Abstract
The examination of endocranial data of archosauriforms has led to advances on the evolution of body size, nerve pathways, and sensory abilities. However, much of that research has focused on bird‐line archosaurs, resulting in a skewed view of Archosauria. Phytosauria, a hypothesized sister taxon to or early‐branching member of Archosauria, provides a potential outgroup condition. Most previous phytosaur endocranial studies were executed without the use of modern technology and focused on derived members of Phytosauria. We present a comparative CT examination of the internal cranial anatomy of Wannia scurriensis, the most basal known parasuchid phytosaur. Wannia scurriensis shows some overall similarity with extant crocodylians and derived phytosaurs in general endocranial shape, a large hypophyseal fossa, and trigeminal (CN V) innervation, but as a whole, the endocast has noticeable differences to crocodylians and other phytosaurs. The pineal region is expanded dorsally as in other phytosaurs but also laterally (previously unrecognized). CN V exits the pons in a more dorsal position than in Parasuchus hislopi, Machaeroprosopus mccauleyi, or Smilosuchus gregorii. Wannia scurriensis also exhibits a larger hypophyseal fossa relative to brain size than observed in P. hislopi or S. gregorii, which may indicate more rapid growth. The well‐preserved semicircular canals have lateral canals that are angled more anteroventrally than in derived phytosaurs. Extensive facial innervation from the large CN V indicates increased rostrum sensitivity and mechanoreceptive abilities as in Alligator mississippiensis. These endocranial similarities among phytosaurs and with Alligator indicate conserved ecological and functional results of an aquatic lifestyle, and highlight a need for further exploration of endocranial anatomy among Archosauriformes.
Keywords: convergence, endocast, plesiomorphy, Triassic
Introduction
The morphology of the brain and associated soft tissue structures (e.g. sinuses, neurovascular pathways) reveals information about intelligence, agility, and sensory abilities in an animal. These soft tissue sensory systems are useful in understanding how an organism responds to environmental stimuli, but they can also inform about evolutionary histories and phylogenetic relationships. This can be translated directly to extinct organisms using fossils and osteological correlates for soft tissue features. By making comparisons between extant and extinct taxa, we can begin to understand the evolution of endocranial morphology. Three‐dimensional reconstructions of soft tissue structures are an emerging source of phylogenetic and comparative data using technologies such as computed tomography (CT). This has been applied in a number of studies on archosaurs (e.g. George & Holliday, 2013; Balanoff et al. 2016; Knoll et al. 2015), which have a rich evolutionary history encompassing thousands of extant species and hundreds of extinct taxa. However, previous focus within Archosauria has been on the avemetatarsalians (bird‐line archosaurs), with less of the focus on the pseudosuchians (crocodylian‐line archosaurs), and much of that focus within pseudosuchians has been on the derived and mostly extant taxa. Little is known about the common ancestor at the junction of these two groups, but there are many members of Phytosauria, a clade recovered as either early‐branching pseudosuchians (e.g. Brusatte et al. 2010; Ezcurra, 2016) or the sister taxon to Archosauria (Nesbitt, 2011).
Phytosaurs are a clade of Late Triassic reptiles that superficially resemble crocodylians (Stocker & Butler, 2013). That resemblance is due to convergence rather than close phylogenetic relationships between phytosaurs and crocodylians (e.g. Brusatte et al. 2010; Nesbitt, 2011; Ezcurra, 2016). Phytosaur endocrania have been examined in the past, though most of those studies utilized more traditional methods of plaster or latex reconstructions and models of the endocranial cavity and focused on the derived leptosuchomorph taxa (e.g. Machaeroprosopus buceros, described by Cope, 1888; Leptosuchus, described by Case, 1928; Machaeroprosopus pristinus, described by Mehl, 1928; Smilosuchus gregorii, described by Camp, 1930). Until recently, Parasuchus hislopi was the only basal phytosaur whose endocast had been described (Chatterjee, 1978). Recent investigations utilizing CT technology to reconstruct the brain endocast of Machaeroprosopus mccauleyi (Holloway et al. 2013) and the brain endocast and paranasal sinuses of Parasuchus angustifrons and Ebrachosuchus neukami (Lautenschlager & Butler, 2016) have allowed for the beginning of more accurate comparisons within Phytosauria and across Archosauriformes (e.g. Triopticus primus, Stocker et al. 2016). These more basal members of Phytosauria contain evidence of soft tissue structures associated with the evolution of morphology related to rostrum elongation and a semiaquatic lifestyle but should also inform on the plesiomorphic endocranial morphology for archosaurs (Stocker & Butler, 2013). Additional investigations of the braincases of other archosauriforms (e.g. Euparkeria capensis, Sobral et al. 2016) and archosaurs (e.g. Stagonolepis, Gower & Walker, 2002; Arizonasaurus, Gower & Nesbitt, 2006; Batrachotomus, Gower, 2002) contribute to further comparisons. However, these comparisons tend to be limited to characters concerning external surfaces and cranial nerves rather than endocrania (Gower & Sennikov, 1996; Nesbitt, 2011). Here we provide a description of the endocranial and paranasal cavities of W. scurriensis in order to establish evolutionary trends and allow more accurate reconstruction of both phytosaur and archosaur plesiomorphic endocranial morphology.
Methods
Specimen
The specimen described here (TTU P‐00539, the holotype specimen) consists of two parts that were described by Langston (1949) and Stocker (2013) (Fig. 1).
Figure 1.
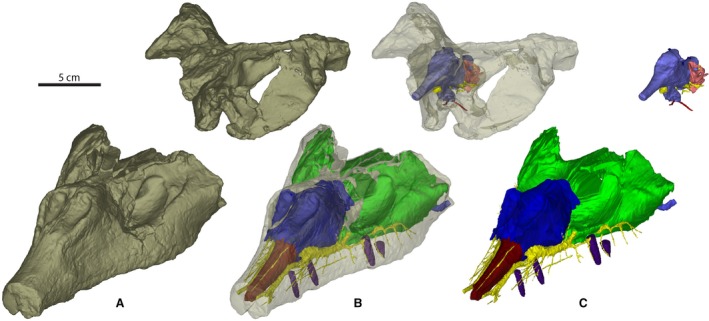
Material of Wannia scurriensis (TTU P‐00539) in (A) anterolateral view of CT scan of skull, (B) anterolateral view of transparent skull, and (C) anterolateral view of endocast. Scale bar = 5 cm.
