Abstract
Co‐localization of dopamine with other classical neurotransmitters in the same neuron is a common phenomenon in the brain of vertebrates. In mammals, some dopaminergic neurons of the ventral tegmental area and the hypothalamus have a glutamatergic co‐phenotype. However, information on the presence of this type of dopaminergic neurons in other vertebrate groups is very scant. Here, we aimed to provide new insights on the evolution of this neuronal co‐phenotype by studying the presence of a dual dopaminergic/glutamatergic neuron phenotype in the central nervous system of lampreys. Double immunofluorescence experiments for dopamine and glutamate in adult sea lampreys revealed co‐localization of both neurotransmitters in some neurons of the preoptic nucleus, the nucleus of the postoptic commissure, the dorsal hypothalamus and in cerebrospinal fluid‐contacting cells of the caudal rhombencephalon and rostral spinal cord. Moreover, co‐localization of dopamine and glutamate was found in dopaminergic fibres in a few brain regions including the lateral pallium, striatum, and the preoptic and postoptic areas but not in the brainstem. Our results suggest that the presence of neurons with a dopaminergic/glutamatergic co‐phenotype is a primitive character shared by jawless and jawed vertebrates. However, important differences in the distribution of these neurons and fibres were noted among the few vertebrates investigated to date. This study offers an anatomical basis for further work on the role of glutamate in dopaminergic neurons.
Keywords: cerebrospinal fluid‐contacting cell, dopamine, glutamate, hypothalamus, lampreys, rhombencephalon
Introduction
More and more studies are showing that the co‐localization of two classical neurotransmitters in the same neuron [for instance, GABA and glycine, dopamine (DA) or serotonin] is a common phenomenon in the central nervous system of vertebrates, including lampreys (see Rodicio et al. 2008; Villar‐Cerviño et al. 2008, 2009; Barreiro‐Iglesias et al. 2009a,b). Although the dopaminergic system is one of the best‐characterized neuronal systems in vertebrates (for a revision, see Smeets & González, 2000), it is only recently that co‐localization of DA with glutamate (GLU; the main excitatory neurotransmitter) has been reported in some neurons. The first demonstration that dopaminergic neurons of the rodent midbrain release GLU at synapses was performed using single‐neuron patch‐clamp recordings in cell cultures (Sulzer et al. 1998; Bourque & Trudeau, 2000). Other studies in rodents have found tyrosine hydroxylase (TH)‐expressing dopaminergic neurons of the ventral tegmental area of the midbrain that co‐express the vesicular GLU transporter 2 (VGlut2) (Dal Bo et al. 2004; Kawano et al. 2006; see Morales & Root, 2014; Yamaguchi et al. 2015). The study by Kawano et al. (2006) also reported co‐expression of VGlut2 in almost half of the TH‐positive neurons in the A11 group of the rat hypothalamus. In the nucleus accumbens of mice, GLU is released by dopaminergic terminals of ventral tegmental area/substantia nigra neurons (Stuber et al. 2010). More recently, Mingote et al. (2015) reported that dopaminergic neurons of the ventral tegmental area elicit glutamatergic signals in specific areas of the forebrain including the nucleus accumbens, olfactory tubercle, entorhinal cortex, central amygdala and cingulate cortex. It has been suggested that VGlut2 may facilitate the vesicular loading of DA in projections of the ventral tegmental area to the ventral striatum of mice (Hnasko et al. 2010). The glutamatergic/dopaminergic co‐phenotype might also be important for the development of mesencephalic dopaminergic neurons (Fortin et al. 2012). Moreover, expression of VGlut2 in dopaminergic neurons decreases as development progresses in the ventral tegmental area of mice (Dal Bo et al. 2008; Mendez et al. 2008) and the ventral tegmental area/substantia nigra of rats (Bérubé‐Carrière et al. 2009).
The co‐localization of glutamatergic and dopaminergic markers in neurons is almost unknown in non‐mammalian vertebrates. A recent study in zebrafish has reported the existence of dopaminergic neurons that express vglut2 transcripts in the posterior tuberculum, but not in other dopaminergic nuclei, which suggests that they may store GLU in vesicles (Filippi et al. 2014). Whether the presence of neurons with a DA/GLU co‐phenotype is a specialization of zebrafish and rodents, or represents an ancestral feature shared with other vertebrates is not known.
Jawless vertebrates such as lampreys are the first vertebrates to arise in evolution, diverging from lines leading to mammals about 560 million years ago. The key outgroup position of lampreys at the base of the vertebrate phylogeny makes them an interesting animal model to understand early vertebrate brain evolution. Various immunohistochemical studies in lampreys have revealed the functional organization and development of the dopaminergic system in lampreys (Pierre et al. 1997; Pombal et al. 1997a,b; Abalo et al. 2005; Barreiro‐Iglesias et al. 2010a,b; Ericsson et al. 2013; Ryczko et al. 2013). The distribution of glutamatergic neurons in the sea lamprey brain has been reported on recently using anti‐GLU immunofluorescence and VGlut in situ hybridization methods (Villar‐Cerviño et al. 2011, 2013). These VGlut in situ hybridization results supported the validity of the immunofluorescence approach with anti‐GLU antibodies to investigate the glutamatergic populations in the brain of the adult sea lamprey (Villar‐Cerviño et al. 2011, 2013). Immunohistochemistry offers clear advantages with respect to in situ hybridization to reveal the morphology of neurons and processes. GLU immunofluorescence stains cell bodies, dendrites and axons in the sea lamprey, allowing a better characterization of glutamatergic cell populations. Regarding the distribution of GLU in the central nervous system, strong immunoreactivity is observed in neuronal perikarya that exhibit the same distribution as that of the VGlut‐positive neurons, except for the giant reticulospinal neurons that express VGlut but are not GLU‐immunoreactive (GLU‐ir) (Villar‐Cerviño et al. 2011, 2013; Fernández‐López et al. 2012). As observed for VGlut expression (Villar‐Cerviño et al. 2011, 2013), ependymal cells are not GLU‐ir in the sea lamprey, which indicates that the anti‐GLU antibodies are not detecting basal metabolic GLU in cells.
