to V3. B: ECG done 14 hours after initiation of isoprenaline infusion shows persistent type 1 Brugada pattern but with the resolution of ST segment. Ventricular ectopic beat is also noted. C: ECG on follow-up while on quinine therapy shows incomplete right bundle branch block pattern with complete resolution of ST segment.
Discussion
BrS is one of the most common causes of VF and sudden cardiac arrest in young individuals, with a mean age at sudden death of 41 ± 15 years. BrS is seen worldwide but is more common in Southeast Asian countries.1 The malignant form of BrS manifests as an electrical storm, which is the most dreaded and lethal complication. BrS patients who suffer electrical storm have an increased propensity to develop recurrent episodes of VF and electrical storms throughout their lifetime.7 BrS patients with repeated episodes of VF and electrical storms continue to experience repetitive shocks resulting in reduced quality of life, decreased device longevity, and complications associated with device replacement. Low-dose isoproterenol infusion administered as a bolus injection intravenously at a dose of 1–2 mg, followed by continuous infusion at a dose of 0.15–0.30 mg/min to maintain a 20% increase in heart rate, is effective in the acute management of electrical storm.3 Quinidine is the only oral medication that is consistently effective in preventing arrhythmias and reducing arrhythmic storms in patients with BrS.4 The clinically relevant effect of quinidine in BrS is due to the inhibition of Ito in the ventricular epicardial cells, thus restoring electrical homogeneity and abolishing phase 2 reentry. The anticholinergic effect of quinidine also contributes to the antiarrhythmic effect.8 There are 2 main drawbacks of treatment with quinidine. Firstly, quinidine often causes side effects, resulting in drug discontinuation in a third of patients owing to diarrhea and hepatoxicity.9 Secondly, quinidine is immediately available in only 19 countries, is unavailable in 99 countries, and is available, but only through restrictive regulatory processes, in 13 countries.5
The present case has several points of interest. Firstly, quinine sulfate as monotherapy was effective in preventing electrical storm and recurrent ICD shocks. Therapy with quinine sulfate also resulted in normalization of ST-segment elevation. Secondly, a diet with high tryptophan ameliorated the auditory side effect (cinchonism) of quinine sulfate. Thirdly, oral cilostazol was ineffective as monotherapy in preventing recurrent shocks and electrical storm. Fourthly, cilostazol therapy increased the episodes of supraventricular arrhythmias, which abated after its withdrawal, and oral quinine sulfate was used as monotherapy.
Mehrotra and colleagues10 reported the use of intravenous quinine in the acute management of arrhythmic storm in a 10-year-old child because of nonavailability of quinidine.10 Quinidine is manufactured in India but is not available for local use. Nonavailability of quinidine in Southeast Asian countries, where the disease is highly prevalent, makes it difficult to manage patients with an electrical storm and recurrent shocks and puts the patient's life at risk.5 Frequent electrical storms, nonavailability of quinidine, and difficulty in procuring the drug prompted us to consider oral quinine sulfate as an alternative. Quinine and its levorotatory diastereomer quinidine have similar pharmacologic activities in many aspects. In experimental arrhythmias, both quinidine and quinine suppress VF thresholds, reverse aconitine-induced atrial fibrillation, decrease ouabain-induced abnormal ventricular beats, and increase atrial refractory periods and His-Purkinje conduction.11 In humans, quinine is effective in suppressing both spontaneous and inducible ventricular arrhythmias without the proarrhythmic potential of QT prolongation, torsades de pointes, or heart block.12 The mean dose of oral quinine sulfate necessary for antiarrhythmic response in adults is 927 mg/day, which is much less than that required for antimalarial activity.12 Quinidine prolongs QTc, but quinine has no effect on the QT interval. The absence of QT prolongation and lower incidence of torsades with quinine is due to its stereoselective effect, resulting in 14 times less potency in blocking the hERG channel, the potassium channel essential for myocardial repolarization.13 Unlike quinidine, quinine has the advantage of being readily available in most countries, and hence, further studies are needed to assess the effectiveness of oral quinine sulfate in the treatment of ventricular arrhythmias in BrS patients. Quinine remains the most commonly used antimalarial therapy despite toxicity concerns, especially in children. A link between quinine toxicity and glucose-6-phosphate dehydrogenase deficiency is known but accounts for only a fraction of adverse response. The cognitive side effects contribute to a significant fraction of adverse reactions to quinine, attributed to tryptophan transport inhibition and tryptophan starvation.14 Tryptophan, an essential amino acid not produced by the body, is a direct precursor for the synthesis of the key neurotransmitter 5-hydroxytryptamine (serotonin). Tryptophan is also a precursor for quinine biosynthesis, and these 2 molecules have marked structural similarity. Quinine binds competitively and reversibly at the tryptophan binding sites in place of tryptophan owing to their similar structures. The symptoms of tryptophan deficiency in humans are neurocognitive and are akin to those seen in cinchonism. The cognitive effects of tryptophan depletion, such as tinnitus, overlap with common quinine side effects. Confirming the hypothesis of low tryptophan levels causing neurocognitive side effects, in malaria patients on quinine therapy side effects are more common in patients with low serum levels of tryptophan, and increasing the dietary intake of tryptophan ameliorates these side effects.6
In our BrS patient, oral cilostazol as monotherapy was ineffective in preventing electrical storms and episodes of ventricular arrhythmias. Similar to our experience, failure of oral cilostazol as monotherapy in preventing electrical storm has been reported.15 Spontaneous AF is seen in 10%–53% of BrS cases and is associated with higher incidence of syncopal episodes and documented VF.1 In the present case, monotherapy with quinine also resulted in suppression of supraventricular arrhythmias, and further studies are needed to validate this finding.