CT scanning and digital reconstruction
TTU P‐00539 was scanned at The University of Texas High‐Resolution CT Facility (UTCT) by Matthew Colbert as Archive 2530 on 8 August, 2011 using an NSI scanner, with additional processing and correction by Jessie Maisano. The specimen was scanned in two parts, a braincase section and a rostral section, at a slice thickness of 0.5 mm, 350 kV and 4.2 mA. Raw scan data were exported in TIFF format. The data comprise 307 slices for the braincase section and 579 for the rostral section, each with dimensions of 1024 × 1024 pixels, a pixel spacing of 0.45 mm, and field of reconstruction at 185 mm. CT datasets are available at http://morphosource.org/Detail/MediaDetail/Show/media_id/12518 for viewing and download.
The CT data files were imported into materialise mimics 19.0 in the form of 16‐bit TIFF images for segmentation and digital reconstruction. The CT data were analyzed on a 64‐bit dual platform Mac‐PC workstation with 32 GB RAM. Structures of interest (e.g. cranial endocast, endosseous labyrinth, paranasal cavities) were digitally extracted using mimics segmentation tools for visualization.
Results
The exceptional preservation of the braincase allows for a detailed endocast accurately depicting the size and position of the brain, endosseous labyrinths, and cranial nerve trunks (Figs 2 and 3; Kley et al. 2010). In addition, the preserved rostrum allows for the detailed segmentation of the paranasal cavities, trigeminal nerve innervations, and dentition, including replacement teeth (Fig. 4).
Figure 2.
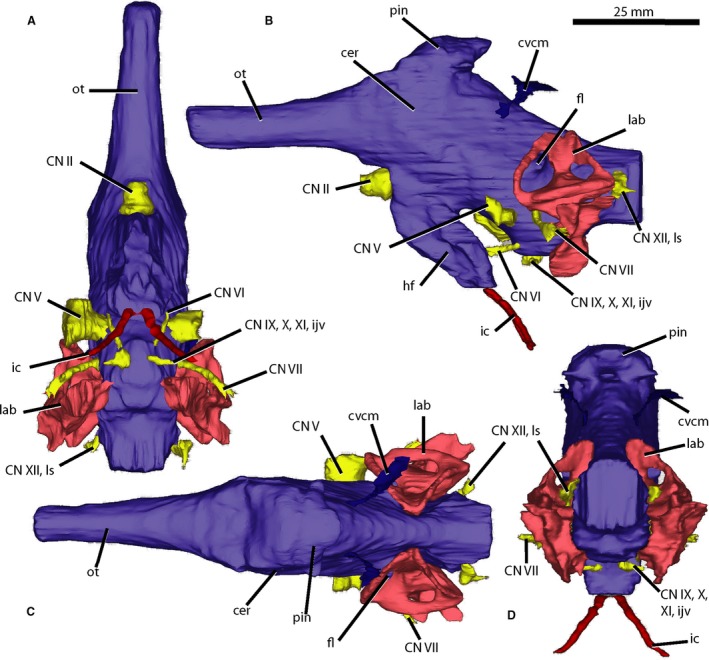
Brain endocast of Wannia scurriensis (TTU P‐00539) in (A) ventral, (B) left lateral, (C) dorsal, and (D) posterior views. cer, cerebrum; CN, cranial nerve; cvcm, caudal middle cerebral vein; fl, flocculus; hf, hypophyseal fossa; ic, internal carotid artery; ijv, internal jugular vein; lab, endosseous labyrinth; ls, longitudinal sinus; ot, olfactory tract; pin, pineal expansion. Scale bar = 25 mm.
Figure 3.
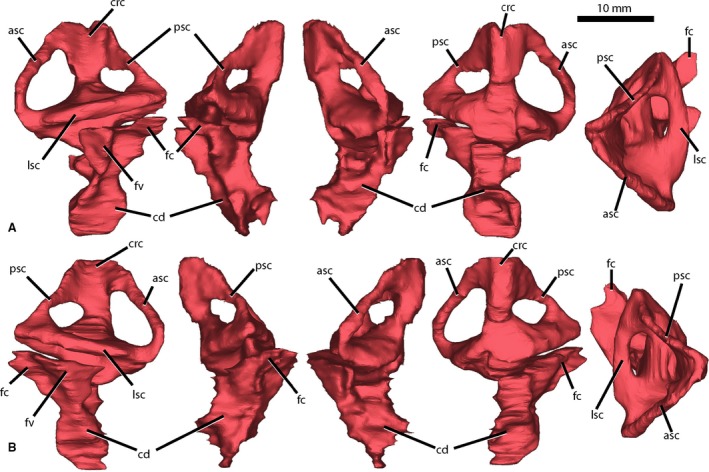
Endosseous labyrinth of Wannia scurriensis (TTU P‐00539) in (A) left lateral, posterior, anterior, medial, and dorsal views and (B) right lateral, posterior, anterior, medial, and dorsal views. asc, anterior semicircular canal; cd, cochlear duct; crc, crus communis; fc, fenestra cochleae; fv, fenestra vestibuli; lsc, lateral semicircular canal; psc, posterior semicircular canal. Scale bar = 10 mm.
Figure 4.