Double immunofluorescence methods and confocal microscopy have been used previously to show the co‐localization of GABA with DA or serotonin (Rodicio et al. 2008; Barreiro‐Iglesias et al. 2009a,b) or GLU with GABA or glycine (Villar‐Cerviño et al. 2011, 2013; Fernández‐López et al. 2012) in neurons of several regions of the sea lamprey central nervous system. Here, we aimed to investigate the possible co‐localization of DA and GLU in the sea lamprey by using double immunofluorescence methods. Our results reveal co‐localization of DA and GLU in some neurons of the lamprey brain and spinal cord, and provide new information for our understanding of the evolution of this neuronal co‐phenotype.
Methods
Animals
All experiments were approved by the Bioethics Committee at the University of Santiago de Compostela and the Consellería do Medio Rural e do Mar of the Xunta de Galicia (code JLPV/IId; Spain) and were performed in accordance with European Union and Spanish guidelines on animal care and experimentation.
Young postmetamorphic adult sea lampreys (Petromyzon marinus L.; n = 7) were used for immunofluorescence experiments. They were collected from the River Ulla (Galicia, northwestern Spain) with permission from the Xunta de Galicia, and maintained in aerated aquaria before use. For experiments, the animals were deeply anaesthetized with 0.02% MS‐222 (Sigma, St. Louis, MO, USA) in fresh water and killed by decapitation. The brain/rostral spinal cord was dissected out and fixed by immersion in 5% glutaraldehyde and 1% sodium metabisulphite in 0.05 m Tris‐buffered saline (TBS; pH 7.4) for 17 h. The fixed brains were embedded in Neg 50™ (Microm International GmbH, Walldorf, Germany), frozen in liquid nitrogen‐cooled isopentane, sectioned on a cryostat in the transverse plane (18‐μm‐thick sections), and mounted on Superfrost Plus glass slides (Menzel, Braunschweig, Germany).
Immunofluorescence
For immunofluorescence, sections were pretreated with 0.2% NaBH4 in deionized water for 45 min at room temperature to quench glutaraldehyde‐induced autofluorescence. The sections were incubated overnight at 4 °C with a mix of a rabbit polyclonal anti‐DA antibody (1 : 750; Dr H. W. M. Steinbusch, Maastricht, the Netherlands) and a mouse monoclonal anti‐GLU antibody (1 : 1000; Swant, Bellinzona, Switzerland) in TBS containing 1% sodium metabisulphite, 15% normal goat serum and 0.2% Triton X‐100. Then the sections were rinsed in TBS and incubated for 1 h at room temperature with Cy3‐conjugated goat anti‐rabbit (1 : 200; Millipore, Temecula, CA, USA) and FITC‐conjugated goat anti‐mouse (1 : 100; Millipore) antibodies.
Controls of specificity
The DA antiserum was raised against a DA‐bovine serum albumin (BSA) conjugate and has very low cross‐reaction with noradrenalin (< 10% cross‐reaction) and other monoamines (< 1% cross‐reaction) (Steinbusch et al. 1991). Control experiments (pre‐adsorption with the corresponding antigen, a DA‐BSA conjugate, and Western blots of lamprey brain protein extracts) previously carried out in our laboratory confirmed the specificity of this antibody (Barreiro‐Iglesias et al. 2008) in lamprey tissue. In addition, there is a good correspondence between the populations revealed by this anti‐DA antibody and those revealed by TH in situ hybridization (Barreiro‐Iglesias et al. 2010b).
The mouse monoclonal anti‐GLU antibody was raised against a glutaraldehyde‐linked L‐GLU‐BSA conjugate by Dr P. Streit (Liu et al. 1989), and this clone was made commercially available through Swant. This antibody has been characterized with respect to cross‐reactivity by antibody dilution experiments as well as by pre‐adsorption experiments (Adám & Csillag, 2006) and it has been used in a previous study of the sea lamprey spinal cord (Fernández‐López et al. 2012). The monoclonal anti‐GLU antibody used in the present investigation showed the same pattern of glutamatergic neuronal populations in the adult sea lamprey brain as that previously described when using two different polyclonal anti‐GLU antibodies. More importantly, all these anti‐GLU antibodies reveal the same distribution of glutamatergic neuronal populations observed by VGlut in situ hybridization (Villar‐Cerviño et al. 2011, 2013).
As a control for the secondary antibodies, the incubation with the primary antibodies was omitted for some sections. No staining was observed in these controls.
Image acquisition
Sections were analysed and photographed with TCS‐SP5 and TCS‐SP2 spectral confocal microscopes (Leica). For co‐localization of neurotransmitters in fibres and terminals, representative fields of fibres throughout the main brain regions were imaged with a high aperture glycerol‐immersion objective (63×, N.A. 1.35). Image processing and confocal projections were done using the lite (Leica) or imagej software. For plate composition, the photomicrographs were adjusted for brightness and contrast using the Adobe photoshop CC software.
