Abstract
Mucinous cystadenocarcinoma is an extremely rare variant of primary breast tumor which is histologically similar to mucinous cystadenocarcinoma of the ovary and pancreas. Herein we report a case of a 63 years old woman diagnosed with diverse histological types of non-synchronous rare primary breast tumors, a medullary carcinoma of the right breast and a mucinous cystadenocarcinoma of the left breast. Macroscopically the neoplasm appeared multilocular filled with mucoid material. Under light microscopy the cystic areas were lined by columnar cells with abundant intracellular and extracellular mucin. Solid areas were composed of tall columnar cells with intracellular mucin. Moderate to marked atypia was noticed and tumor cells stained positive for cytokeratin 7 and negative for cytokeratin 20. Moreover tumor cells displayed a basal like immunophenotype expressed as followed: ER negative, PR negative, HER-2 negative, cytokeratin (CK5/6) positive and EGFR positive.
Key words: Basal like, Breast, Embryogenesis, Mucinous cystadenocarcinoma
Introduction
Mucinous cystadenocarcinoma (MCA) of the breast belongs to the family of mucin producing carcinomas along with mucinous/ colloid carcinoma, signet ring cell carcinoma and columnar cell mucinous carcinoma. Mucinous carcinomas − so called colloid − display malignant cells floating in extracellular mucin; on the other hand signet ring cell carcinomas reveal intracellular mucin whereas columnar cell mucinous carcinoma present elongated glands with compressed lumen lined by columnar cells of clear cytoplasm and basally located nuclei. On cut surface mucinous cystadenocarcinoma of the breast shows multiple cystic spaces filled with mucin, resembling its ovarian and pancreatic counterparts. Microscopically MCA shows both intracellular and extracellular mucin and moreover the cystic spaces are lined by tall columnar cells that contain profuse extracellular mucin.1
The aim of this study is to enrich literature with another single case diagnosed with this rare entity showing exceptional immunophenotype and to shed light on its histogenetical features.
Case Report
A 63 year aged woman was admitted to our hospital due to palpable mass in her left axilla. The patient’ s medical history was significant for a carcinoma of her right breast 24 years ago, for which she underwent lumpectomy and ipsilateral axillary lymph node dissection. Pathology revealed a medullary carcinoma which measured 32 mm in maximum diameter. All lymph nodes resected were free of disease. Imaging revealed no distant disease and the patient was offered adjuvant chemotherapy and radiotherapy.
In 2011 the patient noticed a palpable mass in her left axilla. Clinical examination followed by FNA revealed metastatic carcinoma. Ultrasound examination showed a 16 mm mass in the left upper quadrant of the left breast. The patient had a left lumpectomy along with ipsilateral axillary lymph node dissection. Grossly, the tumor was well circumscribed, comprised of solid and containing mucin cystic areas. Histologically, the tumor consisted of multiple cystic spaces filled in mucin. The cystic wall had several papillary projections with epithelial tufting, was lined by columnar cells showing moderate to severe atypia. Both intracellular (Figure 1A) and extracellular mucin production (Figure 1B) were recognised. Several necroses were noticed and mitotic rate was high. The palpable axillary mass proved to be a lymph node block with a maximum diameter of 47 mm. Two out of the thirteen lymph nodes removed were invaded by the carcinoma. Immunohistochemistry stained positive for CK7 and negative for CK20. Additionally, the neoplasm displayed a basal like immunophenotype being ER, PR and Cerb- 2/HER2 negative and positive for both CK5/6 and EGFR. The patient was discharged 10 days after surgery and received adjuvant chemotherapy and radiotherapy. Forty eight month follow-up she is disease free.
Figure 1.