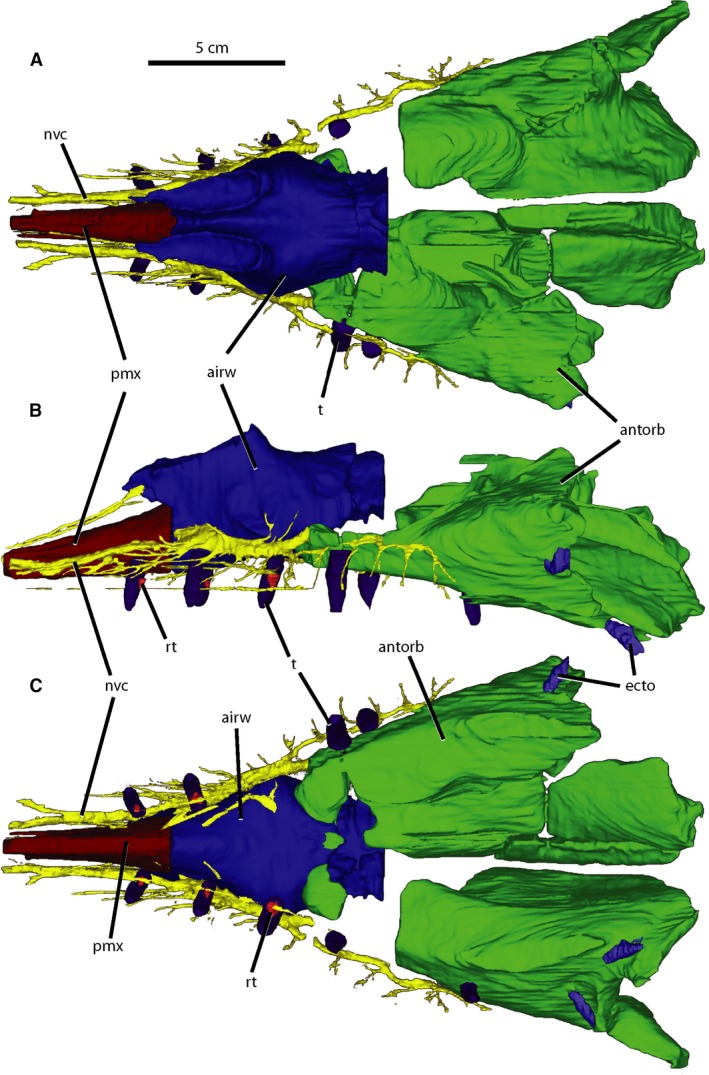
Paranasal cavities of Wannia scurriensis (TTU P‐00539) in (A) dorsal, (B) left lateral, and (C) ventral views. airw, airway; antorb, antorbital sinus; ecto, ectopterygoid sinus; nvc, neurovascular canal; pmx, premaxillary cavity; rt, replacement tooth; t, tooth. Scale bar = 5 cm.
Endocast
As with some other archosauriforms (e.g. T. primus and Tropidosuchus romeri) and most phytosaurs (e.g. M. mccauleyi, S. gregorii, E. neukami, P. angustifrons, and P. hislopi) the brain endocast of W. scurriensis is almost completely enclosed in bone, allowing for reconstruction of the entire endocast. The endocast is nearly symmetrical and shows few signs of deformation. Only the anterior portion of the braincase is missing, eliminating boundaries for the anteriormost portions of the olfactory tract and the olfactory bulbs. Excluding the endosseous labyrinths and the cranial nerves, the volume of the endocast is 21.24 cm3 (compared with 18.68 cm3 for M. mccauleyi, Holloway et al. 2013, the approximate volumes of 10.22 cm3 and 8.77 cm3 for P. angustifrons and E. neukami, respectively, Lautenschlager & Butler, 2016, and the maximum volume of 0.7 cm3 for Tro. romeri, Trotteyn & Paulina‐Carabajal, 2016) The endocast of W. scurriensis is elongate and mediolaterally narrow, similar to those of M. mccauleyi, E. neukami, and P. angustifrons (Lautenschlager & Butler, 2016).
The fore‐brain is defined as comprising the olfactory bulbs, olfactory tract, cerebrum, hypothalamus, thalamus, and cranial nerves I and II. The olfactory tract extends anteriorly from the main body of the endocast, is unexpanded dorsally, and connects in a horizontal plane posteriorly to the dorsoventrally and mediolaterally expanded cerebral hemispheres (Fig. 2B,C). The anterior portions of the olfactory tract, including the olfactory bulbs, are unable to be reconstructed because of the incomplete preservation of the skull. The olfactory tract is cylindrical in cross‐section, linear, and bounded ventrally by the laterosphenoid and dorsally by the frontals. The posterior portion of the olfactory tract widens laterally to the point where it feeds into the cerebral hemispheres. The cerebral hemispheres are the most laterally expanding portions of the brain endocast and narrow posteriorly, though less so than in M. mccauleyi. The caudal middle cerebral veins exit the posterior cerebral hemispheres and extend posterodorsally (Fig. 2B‐D). Dorsal to the cerebral hemispheres, there is a dorsal projection exhibiting lateral and slight anterior extensions which has been described previously as the epiphysis or pineal expansion by Holloway et al. (2013) (Fig. 2B‐D). A similar, though more posteriorly placed, dorsal expansion in E. neukami and P. angustifrons was described as the dural venous sinus or possibly the paratympanic sinus by Lautenschlager & Butler (2016). However, it is difficult to differentiate between brain endocast and sinus in W. scurriensis because of the lack of osteological dividers in this region. This dorsal expansion extends dorsally to its contact with the parietal and is interpreted as a pineal expansion here for W. scurriensis because of its location and the presence of a pineal complex in all extant reptiles except crocodylians (Quay, 1979). There is a depression in the dorsal surface of the parietal just anterodorsal to the most anterodorsal part of the pineal expansion. Our CT scan data suggest that the pineal expansion does not exit the dorsal surface of the skull roof of TTU P‐00539 through any kind of foramen in the parietal, similar to the condition in T. primus (Stocker et al. 2016). This is contrary to the interpretation of Langston (1949) who suggested a parietal foramen was present in W. scurriensis, but confirms the hypothesis of Stocker (2013) that this is not an archosauriform character. Posterior to the pineal hemisphere and cerebral hemispheres, the medulla has a dorsal surface that slopes posteroventrally and is slightly concave posterodorsally.