Quantification of double‐labelled cells
To quantify the percentage of dopaminergic cells that showed GLU immunoreactivity in each nucleus, one of every three (preoptic and the postoptic commissure nuclei) and one of every four (dorsal hypothalamus) sections were analyzed from rostral to caudal in each nucleus. The total number of DA‐immunoreactive (DA‐ir) cells and double‐labelled cells was manually counted by going through the stack of confocal optical sections viewed with imageJ. The percentage of DA‐ir cells that were also GLU‐ir was calculated for each section. Then, the mean percentage of co‐localization was calculated for each nucleus of each animal. The mean (± standard error of the mean) percentage of co‐localization is given based on the quantification of several animals.
Nomenclature
For the description of DA‐ir neuronal populations and brain regions, we followed the nomenclature used in Barreiro‐Iglesias et al. (2009b).
Results
General organization of the dopaminergic and glutamatergic neurons in the sea lamprey brain
Numerous DA‐ir neurons were distributed in discrete groups of the forebrain (olfactory bulbs, striatum, preoptic nucleus, postoptic commissure nucleus, dorsal and ventral hypothalamic nuclei, mammillary nucleus and paratubercular nucleus), rhombencephalon (ventral isthmus and caudal rhombencephalon) and spinal cord of adult sea lampreys, confirming the results of previous studies of this lamprey species (Abalo et al. 2005; Barreiro‐Iglesias et al. 2008, 2009b). Double DA⁄GLU immunofluorescence showed that glutamatergic cell populations are far more numerous and widely distributed in the sea lamprey than are dopaminergic cells and that most of them do not show DA immunoreactivity. The distribution of the lamprey glutamatergic populations has been reported recently using both GLU immunohistochemistry and VGlut in situ hybridization (Villar‐Cerviño et al. 2011, 2013) and its detailed study is beyond the scope of the present investigation. Accordingly, study of co‐localization of DA and GLU immunoreactivities in neurons was centred on the DA‐ir brain nuclei. The topological organization of the dopaminergic populations in the adult sea lamprey and the location of DA⁄GLU double immuno‐labelled neurons are schematically illustrated in Fig. 1. Only clearly double‐labelled neurons, as revealed by orthogonal reconstructions of single‐plane confocal sections, were considered in the present investigation. The following dopaminergic neuronal populations were considered: (i) the DA‐ir granule‐like cells of the olfactory bulbs; (ii) the scarce DA‐ir neurons located in the striatum; (iii) the DA‐ir neurons of the preoptic nucleus; (iv) the DA‐ir neurons of the postoptic commissure nucleus; (v) the numerous DA‐ir cells of the dorsal and ventral hypothalamic regions; (vi) the numerous DA‐ir cerebrospinal fluid‐contacting (CSF‐c) cells of the mammillary nucleus; (vii) the DA‐ir paratubercular nucleus; (viii) the scarce DA‐ir cells of the ventral isthmus; (ix) the group of DA‐ir CSF‐c cells of the caudal rhombencephalon; (x) the DA‐ir CSF‐c cells of the ventral spinal cord and (xi) the DA‐ir cells located in the ventral midline of the spinal cord. We did not observe any rostro‐caudal differences in the distribution of double‐labelled cells in the nuclei that contained these type of neurons.
Figure 1.
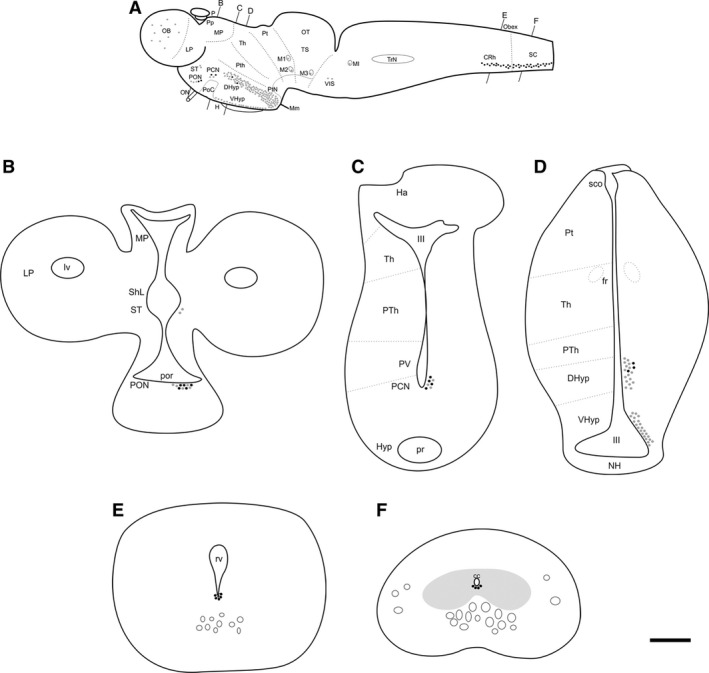
Schematic drawings of a lateral view (A) and transverse sections (B–F) of the brain of the sea lamprey showing the distribution and location of double‐labelled DA‐ir⁄GLU‐ir cells (black dots) in comparison with the distribution of DA‐ir cells (grey dots). Note that a dot may not represent a single neuron. Black lines on top and bottom of (A) indicate the plane of section of the transverse schematics in (B–F). In (B–F) the location of the cells is shown only for the right side with the name of the regions on the left side. cc, central canal; CRh, caudal rhombencephalon; DHyp, dorsal hypothalamus; H, hypophysis; Ha, habenula; III, third ventricle; LP, lateral pallium; lv, lateral ventricle; M1‐3 and MI, giant Müller cells; Mm, mammillary nucleus; MP, medial pallium; NH, neurohypophysis; OB, olfactory bulbs; ON, optic nerve; OT, optic tectum; P, pineal organ; PCN, postoptic commissure nucleus; PON, preoptic nucleus; PoC, postoptic commissure; por, preoptic recess; Pp, parapineal organ; pr, posterior hypothalamic recess; Pt, pretectum; PTh, prethalamus; PtN, paratubercular nucleus; PV, paraventricular nucleus; rv, rhombencephalic ventricle; SC, spinal cord; sco, subcommissural organ; ShL, subhippocampal lobe; ST, striatum; Th, thalamus; TrN, trigeminal nucleus; TS, torus semicircularis; VIS, ventral isthmus; VHyp, ventral hypothalamus. Scale bar: 800 μm (A) and 125 μm (B–F). Modified from Barreiro‐Iglesias et al. (2009b) and Villar‐Cerviño et al. (2011, 2013).