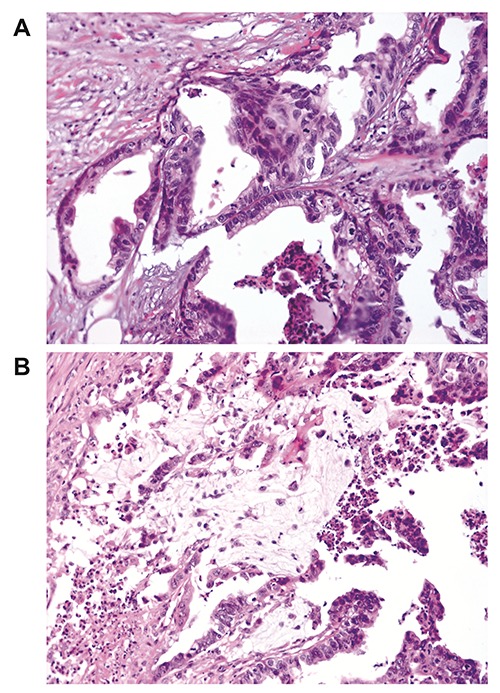
A) The epithelium is moderately differentiated with single or pseudostratified basal nuclei that present mild atypia with intracellular mucin production. B) Extracellular mucin production in cystadenocarcinoma of the breast.
Discussion
Mucinous carcinoma typically appears in two different subtypes, the cystic (mucinous cystadenocarcinoma) and solid (columnar cell mucinous carcinoma).1 Both belong to the family of mucin producing breast carcinomas along with mucinous/colloid carcinoma and signet ring cell carcinoma listed as so in the 2003 WHO edition. Mucinous/colloid carcinoma displays malignant cells floating in extracellular mucin and signet ring cell carcinoma in which the mucin is intracellular. Mucinous cystadenocarcinoma of the breast shows both intracellular and extracellular mucin production consisting of sulfomucin and sialomucin that stain positive for periodic acid-Schiff and alcian blue pH 2.5 and 1.0 as well as mucicarmine.2 Unlike histological counterparts of the ovary and pancreas, MCA of the breast is extremely rare and was firstly coined by Koenig and Tavassoli in 1998.1
Microscopically it shows both intracellular and extracellular mucin. The epithelium is well differentiated with single or pseudostratified basal nuclei that present mild atypia. Moreover the cystic spaces are lined by a single layer of tall columnar cells that contain profuse extracellular mucin, with absent myoepithelium. In the present case a mucinous cystadenocarcinoma, of great resemblance to what Koenig and Tavassoli have described is reported. However the intriguing in this case is that the patient suffered of bilateral metachronous breast tumors, with an interval of 24 years, yet of indolent behavior. To the best of our knowledge, since 1998 a total of twenty cases have been reported in literature, one of which with bilateral tumors. Features of the 20 previous published cases are presented in (Table 1).1-13
Table 1.
Reported instances of primary breast mucinous cystadenocarcinomas.
| Case (Ref.) | Age, years | Size, mm | Stage (pTNM) | ER | PR | c-erbB2 | Ki-67, % | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 13 | 79 | 60 | T3N0M0 | NA | NA | NA | NA | M, | DOR 2 years |
| 21 | 54 | 190 | T4N1M0 | – | – | NA | 40 | M, LND | ANED 24 months |
| 31-Jan | 67 | 23 | T2N0M0 | – | – | NA | 30 | M, LND | ANED 22 months |
| 41 | 9 | 85 | T3N0M0 | – | – | NA | 70 | M, LND, C, R | ANED 11 months |
| 51 | 61 | 8 | T1N0M0 | – | – | NA | 50 | L, LND | NA |
| 62 | 74 | 100 | T3N0M0 | – | NA | NA | 21.8 | M, LND | ANED |
| 74 | 96 | 20 | T2N2M0 | – | – | – | 35 | L, LND | DOR 46 months |
| 85 | 65 | 30 | T2N0M0 | – | – | – | 20.5 | M, LND, C | ANED 8 months |
| 96 | 51 | 40 | T2N0M0 | – | – | NA | NA | L | NA |
| 107 | 55 | 25 | T2N0M0 | – | – | – | 90 | L | ANED 6 months |
| 118 | 52 | 100 | T3N0M0 | + | – | – | NA | M, LND | ANED 24 months |
| 6-May | 61 | 30 | T2N0M0 | – | – | – | NA | M, LND | ANED 6 months |
| 139 | 73 | 45 | T2N0M0 | – | – | 2+ | NA | M, LND | NA |
| 1410 | 65 | 30 | T2N0M0 | – | – | NA | NA | PM, LND | ANED 6 months |
| 157 | 52 | 65 | T3N0M0 | – | – | – | 10 | NA | NA |
| 1611 | 41 | 70, 50, 25 | T3N1M0 | – | – | 50 | M, LND | ANED 24 months | |
| 177 | 59 | 9 | T1N0M0 | – | – | 2+ | 5 | PM, LND, C | ANED 3 months |
| 187 | 62 | 56 | T3N0M0 | – | – | – | NA | M, LND | ANED 5 months |
| 1912 | 55 | 20 | T1N0M0 | – | – | 2+ | 30 | L, LND, C, R | ANED 10 months |
| 2013 | 91 | 75 | T3N0M0 | – | – | – | 40 | M, R | DOR 14 months |
ANED, Alive with no evidence of disease; C, chemotherapy; DOR, died of other reason; ER, estrogen receptor; L, lumpectomy; LND, with lymph node dissection; M, modified radical mastectomy; NA, not available; PM, partial mastectomy; PR, progesterone receptor; R, radiation therapy; TNM, tumor, node, metastasis.