A hypophyseal or pituitary fossa is clearly present ventral to the cerebral hemispheres (Fig. 2B). This is in contrast to the apparent lack of a hypophysis in M. mccauleyi (Holloway et al. 2013) but consistent with the presence in P. angustifrons, E. neukami, T. romeri, and T. primus (Trotteyn & Paulina‐Carabajal, 2016; Lautenschlager & Butler, 2016; Stocker et al. 2016). The large hypophysis, or pituitary body, extends posteroventrally and is the ventralmost extending part of the brain; it is larger in W. scurriensis than in P. angustifrons, and similar in size to that of E. neukami (Lautenschlager & Butler, 2016). The hypophysis expands laterally to its ventralmost point, where the internal carotid arteries extend posteroventrally (Fig. 2A,B,D). The internal carotid arteries exit the braincase ventrally at the lateral sides of the basisphenoid tubera, as also observed in UMMP 8409 (Case, 1928), S. gregorii (UCMP 27200 [Camp, 1930]), Machaeroprosopus lottorum (TTU P‐10076 [Hungerbühler et al. 2013]), Pravusuchus hortus (AMNH FR 30646 [Stocker, 2010]), and Mystriosuchus westphali (GPIT 261/001 [Hungerbühler, 2002]).
The cephalic flexure, which refers to the curve of the region between the fore‐ and mid‐brain, is 148° in TTU P‐00539 (following Lautenschlager & Hübner, 2013). This is similar to the orientation seen in other phytosaurs (e.g. P. hislopi, S. gregorii) and aetosaurs (e.g. Desmatosuchus spurensis) but is different from the elongate endocast of the archosauriforms T. romeri (Trotteyn & Paulina‐Carabajal, 2016) and T. primus (Stocker et al. 2016). The posterior part of the brain comprises the mid‐ and hind‐brain, contains the floccular lobes, and is the source of many of the cranial nerves. The mid‐brain refers to the tectum and includes cranial nerves III and IV, and the hind‐brain includes the pons, cerebellum, medulla, spinal cord, and cranial nerves V through XII. The pontine flexure, the curve of the region between the mid‐ and hind‐brain, is 148° following Lautenschlager & Hübner (2013). The floccular lobes (Fig. 2B,C) are short projections extending laterally from the cerebellum into the vestibular apparatus of the endosseous labyrinth as in other phytosaurs (e.g. E. neukami, P. angustifrons, P. hislopi, Lautenschlager & Butler, 2016; Stocker et al. 2016). These structures extend laterally, just barely beyond the anterior semicircular canal.
Cranial nerves
At the juncture of the hypophysis and the cerebrum, cranial nerve II (optic nerve) extends anteriorly through a large foramen in the laterosphenoid. The foramen for CN II is large in phytosaurs (e.g. M. buceros, S. gregorii, P. hislopi) and the archosauriform T. primus.
Cranial nerve V (trigeminal nerve) extends anterolaterally from the anteroventral portion of the hind‐brain, just anterior to the semicircular canals through a large foramen in the prootic (Fig. 2A‐C). The trigeminal fossa is surrounded by bone in W. scurriensis and also crocodylians and nonavian dinosaurs (George & Holliday, 2013). The trigeminal nerve is present in the rostral section as the maxillary branch and exits the antorbital cavity anteriorly on both sides of the rostrum (Fig. 4). The maxillary branch extends both anteriorly and posteriorly, sending off small accessory nerves to the alveoli and facial region, similar to the branching trigeminal in extant crocodylians (Leitch & Catania, 2012; George & Holliday, 2013). Posteriorly, the canal runs lateral to the alveoli to the posteriormost end of the maxilla with small ventrally extending branches both anterolateral and posterolateral to each alveolus. Some of these small branches have additional smaller branches off them. Anteriorly, the canal passes lateral to the airway and premaxillary cavity. It is largest in diameter lateral to the external nares and anteroventral to the antorbital fenestra, and many fine branches extend anterolaterally from this region, branching further and extending to randomly distributed foramina on the lateral surface of the maxilla anterior to the antorbital fenestra (Stocker, 2013). The anterior branch continues anteriorly, narrowing as it passes lateral to the premaxillary cavity where it forks into two branches, one parallelling the cavity, and another curving laterally.
On the anteroventral surface of the hind‐brain, the small, thin cranial nerve VI (abducens nerve) exits the endocast and trends anteriorly (Fig. 2A,B). Just posterior to the base of cranial nerve V and just ventral to the anterior semicircular canal, cranial nerve VII (facial nerve) extends laterally from the lateral portion of the hind‐brain dorsal to the location observed in E. neukami and P. angustifrons (Lautenschlager & Butler, 2016). CN VII is long and thin and gently curves posteriorly to exit the prootic posterior to where cranial nerve V exits the braincase (Fig. 2). There are thin extensions from the most ventral surface of the hind‐brain that may be the pathways of the internal jugular vein or cranial nerves IX (glossopharyngeal nerve), X (vagus nerve) and XI (spinal accessory nerve) (Fig. 2A,B,D). The final observable pathway, either cranial nerve XII (hypoglossal nerve) or the longitudinal sinus (Lautenschlager & Butler, 2016), is visible posterior to the junction of the lateral and posterior semicircular canals on the hind‐brain (Fig. 2). A crack that runs through the braincase posterior to the hind‐brain makes it difficult to trace this structure any farther, but it does appear to exit through the exoccipital.
Endosseous labyrinth
The endosseous labyrinth of W. scurriensis is robust and complete on both the left and right sides (Fig. 3). The anterior semicircular canals are the longest (2.23 cm), remain narrow from the crus communis to the ampulla, and are convex anteriorly. The posterior semicircular canals are the shortest (1.45 cm), are linear from the crus communis to their intersection with the lateral semicircular canals, and are thicker in diameter at these intersections. The lateral semicircular canals are the thickest in diameter (2.52 mm) and are convex laterally. The canals are oriented at roughly right angles to one another, similar to the orientations of the canals of E. neukami, P. angustifrons, and P. hislopi. However, the endosseous labyrinths of E. neukami and P. angustifrons are dorsoventrally compressed compared with the canals of W. scurriensis, though this may be preservational; when retrodeformed, the anterior and posterior canals of E. neukami and P. angustifrons are dorsally convex from the crus communis. Wannia scurriensis exhibits anterior and posterior canals that are less dorsally convex.