Co‐localization of DA and GLU in neurons of the forebrain
No co‐localization of DA and GLU was observed in the DA‐ir granule‐like cells of the olfactory bulbs or in the scarce DA‐ir cells of the striatum (not shown). In the preoptic nucleus, spindle‐shaped dopaminergic cells showed co‐localization of DA and GLU immunoreactivities (59.25 ± 14.47% of the dopaminergic neurons, n = 5 brains, n = 255 neurons; Fig. 2A–A″). Co‐localization with GLU was observed in the somata of dopaminergic cells (Fig. 2A–A″) and in thick beaded cell processes coursing in the neuropil of this area (Fig. 3A–A″). Some spindle‐shaped cells of the postoptic commissure nucleus showed co‐localization of DA and GLU immunoreactivities (68.18 ± 11.94% of the dopaminergic neurons, n = 4 brains, n = 98 neurons; Fig. 2B–B″). In this nucleus, however, most neurons were only GLU‐ir. Some beaded processes coursing in the neuropil of this nucleus were DA/GLU double‐labelled (Fig. 3B–B″).
Figure 2.
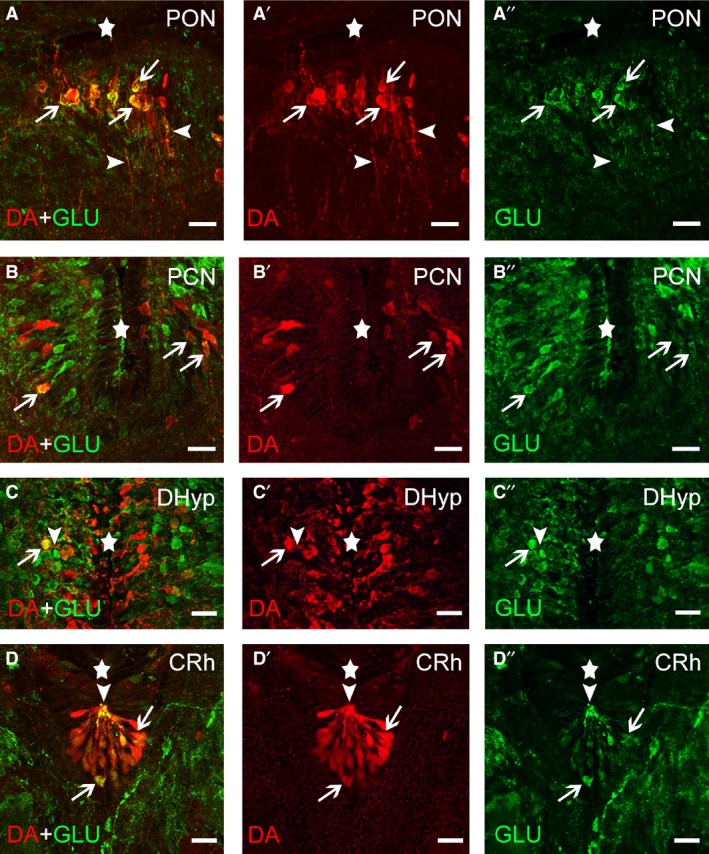
Photomicrographs of transverse sections of the sea lamprey brain showing the presence of double‐labelled DA‐ir (red channel)/GLU‐ir (green channel) neurons (arrows) in the preoptic (PON; A–A″) and postoptic commissure (PCN; B–B″) nuclei, the dorsal hypothalamus (DHyp; C–C″) and the caudal rhombencephalon (CRh; D–D″). Arrowheads indicate the presence of double‐labelled DA‐ir/GLU‐ir processes. The stars indicate the ventricles. Scale bars: 25 μm.
Figure 3.