Literature reports that patients diagnosed with this entity are typically postmenopausal females aged between 47 and 96 years. Our patient was 63 years old which falls within the literature age average.
As a clinicopathological finding the lymph node metastases are infrequent; three of the previous twenty patients of the literature have developed metastasis.1,4,11 Similarly our case revealed lymph node involvement in two of thirteen nodes besides to a block. Commonly, this tumor is associated to a favorable prognosis even in case of lymph node invasion, especially when complete resection of the tumor has been achieved. In line with this observation the patient of our study showed no recurrence or distant metastases, despite the invasion of a lymph node block and 2 out of 13 lymph nodes retrieved.
As far as tumor size is concerned, this tumor typically measures 8 to 190 mm. Our patient’s primary tumor had maximum diameter of 16mm which is within the bibliography range. Multicentricity was described in one case consisted of three foci 70 mm, 50 mm and 25 mm.11
The differential diagnosis of MCA includes metastasis from other organs and cystic hypersecretory breast carcinoma due to the fact that they both show a cystic appearance,1 and likewise cytologic image.14,15 The differential diagnosis lay on the immunohistochemical profile in the former and on the presence of tall columnar cells in the latter.
The immunohistochemical profile of MCA of the breast is typically CK7 (+), CK20 (-) and CDX-2 (-), GCDFP-15 (+), mammoglobin (+) and triple negative ER, PR, HER2 expression. Ovarian and pancreatic MCAs are commonly CK7 (+), CK20 (+) and CDX-2 (+). Expression of ER and PR is common in cystic hypersecretory carcinoma whereas in MCA only one case was ER positive,8 and this was disputed by Dong-Liang et al. who noticed different morphological features from all other cases.7 Interestingly, there is evidence that rarely HER2 protein may stain positive in MCA. In these cases, the HER2 positivity has been confirmed by gene amplification via FISH assessment.9,12 Another paper reported a case with a basal- like immunophenotype classified as ER-, PR-, HER2-, CK5/6+ and EGFR+.6 The current case is CK7 (+), CK20 (-), with a basal like immunophenotype supporting the breast origin of this particular tumor.
The cumulative data suggests a favorable prognosis of breast MCAs despite its immunoprofile which is usually considered as a poor prognostic factor in typical invasive ductal carcinomas. Patients of most reported cases are alive with no evidence of disease or passed away of other non-MCA related causes. However, more cases should be reported and discussed thoroughly to maintain such assertion. The patient of the discussed case is alive with no evidence of disease forty eight months after diagnosis.
Lee and Chaung ensued that the histogenesis of the neoplasm is result of mucinous accumulation within the lumen of intraductal papillary carcinoma which present mucin metaplasia and extracellular mucin production. This phenomenon causes cystic dilation of the lumen duct, loss of myoepithelial cells and invasion of adjacent stroma.6 However, this assumption does not interpret the favorable prognosis of MCAs despite the poor prognostic factor of their immunoprofile. Another theory supports a mucinous metaplasia from ordinary ductal carcinoma,5 which seems less possible. Also, a mucocele-like lesion with malignant transformation is included in differential diagnosis, unless ordinary mammary carcinoma is found in the adjacent tissue.
The similarities of the MCA of the breast with its ovarian and pancreatic counterpart prompt us to investigate the literature and find possible common embryological origin or genetic profile. The ovary as an indifferent genital ridge derived from the mesonephros mesenchyme, likewise the pancreatic primordial first appear during the 5th week of gestation and develops from the splanchnic mesoderm.