The cochlear ducts extend ventrally from the endosseous labyrinths and are concave medially. These ducts are robust and elongate ventrally, similar to those of D. spurensis (Stocker et al. 2016) but more so than in E. neukami and P. angustifrons. The cochlear ducts of W. scurriensis have three extensions, including a small anteriorly extending trunk one‐third of the way ventrally from the semicircular canals and lateral and posterolateral expansions at the top of the duct, whereas the endocrania of E. neukami and P. angustifrons possess a single posterolateral expansion. The lateral expansions in W. scurriensis likely enter the fenestrae vestibuli (= oval window) and the posterolateral expansions likely enter the fenestrae cochleae (= round window) (Fig. 3), based on comparisons with T. primus (Stocker et al. 2016). The generally triangular vestibule and ventrally elongate cochlear duct are similar to those of extant crocodylians, including Alligator, Crocodylus, and Gavialis (Witmer et al. 2008), as are the locations of the fenestrae vestibuli and cochleae. The inner ear is robust and the cochlear duct is concave medially in these taxa as well.
Paranasal cavities
The paranasal cavity of W. scurriensis was segmented into three sections in accordance with the methods of Lautenschlager & Butler (2016), representative of the antorbital sinus, airway, and premaxillary sinus (Fig. 4). The antorbital cavity is large, filling the posterior half of the rostrum section. As with extant archosaurs, the antorbital cavity of W. scurriensis plays a role in housing the antorbital sinus and maxillary neurovasculature as evidenced by the anterior extension of the trigeminal nerve from the antorbital cavity (Witmer, 1995). The antorbital cavity is both lateral and medial to the airway, which is anteroposteriorly short and unbranched in W. scurriensis. The airway extends posteriorly from the nares at its anterior end to halfway posteriorly in the rostrum section, narrowing posteriorly, similar to those of E. neukami and P. angustifrons. The airway in W. scurriensis rises dorsally to the external nares and ventrally to where it is bounded by the maxillae and premaxillae. The premaxillary cavity, hypothesized to be an anterior evagination of the antorbital air sinus that results in a structurally sound rostrum (Witmer, 1997), extends anteriorly from the nares to the anteriormost edge of the skull, narrowing anteriorly, again similar to the morphology of E. neukami and P. angustifrons. There are small cavities within the ectopterygoids of W. scurriensis similar to those in P. angustifrons (Lautenschlager & Butler, 2016) that do not connect to neurovascular canals or the antorbital cavity and are considered separate ectopterygoid sinuses (Fig. 4B,C). These recesses are present in theropod dinosaurs and other phytosaurs and seem to be pneumatic as well, though the source of pneumatization is uncertain (Witmer, 1997).
Dentition
The preserved posterior end of the right premaxilla only preserves a single alveolus; the right maxilla contains 18 alveoli (corrected from 16 reported by Stocker, 2013). The preserved posterior of the left premaxilla contains one alveolus as well, and the left maxilla contains 17 alveoli. There are 14 teeth preserved in nine alveoli, including nine robust, broken erupted teeth and five small replacement teeth (Fig. 4B,C). The erupted teeth are round in cross‐section, conical in profile, angled laterally, and the roots extend dorsally within the skull to the antorbital cavity or trigeminal nerve pathway. Replacement teeth are present medially and parallel to the erupted teeth, located about halfway down the length of the tooth alongside the erupted teeth. The crowns of the replacement teeth are round in cross‐section and triangular in profile.
Discussion
We provide a potentially plesiomorphic condition for Archosauria by investigating the earliest branching Late Triassic phytosaur. The contested position of Phytosauria at the base of Archosauria leads us to focus on the similarities between members of Archosauriformes, Phytosauria, and Archosauria. All known phytosaur endocrania are similar in their generally elongate shape, mediolateral narrowness, and horizontal organization (Fig. 5). Generally, the more derived taxa within Phytosauria have endocasts that are even more elongate and horizontally oriented than those of the more basal members of the group, which appear more robust and verticalized (Lautenschlager & Butler, 2016). Endocasts of other archosauriforms (e.g. T. primus, T. romeri) and some archosaurs (e.g. aetosaurs, notosuchians, crocodylians) are also generally horizontal, placing fore‐, mid‐, and hind‐brain in a single plane, whereas dinosaurs and poposauroids differ in having more verticalized, robust endocasts (Witmer et al. 2008; Kley et al. 2010; Holloway et al. 2013; Stocker et al. 2016).
Figure 5.
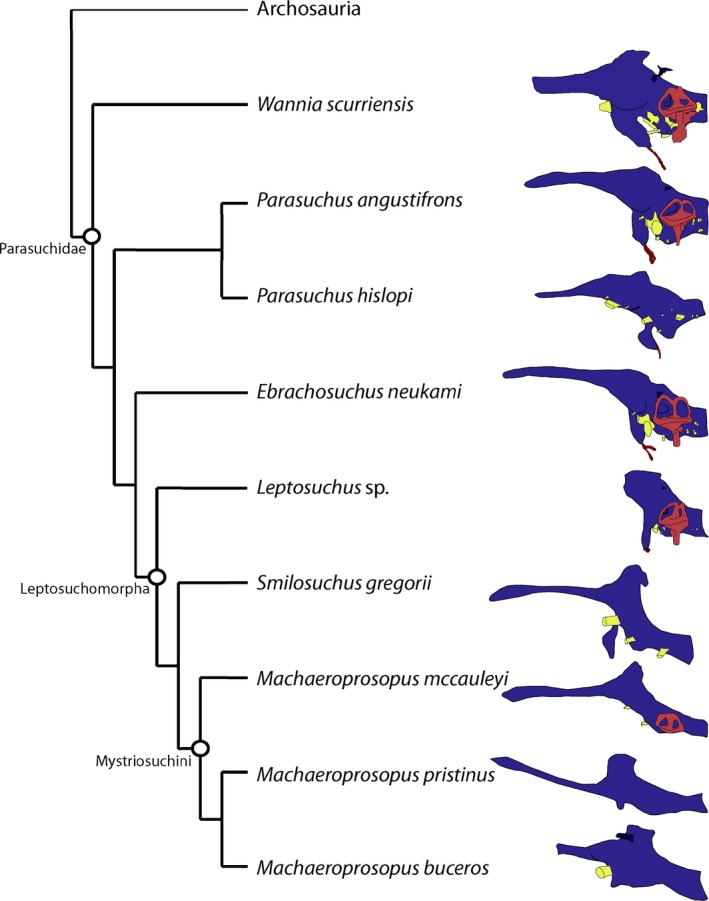
Comparisons to phytosaurs investigated using both traditional methods and CT scanning, simplified to line drawings, emphasizing elongate shape and horizontal orientation (modified from Lautenschlager & Butler, 2016).