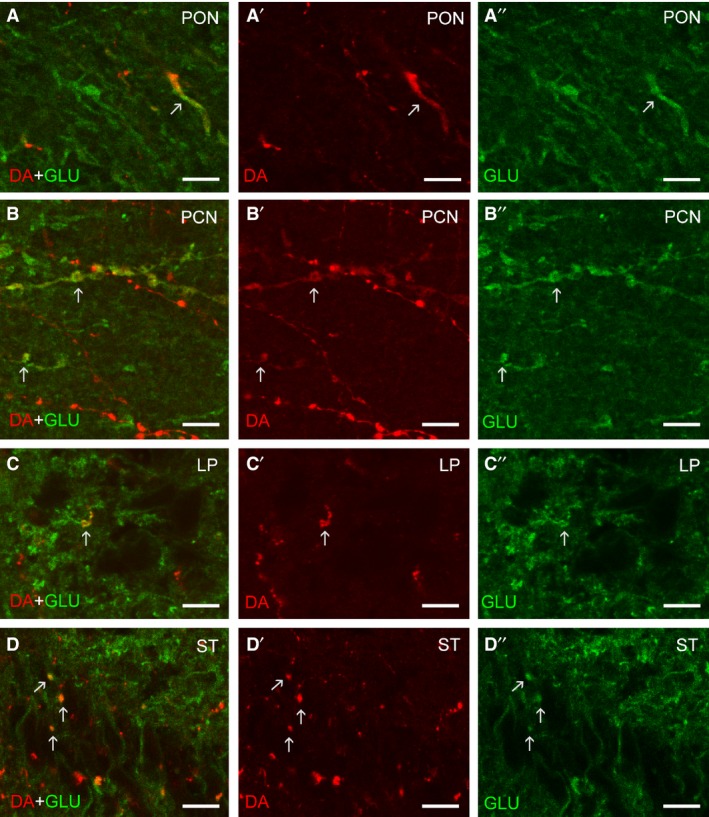
High magnification confocal photomicrographs showing DA/GLU double‐labelled processes (arrows) in the preoptic nucleus (A–A″), postoptic commissure nucleus (B–B″), lateral pallium (C–C″) and striatum (D–D″). The left column represents merged channels, the middle column represents DA immunoreactivity (red channel), and the right column represents GLU immunoreactivity (green channel). Images are projections of two to three 0.5‐μm‐thick confocal sections. Scale bars: 5 μm.
The hypothalamus of the sea lamprey contained numerous DA‐ir cells in dorsal, ventral and mammillary regions. A few rounded perikarya of the dorsal hypothalamus showed co‐localization of GLU and DA immunoreactivities (13.40 ± 4.23% of the dopaminergic neurons, n = 4 brains, 1289 neurons; Fig. 2C–C″). Usually these double‐labelled cells were observed in the second or third layer of periventricular cells away from the ventricle. Co‐localization of DA and GLU immunoreactivities was also observed in the ventricular process of some of these cells (Fig. 2C–C″). In this nucleus, however, most neurons exhibited either DA or GLU immunoreactivity. In addition, the mammillary region and the ventral hypothalamus showed numerous DA‐ir and GLU‐ir neurons, but co‐localization of these immunoreactivities in the same neuron was not observed (not shown). The retro‐mammillary/posterior tubercle region of the sea lamprey contains a group of non‐CSF‐contacting TH‐ir and DA‐ir neurons with prominent dendrites, the paratubercular nucleus (Abalo et al. 2005; Barreiro‐Iglesias et al. 2010b; Ryczko et al. 2013). No co‐localization of DA and GLU immunoreactivities was observed in DA‐ir cells of the paratubercular nucleus, or in processes of these cells.
Co‐localization of DA and GLU in neurons of the rhombencephalon and rostral spinal cord
In the rostral rhombencephalon, no co‐localization with GLU immunoreactivity was observed in the scarce DA‐ir cells of the ventral isthmus (not shown). The largest population of dopaminergic neurons of the rhombencephalon is located in its caudal region and consists of small CSF‐contacting cells. Co‐localization of DA and GLU was observed in almost all, if not all, of the CSF‐contacting DA‐ir cells (Fig. 2D–D″). Co‐localization was observed in apical dendrites and perikarya of these cells (Fig. 2D–D″). The GLU‐ir cells observed in surrounding regions were negative for DA. The DA‐ir CSF‐c cells of the caudal rhombencephalon continued along the rostral spinal cord as ventral CSF‐c cells. As in the rhombencephalon, all these cells were also GLU‐ir (Fig. 4). Co‐localization was observed in apical dendrites and perikarya of these cells (Fig. 4). The non‐CSF‐c DA‐ir cells located in the ventral midline of the spinal cord were not GLU‐ir (not shown).
Figure 4.