On the other hand breast is considered an epidermal gland which develops at the 4th week when ectoderm and underlying mesoderm proliferate and differentiate into skin.
The genetic profile of ovarian MCAs comprises of somatic mutations in KRAS and HER2 amplification in 15-20% of tumors that do not harbor KRAS mutations. Similarly in pancreatic MCAs activating point mutations in codon 12 of the KRAS gene have been observed in non as well as invasive tumors while alteration of TP53, CDKN2A (p16) and SMAD4 (DPC4) are associated with invasive component. Literature lacks information of the genetic profile of mammarian MCAs which is reasonable considering the rarity of the tumor. It would be of great interest to gather relative data of all published cases in a joined article.
Conclusions
Mucinous cystadenocarcinoma of the breast is a very rare primary breast carcinoma with only twenty cases being reported. It has unique morphology among breast carcinomas and its clinical behavior is distinct showing a favorable prognosis which is unrelated to the size of the tumor, lymph node metastasis or the molecular subtype. Despite its morphological similarities to pancreatic and ovary cystadenocarcinoma the embryogenesis and immunophenotype is diverse.
References
- 1.Koenig C, Tavassoli FA, Mucinous cystadenocarcinoma of the breast. Am J Surg Pathol 1998;22:698-703. [DOI] [PubMed] [Google Scholar]
- 2.Domoto H, Terahata S, Yamazaki T, et al. Mucinous cystadenocarcinoma of the breast showing sulfomucin production. Histopathology 2000;36:567-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol 1984;8:31-41. [DOI] [PubMed] [Google Scholar]
- 4.Honma N, Sakamoto G, Ikenaga M, et al. Mucinous cystadenocarcinoma of the breast: a case report and review of the literature. Arch Pathol Lab Med 2003;127:1031-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen WY, Chen CS, Chen HC, et al. Mucinous cystadenocarcinoma of the breast coexisting with infiltrating ductal carcinoma. Pathol Int 2004;54:781-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Chaung CR. Mucinous metaplasia of breast carcinoma with macrocystic transformation resembling ovarian mucinous cystadenocarcinoma in a case of synchronous bilateral infiltrating ductal carcinoma. Pathol Int 2008; 58:601-5. [DOI] [PubMed] [Google Scholar]
- 7.Dong-Liang L, Ji-Lin H, Shi-Hong S, et al. Primary mucinous cystadenocarcinoma of the breast with endocervicallike mucinous epithelium. Breast Care 2013;8:445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakici S, Gonullu G, Gursel SB, et al. Mucinous cystadenocarcinoma of the breast with estrogen receptor expression: A case report and review of the literature. Case Rep Oncol 2009;2:210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersson F, Pang B, Thamboo TP, Putti TC. Mucinous cystadenocarcinoma of the breast with amplification of the HER2-gene confirmed by FISH: the first case reported. Hum Pathol 2010;41:910-3. [DOI] [PubMed] [Google Scholar]
- 10.Sentani K, Tashiro T, Uraoka N, et al. Primary mammary mucinous cystadenocarcinoma: cytological and histological findings. Diagn Cytopathol 2012;40:624-8. [DOI] [PubMed] [Google Scholar]
- 11.Deng Y, Xue D, Wang X, et al. Mucinous cystadenocarcinoma of the breast with a basal-like immunophenotype. Pathol Int 2012;62:429-32. [DOI] [PubMed] [Google Scholar]
- 12.Kucukzeybek BB, Yigit S, Sari AA, et al. Primary mucinous cystadenocarcinoma of the breast with amplification of the HER2 gene confirmed by FISH – case report and review of the literature. Pol J Pathol 2014;65:70-3. [DOI] [PubMed] [Google Scholar]
- 13.Witherspoon LE, Oxenhandler RW. A rare tumor: mucinous cystadenocarcinoma of the breast. Am Surg 2015;8:E106-8. [PubMed] [Google Scholar]
- 14.Kim MK, Kwon GY, Gong GY. Fine needle aspiration cytology of cystic hypersecretory carcinoma of the breast. A case report. Acta Cytol 1997;41:892-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee WY, Cheng I, Chang TW. Diagnosing invasive cystic hypersecretory duct carcinoma of the breast with fine needle aspiration cytology: a case report. Acta Cytol 1999;43:273-6. [DOI] [PubMed] [Google Scholar]