Most phytosaurian taxa exhibit enlarged hypophyses, enlarged pineal regions, and elongate olfactory tracts. An enlarged hypophyseal region is representative of rapid growth and a resultant large body size (Edinger, 1942; Witmer et al. 2008; Sander et al. 2011). Whether the expanded pineal region is a result of the presence of a dural venous sinus or paratympanic sinus or simply an enlarged pineal body is indeterminable without osteological boundaries and additional comparative material. An elongate olfactory tract is likely related to the elongate rostrum (Wyneken, 2007). Additionally, the orientation of the lateral semicircular canal with respect to horizontal has been associated with head position in extant animals exhibiting an ‘alert posture’ (Witmer et al. 2003, 2008). Orienting the lateral semicircular canals of W. scurriensis parallel to the horizontal plane results in an upturned olfactory tract and skull roof rather than the level olfactory tract and skull roof observed in E. neukami and P. angustifrons. The angle between the dorsal border of the olfactory tract and the lateral semicircular canal is 13° in W. scurriensis (compared with 7–9° in E. neukami, 6–8° in P. angustifrons, and ~ 0° in M. mccauleyi).
The phytosaurian taxa that preserve endosseous labyrinths also have floccular lobes extending laterally within the anterior semicircular canals (Holloway et al. 2013; Lautenschlager & Butler, 2016; Stocker et al. 2016). The flocculus is responsible for rapid eye, head, and neck movements in extant organisms (Witmer et al. 2003, 2008), and its presence may indicate the potential for similar abilities in phytosaurs. Phytosaur endocasts retain a relatively small flocculus, and this structure is clearly present in the archosauriform T. primus, possibly present in E. capensis, and is small in the archosaurs Postosuchus kirkpatricki, Gracilisuchus stipanicicorum, Herrerasaurus ischigualastensis, and in sauropods (Witmer et al. 2008; Holloway et al. 2013; Stocker et al. 2016). Across Archosauria, there is a reduction of the pineal region and a curve to the olfactory tract in more derived members (Holloway et al. 2013). Cranial nerves and arteries exit the brain endocast in similar locations, though with slight variations, among phytosaurian taxa and across Archosauriformes as well. For example, among phytosaurs, the optic nerve foramen is a single foramen with no bony prominence separating the optic nerves similar to the condition in Crocodylus johnstoni (Witmer et al. 2008). This is in contrast to the condition in some early archosauriforms (e.g. T. primus and Proterosuchus fergusi) and dinosaurs (e.g. theropods, sauropods, and Aves) in which bone divides the optic nerve trunk into paired apertures (Stocker et al. 2016; Clark et al. 1993; Sampson & Witmer, 2007; Witmer & Ridgely, 2009; Witmer et al. 2008). Unlike the single ventral extension in W. scurriensis, in M. mccauleyi there are separate pathways for the internal jugular and CN IX, X, and XII (Holloway et al. 2013), and in E. neukami and P. angustifrons, the trunk of CN IX, X, and XII is present posteroventral to the semicircular canals (Lautenschlager & Butler, 2016). Additionally, the ventral exit of the internal carotid arteries occurs in both non‐archosaurian archosauriforms (e.g. E. capensis) and archosaurs (e.g. Arizonasaurus) (Gower & Sennikov, 1996; Nesbitt, 2011). Finally, many archosaurs (e.g. theropod dinosaurs, aetosaurs) have accessory cavities in addition to the antorbital cavity, that appear to be pneumatic sinuses (Witmer, 1997), and these are observed in W. scurriensis as well.
Typically, phytosaurs and crocodylians are considered convergent because of their elongate rostra, sprawling posture, elongate tails, osteoderm covered bodies, and carnivorous dentition (e.g. Hunt, 1989; Stocker & Butler, 2013). This convergence in overall external morphology is also observed in endocranial comparisons. Phytosaurs and crocodylians share a generally streamlined shape, elongate olfactory tract, large hypophysis, and cranial nerves located on the ventral surface of the brain (Holloway et al. 2013; Lautenschlager & Butler, 2016). The large hypophyseal fossa, which houses a relatively large pituitary in crocodylians and likely housed a large pituitary in phytosaurs, is responsible for the large body size of both groups (Edinger, 1942; Saint Girons, 1970). In addition, the endosseous labyrinths of phytosaurs, specifically W. scurriensis, are extremely similar in morphology to those of Alligator and Crocodylus (e.g. curvature, relative length, and angles and directions in which semicircular canals branch from the crus communis). Similar endosseous labyrinth morphology is indicative of similar postures, specifically the alert head posture (Witmer et al. 2003, 2008). An elongate cochlear duct is present in phytosaurs, Alligator, Crocodylus, Gavialis, and extinct species of crocodylians. The elongate cochlear duct correlates with auditory capability and sensitivity to low‐frequencies (Gleich & Manley, 2000; Gleich et al. 2005), which in extant crocodylians are produced below the surface of the water (Vergne et al. 2009; Dinets, 2011). The similar morphologies of these endocranial features indicate convergence in behavior and ecology possibly related to a semiaquatic lifestyle (e.g. communication and prey acquisition).