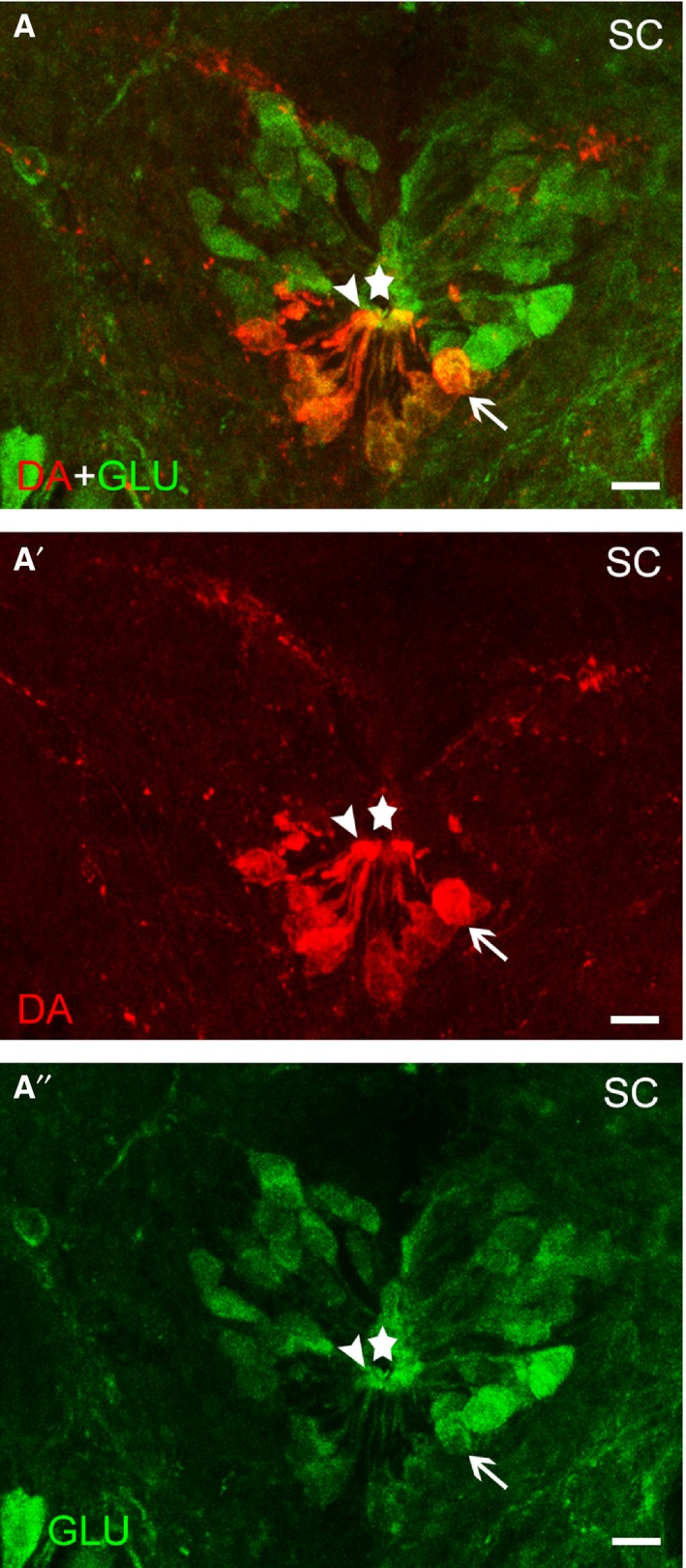
Photomicrograph of a transverse section of the sea lamprey rostral spinal cord (SC) showing the presence of double‐labelled (A) DA‐ir (red channel; A′)/GLU‐ir (green channel; A′′) CSF‐c neurons (arrows). Arrowheads indicate the presence of double‐labelled DA‐ir/GLU‐ir dendrites ending as a club on the central canal. The stars indicate the central canal. Scale bars: 12 μm.
Co‐localization of DA and GLU in fibres of the brain
To investigate possible targets of DA/GLU double‐labelled neurons, we examined a number of brain areas receiving abundant dopaminergic fibres. These regions included: the ventral areas of the lateral pallium, the striatum (both periventricular and ventrolateral regions), the preoptic area, the neuropil region of the nucleus of the post‐optic commissure, the hypothalamus including the mammillary recess, and the dorsal thalamus (lateral neuropil) in the forebrain, the torus semicircularis in the midbrain, the dorsal, intermediate and ventral neuropil in the isthmic region, and the region of the dorsal isthmic (‘cerebellar’) commissure and the region ventrolateral to the trigeminal motor nucleus in the rhombencephalon. Of these regions, only the lateral pallium, the striatum (both periventricular and ventrolateral regions), the preoptic area and the neuropil region of the nucleus of the postoptic commissure showed DA/GLU double‐labelled fibres (Fig. 3A–D). The telencephalon was richly innervated by DA‐ir fibres, which were abundant in the lateral pallium and striatum. In the lateral pallium and striatum, a few DA‐ir fibres were clearly double‐labelled with GLU immunoreactivity (Fig. 3C,D), but most were single‐labelled. Although many dopaminergic fibres were observed in the tuberal and mammillary hypothalamic regions, no double‐labelled fibres were observed (not shown). The same occurred in dorsal thalamus, posterior tubercular region, torus semicircularis, isthmus and trigeminal tegmental regions (not shown).
Discussion
This study shows that in the sea lamprey brain some dopaminergic neurons in the forebrain, rhombencephalon and spinal cord present a dual DA/GLU phenotype, as revealed by DA and GLU double immunofluorescence experiments. These neurons were observed in nuclei that contain both neurons expressing lamprey TH mRNA (Barreiro‐Iglesias et al. 2010b) and neurons expressing lamprey VGlut mRNA (Villar‐Cerviño et al. 2011, 2013), as shown by in situ hybridization. Taken together, these results in sea lamprey suggest that DA/GLU double‐immunolabelled cells both synthesize DA and accumulate GLU in synaptic vesicles, probably releasing them together. In the nuclei where this double phenotype was observed, these cells represent only a fraction of the dopaminergic population with the exception of the rhombencephalic caudal population. In addition to double‐labelled perikarya, double‐labelled fibres and terminals were observed in a few brain regions, including the preoptic and postoptic area, the striatum and lateral pallium. This dual DA/GLU neuronal phenotype has been mainly described and studied in the midbrain ventral tegmental area and the hypothalamic A11 population of rodents (Kawano et al. 2006; see Morales & Root, 2014; Yamaguchi et al. 2015). As in most nuclei of the sea lamprey, only a portion of the dopaminergic neurons in these rodent populations showed a double phenotype. In addition, projections of these dual phenotype cells to specific areas of the forebrain have been reported recently (Mingote et al. 2015). Restricted co‐localization of TH immunoreactivity and VGlut2a/b expression was observed in the brain only in the posterior tubercular/hypothalamus region of larval zebrafish, being absent in other catecholaminergic nuclei of the zebrafish brain (Filippi et al. 2014).