In conclusion, oral quinine sulfate is effective in the treatment of electrical storm and prevention of recurrent ICD shocks in BrS patients. High tryptophan diet is helpful in the management of common side effects of quinine, such as cinchonism. In countries where quinidine is not available, oral quinine sulfate should be considered as an alternative to prevent recurrent ICD shocks and electrical storms.
References
- 1.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS Expert Consensus Statement on the Diagnosis and Management of Patients with Inherited Primary Arrhythmia Syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Brugada P., Brugada R., Mont L., Rivero M., Geelen P., Brugada J. Natural history of Brugada syndrome. J Cardiovasc Electrophysiol. 2003;14:455–457. doi: 10.1046/j.1540-8167.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- 3.Ohgo T., Okamura H., Noda T., Satomi K., Suyama K., Kurita T., Aihara N., Kamakura S., Ohe T., Shimizu W. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Belhassen B., Glick A., Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S., Wilde A.A.M., Guevara-Valdivia M.E. Quinidine, a life-saving medication for brugada syndrome, is inaccessible in many countries. J Am Coll Cardiol. 2013;61:2383–2387. doi: 10.1016/j.jacc.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 6.Islahudin F., Pleass R.J., Avery S.V., Ting K.N. Quinine interactions with tryptophan and tyrosine in malaria patients, and implications for quinine responses in the clinical setting. J Antimicrob Chemother. 2012;67:2501–2505. doi: 10.1093/jac/dks253. [DOI] [PubMed] [Google Scholar]
- 7.Shvilkin A. Treatment of ventricular fibrillation storm in Brugada syndrome: weathering the storm but staying in hot water? Heart Rhythm. 2007;4:701–702. doi: 10.1016/j.hrthm.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Márquez M.F., Salica G., Hermosillo A.G., Pastelín G., Gómez-Flores J., Nava S., Cárdenas M. Ionic basis of pharmacological therapy in Brugada syndrome. J Cardiovasc Electrophysiol. 2007;18:234–240. doi: 10.1111/j.1540-8167.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 9.Márquez M.F., Bonny A., Hernández-Castillo E., De Sisti A., Gómez-Flores J., Nava S., Hidden-Lucet F., Iturralde P., Cárdenas M., Tonet J. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm. 2012;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra S., Juneja R., Naik N., Pavri B.B. Successful use of quinine in the treatment of electrical storm in a child with brugada syndrome. J Cardiovasc Electrophysiol. 2011;22:594–597. doi: 10.1111/j.1540-8167.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 11.Klevans L.R., Kelly R.J., Kovacs J.L. Comparison of the antiarrhythmic activity of quinidine and quinine. Arch Int Pharmacodyn Ther. 1977;227:57–68. [PubMed] [Google Scholar]
- 12.Sheldon R., Duff H., Koshman M.L. Antiarrhythmic activity of quinine in humans. Circulation. 1995;92:2944–2950. doi: 10.1161/01.cir.92.10.2944. [DOI] [PubMed] [Google Scholar]
- 13.Yan M., Fan P., Shi Y., Feng L., Wang J., Zhan G., Li B. Stereoselective blockage of quinidine and quinine in the hERG channel and the effect of their rescue potency on drug-induced hERG trafficking defect. Int J Mol Sci. 2016;17:1648. doi: 10.3390/ijms17101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khozoie C., Pleass R.J., Avery S.V. The antimalarial drug quinine disrupts Tat2p-mediated tryptophan transport and causes tryptophan starvation. J Biol Chem. 2009;284:17968–17974. doi: 10.1074/jbc.M109.005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abud A., Bagattin D., Goyeneche R., Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:210–212. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]