The ability of the rostrum to sense changes in the water may be convergent across these groups as well. Extant crocodylians have integumentary sensory organs or dome pressure receptors in their body, face, and mandibles that are responsible for sensing and orienting to changes in the water associated with disturbances (Soares, 2002). Nerve fibers run through the dentary and maxilla, exiting through many small foramina and project both anteriorly and posteriorly out of the lateral surface of those elements (Leitch & Catania, 2012). These foramina, innervated by the trigeminal nerve, are present on the palate, gums, and face in a ‘beehive’ pattern only in extant crocodylians and extinct crocodylians that are hypothesized to be semi‐aquatic back to the Early Jurassic (Soares, 2002). Among reptiles, the majority of trigeminal nerve foramina appear in linear arrangements and in relatively low foramina density to crocodylians (Soares, 2002; Morhardt, 2009). The ‘beehive’ pattern in crocodylians, in addition to high foramina density relative to other reptiles, is similar to the morphology exhibited by W. scurriensis and other phytosaurs [e.g. Angistorhinus (ROM 7977), Rutiodon carolinensis (USNM 214513)] in which branches off the main trigeminal canal extend to scattered foramina on the maxilla and premaxilla (Fig. 4). Therefore, we hypothesize that these foramina are osteological correlates for similar sensory organs in phytosaurs, which is consistent with phytosaurs having a semi‐aquatic paleoecology (Stocker & Butler, 2013). In addition to establishing morphological evidence for semi‐aquatic sensory systems rather than depending on depositional environment and overall external morphology, W. scurriensis confirms similarities in endocranial morphology in phytosaurs and among Archosauriformes.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author contributions
E.J.L. contributed to the concept and design, data analysis and interpretation, and drafting of the manuscript and figures. M.R.S. contributed to the concept and design, acquisition of data, data analysis and interpretation, and critical revision of the manuscript and figures.
Acknowledgements
We thank Bill Mueller for loaning the specimen to MRS for study. We thank Matthew Colbert and The University of Texas CT facility (UTCT) for scanning the specimen. Funding for the scans was provided by the Jackson School of Geosciences to MRS. Stephan Lautenschlager, Casey Holliday, Waymon Holloway, Sterling Nesbitt, Richard Butler, Lawrence Witmer, Christopher J. Bell, and the Paleobiology & Geobiology Research Group at Virginia Tech provided constructive discussion.
References
- Balanoff AM, Bever GS, Colbert MW, et al. (2016) Best practices for digitally constructing endocranial casts: examples from birds and their dinosaurian relatives. J Anat 229, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusatte SL, Benton MJ, Desojo JB, et al. (2010) The higher‐level phylogeny of Archosauria (Tetrapoda: Diapsida). J Syst Paleontol 8, 3–47. [Google Scholar]
- Camp CL (1930) A Study of the Phytosaurs: With Description of New Material from Western North America. Berkeley, California: Memoirs of the University of California; 10, 1–174. [Google Scholar]
- Case EC (1928) An endocranial cast of a phytosaur from the upper Triassic beds of Western Texas. J Comp Neurol 45, 161–168. [Google Scholar]
- Chatterjee S (1978) A primitive parasuchid (phytosaur) reptile from the Upper Triassic Maleri Formation of India. Palaeontology 21, 83–127. [Google Scholar]
- Clark JM, Welman J, Gauthier JA, et al. (1993) The laterosphenoid bone of early archosauriforms. J Vert Paleontol 13, 48–57. [Google Scholar]
- Cope ED (1888) The pineal eye in extinct vertebrates. Am Nat 22, 914–917. [Google Scholar]
- Dinets V (2011) Effects of aquatic habitat continuity on signal composition in crocodilians. Anim Behav 82, 191–201. [Google Scholar]
- Edinger T (1942) The pituitary body in giant animals fossil and living: a survey and a suggestion. Q Rev Biol 17, 31–45. [Google Scholar]
- Ezcurra MD (2016) The phylogenetic relationships of basal archosauromorphs, with an emphasis on the systematics of proterosuchian archosauriforms. PeerJ 4, e1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ID, Holliday CM (2013) Trigeminal nerve morphology in Alligator mississippiensis and its significance for crocodyliform facial sensation and evolution. Anat Rec 296, 670–680. [DOI] [PubMed] [Google Scholar]
- Gleich O, Manley GA (2000) The hearing organ of birds and Crocodilia In: Comparative Hearing: Birds and Reptiles (eds Dooling RJ, Fay RR, Popper AN.), pp. 70–138. New York: Springer. [Google Scholar]
- Gleich O, Dooling RJ, Manley GA (2005) Audiogram, body mass, and basilar papilla length; correlations in birds and predictions for extinct archosaurs. Naturwissenschaften 92, 595–598. [DOI] [PubMed] [Google Scholar]
- Gower DJ (2002) Braincase evolution in suchian archosaurs (Reptilia: Diapsida): evidence from the rauisuchian Batrachotomus kupeferzellensis . Zool J Linn Soc 136, 49–76. [Google Scholar]
- Gower DJ, Nesbitt SJ (2006) The braincase of Arizonasaurus babbitti – further evidence for the non‐monophyly of ‘rauisuchian’ archosaurs. J Vert Paleontol 26, 79–87. [Google Scholar]
- Gower DJ, Sennikov AG (1996) Morphology and phylogenetic informativeness of early archosaur braincases. Palaeontology 39, 883–906. [Google Scholar]
- Gower DJ, Walker AD (2002) New data on the braincase of the aetosaurian archosaur (Reptilia: Diapsida) Stagonolepis robertsoni Agassiz. Zool J Linn Soc 136, 7–23. [Google Scholar]