The absence of dopaminergic populations in the lamprey mesencephalon precludes any direct comparison between midbrain DA/GLU populations of mammals and the DA/GLU populations of the sea lamprey brain. However, comparative studies of the development of midbrain‐diencephalic dopaminergic groups in tetrapods using a segmental approach suggest a common process for the generation of these neurons along the rostrocaudal brain axis (Marín et al. 1998), and thus the posterior tubercle and midbrain populations might be considered as segmental homologues. In both lampreys and zebrafish, an ascending projection system from the dopaminergic posterior tubercle populations toward the basal telencephalon has been demonstrated experimentally (Pombal et al. 1997a; Rink & Wullimann, 2001), and these projections may be considered to represent the equivalent (analogue) of the nigro‐striatal projection of tetrapods due to the lack of mesencephalic dopaminergic populations. The posterior tuberculum/paratubercular region‐striatal circuit of lampreys has been suggested to be functionally organized as the mammalian nigro‐striatal system (Ericsson et al. 2013). Some recent authors refer to these lamprey posterior tubercle populations as substantia nigra pars compacta (see Ericsson et al. 2013), but homology of these nuclei is unclear or incomplete from an embryologic point of view (Yamamoto & Vernier, 2011; Pérez‐Fernández et al. 2014) and this name should be avoided. In zebrafish, some dopaminergic neurons of the posterior tuberculum show VGlut2 expression (Filippi et al. 2014). Those authors suggested that these dopaminergic neurons of the zebrafish posterior tuberculum could elicit a dual DA/GLU response in the striatum similar to that reported in the nigro‐striatal system of rodents. Unlike that reported in zebrafish, present results in lampreys show that the posterior tuberculum/paratubercular dopaminergic neurons do not co‐localize GLU immunoreactivity at the levels detected by the current immunohistochemical approach. Therefore, this represents an important difference between the paratubercular dopaminergic neurons of the sea lamprey and the dopaminergic cells of the zebrafish posterior tuberculum (Filippi et al. 2014) and the rodent ventral tegmental area/substantia nigra (see above).
In rodents, half of the TH‐positive neurons of the A11 hypothalamic dopaminergic nucleus show expression of VGlut2 (Kawano et al. 2006). A few neurons with the DA‐GLU co‐phenotype were also observed in the dorsal hypothalamus of the sea lamprey. This finding, together with the observation of numerous TH‐VGlut2 expressing neurons in the posterior tuberal nucleus of zebrafish (Filippi et al. 2014), suggests that the presence of hypothalamic neurons with a DA/GLU co‐phenotype is a feature that appeared early during vertebrate evolution. However, whether the dorsal hypothalamic neurons of lampreys, the A11 neurons of mice and the posterior tuberal neurons of zebrafish are homologous is not known. Interestingly, descending dopaminergic diencephalic projections to the spinal cord arise from A11 neurons in mice (Qu et al. 2006), the periventricular nucleus of the posterior tuberculum in zebrafish (Kuscha et al. 2012) and the paratubercular and mammillary nuclei in sea lamprey (Barreiro‐Iglesias et al. 2008). In lampreys, these nuclei did not show DA/GLU co‐localization (present results).
Our study revealed co‐localization of DA and GLU immunoreactivity in a population of CSF‐contacting cells located in the ventral midline of the caudal rhombencephalon. As far as we are aware, this is the first time this DA/GLU co‐phenotype has been reported in the rhombencephalon of any vertebrate species. A different phenotype expressing TH and VGlut2 has been reported in the zebrafish rhombencephalon (Filippi et al. 2014). These cells lack CSF‐contacting processes and were considered to be noradrenergic neurons, although a mix of dopaminergic and noradrenergic neurons has been reported in the zebrafish medulla (Kaslin & Panula, 2001). As TH is a marker of both noradrenergic and dopaminergic neurons, results of Filippi et al. (2014) using TH as a marker do not rule out the possibility that some of these dual cells could be dopaminergic. Regarding the DA‐ir CSF‐contacting cells of the lamprey caudal rhombencephalon, some of them originate descending projections to the rostral spinal cord (Barreiro‐Iglesias et al. 2008); therefore, they could be a source of dual DA‐GLU descending transmission to the spinal cord, whereas in jawed vertebrates it should be of diencephalic origin (see previous paragraph). Previous studies reported that all the DA‐ir CSF‐c neurons of the caudal rhombencephalon of the sea lamprey were also GABA‐ir (Barreiro‐Iglesias et al. 2009b), which indicates that they actually have a triple co‐phenotype (DA, GLU and GABA) in terms of classical neurotransmitters. The existence of neurons with a similar triple neurotransmitter phenotype has been described previously in rodents, in which some neurons of the ventral tegmental area are TH/VGlut2/glutamic acid decarboxylase (GAD)‐positive (Barker et al. 2016). Whether these lamprey medullary cells release DA, GABA and GLU at synapses needs to be investigated. The DA‐ir CSF‐c cells of the caudal rhombencephalon continue along the ventral spinal cord where they also were GLU‐ir in the spinal cord (present results). These neurons are also GABA‐ir (Rodicio et al. 2008) and therefore also have a triple co‐phenotype.
Finally, we observed neurons with the DA/GLU co‐phenotype in the postoptic commissure and preoptic nuclei of the sea lamprey. Neurons in the postoptic commissure and preoptic nuclei could also have a triple DA/GLU/GABA phenotype, as some neurons of these nuclei co‐localize DA and GABA (Barreiro‐Iglesias et al. 2009b). To our knowledge, the presence of dual DA/GLU neurons in preoptic and postoptic brain regions has not been described in other vertebrates. This indicates that its presence is a feature of the lamprey, but whether it is a derived character or represents an ancestral condition is not known. More studies are needed in other vertebrate lineages, including basal jawed vertebrates such as elasmobranches, for understanding the evolution of this neuronal co‐phenotype.