- Holloway WL, Claeson KM, O'Keefe FR (2013) A virtual phytosaur endocast and its implications for sensory system evolution in archosaurs. J Vert Paleontol 33, 848–857. [Google Scholar]
- Hungerbühler A (2002) The Late Triassic phytosaur Mystriosuchus westphali, with a revision of the genus. Palaeontology 45, 377–418. [Google Scholar]
- Hungerbühler A, Mueller B, Chatterjee S, et al. (2013) Cranial anatomy of the Late Triassic phytosaur Machaeroprosopus with the description of a new species from West Texas. Earth Environ Sci Trans R Soc Edinb 103, 269–312. [Google Scholar]
- Hunt AP (1989) Cranial morphology and ecology among phytosaurs In: Dawn of the Age of Dinosaurs in the American Southwest. (ed. Lucas S. G. and Hunt A. P.), pp. 349–354, Albuquerque: New Mexico Museum of Natural History. [Google Scholar]
- Kley NJ, Sertich JJ, Turner AH, et al. (2010) Craniofacial morphology of Simosuchus clarki (Crocodyliformes: Notosuchia) from the Late Cretaceous of Madagascar. J Vert Paleontol 30, 13–98. [Google Scholar]
- Knoll F, Witmer LM, Ridgely RC, et al. (2015) A new titanosaurian braincase from the Cretaceous ‘Lo Hueco’ locality in Spain sheds light on neuroanatomical evolution within Titanosauria. PLoS One 10, e0138233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston W (1949) A new species of Paleorhinus from the Triassic of Texas. Am J Sci 247, 324–341. [Google Scholar]
- Lautenschlager S, Butler RJ (2016) Neural and endocranial anatomy of Triassic phytosaurian reptiles and convergence with fossil and modern crocodylians. PeerJ 4, e2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager S, Hübner T (2013) Ontogenetic trajectories in the ornithischian endocranium. J Evol Biol 26, 2044–2050. [DOI] [PubMed] [Google Scholar]
- Leitch DB, Catania KC (2012) Structure, innervation and response properties of integumentary sensory organs in crocodilians. J Exp Biol 215, 4217–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl MG (1928) Pseudopalatus pristinus: A New Genus and Species of Phytosaurs from Arizona. Columbia, Missouri: The University of Missouri Studies, 3–25. [Google Scholar]
- Morhardt AC (2009) Dinosaur smiles: do the texture and morphology of the premaxilla, maxilla, and dentary bones of sauropsids provide osteological correlates for inferring extra‐oral structures reliably in dinosaurs? Masters thesis, Western Illinois University.
- Nesbitt SJ (2011) The early evolution of archosaurs: relationships and the origin of major clades. Bull Am Mus Nat Hist 392, 1–292. [Google Scholar]
- Quay W (1979) The parietal eye‐pineal complex In: Biology of the Reptilia 9. (ed. Gans C., Northcutt R. G. and Ulinski P.), pp. 245–406, New York: Academic Press. [Google Scholar]
- Saint Girons H (1970) The pituitary gland In: Biology of the Reptilia 3. (ed. Gans C. and Parsons T. S.), pp. 245–406, London: Academic Press. [Google Scholar]
- Sampson SD, Witmer LM (2007) Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. J Vertebr Paleontol 27, 32–102. [Google Scholar]
- Sander MP, Christian A, Clauss M, et al. (2011) Biology of the sauropod dinosaurs: the evolution of gigantism. Biol Rev 86, 117–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D (2002) Neurology: an ancient sensory organ in crocodilians. Nature 417, 241–242. [DOI] [PubMed] [Google Scholar]
- Sobral G, Sookias RB, Bhullar B‐AS, et al. (2016) New information on the braincase and inner ear of Euparkeria capensis Broom: implications for diapsid and archosaur evolution. R Soc Open Sci 3, 91–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker MR (2010) A new taxon of phytosaur (Archosauria: Pseudosuchia) from the Late Triassic (Norian) Sonsela Member (Chinle Formation) in Arizona, and a critical reevaluation of Leptosuchus . Palaeontology 53, 997–1022. [Google Scholar]
- Stocker MR (2013) A new taxonomic arrangement for Paleorhinus scurriensis . Earth Environ Sci Trans R Soc Edinb 103, 251–263. [Google Scholar]
- Stocker MR, Butler RJ (2013) Phytosauria. Geological Society, London, Special Publications 379.
- Stocker MR, Nesbitt SJ, Criswell KE, et al. (2016) A dome‐headed stem archosaur exemplifies convergence among dinosaurs and their distant relatives. Curr Biol 26, 2674–2680. [DOI] [PubMed] [Google Scholar]
- Trotteyn MJ, Paulina‐Carabajal A (2016) Braincase and neuroanatomy of Pseudochampsa ischigualastensis and Tropidosuchus romeri (Archosauriformes, Proterochampsia). Ameghiniana 53, 527–542. [Google Scholar]
- Vergne AL, Pritz MB, Mathevon N (2009) Acoustic communication in crocodilians: from behavior to brain. Biol Rev 84, 391–411. [DOI] [PubMed] [Google Scholar]
- Witmer LM (1995) Homology of facial structures in extant archosaurs (birds and crocodilians), with special reference to paranasal pneumaticity and nasal conchae. J Morphol 225, 269–327. [DOI] [PubMed] [Google Scholar]
- Witmer LM (1997) The evolution of the antorbital cavity of archosaurs: a study in soft‐tissue reconstruction in the fossil record with an analysis of the function of pneumaticity. J Vert Paleontol 17, 1–75. [Google Scholar]
- Witmer LM, Ridgely RC (2009) New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec 292, 1266–1296. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Chatterjee S, Franzosa J, et al. (2003) Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature 425, 950–953. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Ridgely RC, Dufeau DL, et al. (2008) Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs In: Anatomical Imaging: Towards A New Morphology (eds. Endo H, Frey R.), pp. 67–88. Tokyo: Springer‐Verlag. [Google Scholar]
- Wyneken J (2007) Reptilian neurology: anatomy and function. Vet Clin North Am Exot Anim Pract 10, 837–853. [DOI] [PubMed] [Google Scholar]