In conclusion, the presence of DA/GLU‐containing neurons is shared by jawless and jawed vertebrates. The present study extends our knowledge on the distribution of neurons with a dual DA/GLU phenotype in the central nervous system of vertebrates, revealing a notable diversity in terms of brain location and possible functions of these cells.
Author contributions
Acquisition of data: B.F.L., D.S.C. and A.B.I. Analysis and interpretation of data: B.F.L., D.S.C., M.C.R., R.A. and A.B.I. Drafting of the manuscript: A.B.I. Preparation of figures: B.F.L. and A.B.I. Critical revision of the manuscript: M.C.R. and R.A. Obtained funding: M.C.R. and A.B.I. Study concept and design and study supervision: A.B.I. All authors have approved the submitted manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We sincerely thank the Servicio de Microscopía and Mercedes Rivas Cascallar (Universidade de Santiago de Compostela) for confocal microscope facilities and assistance and the staff of Ximonde Biological Station for providing the lampreys used in this study. This work was supported by grants from the Spanish Ministry of Science and Education and the European Regional Development Fund 2007–2013 (BFU2004‐01080; BFU2007‐61056/BFI), the Spanish Ministry of Economy and Competitiveness and the European Regional Development Fund 2007–2013 (BFU2014‐56300‐P) and the Xunta de Galicia (GPC2014/030). A.B.I. was supported by a grant from the Xunta de Galicia (2016‐PG008).
References
- Abalo XM, Villar‐Cheda B, Anadón R, et al. (2005) Development of the dopamine‐immunoreactive system in the central nervous system of the sea lamprey. Brain Res Bull 66, 560–564. [DOI] [PubMed] [Google Scholar]
- Adám AS, Csillag A (2006) Differential distribution of l‐aspartate‐ and l‐glutamate‐immunoreactive structures in the arcopallium and medial striatum of the domestic chick (Gallus domesticus). J Comp Neurol 498, 266–276. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Zhang S, et al. (2016) Multiplexed neurochemical signaling by neurons of the ventral tegmental area. J Chem Neuroanat 73, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A, Villar‐Cerviño V, Anadón R, et al. (2008) Descending brain‐spinal cord projections in a primitive vertebrate, the lamprey: cerebrospinal fluid‐contacting and dopaminergic neurons. J Comp Neurol 511, 711–723. [DOI] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A, Cornide‐Petronio ME, Anadón R, et al. (2009a) Serotonin and GABA are colocalized in restricted groups of neurons in the larval sea lamprey brain: insights into the early evolution of neurotransmitter colocalization in vertebrates. J Anat 215, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A, Villar‐Cerviño V, Anadón R, et al. (2009b) Dopamine and gamma‐aminobutyric acid are colocalized in restricted groups of neurons in the sea lamprey brain: insights into the early evolution of neurotransmitter colocalization in vertebrates. J Anat 215, 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A, Anadón R, Rodicio MC (2010a) New insights on the neuropeptide Y system in the larval lamprey brain: neuropeptide Y immunoreactive neurons, descending spinal projections and comparison with tyrosine hydroxylase and GABA immunoreactivities. Neuroscience 167, 396–413. [DOI] [PubMed] [Google Scholar]
- Barreiro‐Iglesias A, Laramore C, Shifman MI, et al. (2010b) The sea lamprey tyrosine hydroxylase: cDNA cloning and in situ hybridization study in the brain. Neuroscience 168, 659–669. [DOI] [PubMed] [Google Scholar]
- Bérubé‐Carrière N, Riad M, Dal Bo G, et al. (2009) The dual dopamine‐glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol 517, 873–891. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE (2000) GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci 12, 3172–3180. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, St‐Gelais F, Danik M, et al. (2004) Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem 88, 1398–1405. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, Bérubé‐Carrière N, Mendez JA, et al. (2008) Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6‐hydroxydopamine lesion. Neuroscience 156, 59–70. [DOI] [PubMed] [Google Scholar]
- Ericsson J, Stephenson‐Jones M, Pérez‐Fernández J, et al. (2013) Dopamine differentially modulates the excitability of striatal neurons of the direct and indirect pathways in lamprey. J Neurosci 33, 8045–8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐López B, Villar‐Cerviño V, Valle‐Maroto SM, et al. (2012) The glutamatergic neurons in the spinal cord of the sea lamprey: an in situ hybridization and immunohistochemical study. PLoS ONE 7, e47898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi A, Mueller T, Driever W (2014) vglut2 and gad expression reveal distinct patterns of dual GABAergic versus glutamatergic cotransmitter phenotypes of dopaminergic and noradrenergic neurons in the Zebrafish brain. J Comp Neurol 522, 2019–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin GM, Bourque MJ, Mendez JA, et al. (2012) Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci 32, 17477–17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, et al. (2010) Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron 65, 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Panula P (2001) Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J Comp Neurol 440, 342–377. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kawasaki A, Sakata‐Haga H, et al. (2006) Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol 498, 581–592. [DOI] [PubMed] [Google Scholar]
- Kuscha V, Barreiro‐Iglesias A, Becker CG, et al. (2012) Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J Comp Neurol 520, 933–951. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Grandes P, Matute C, et al. (1989) Glutamate‐like immunoreactivity revealed in rat olfactory bulb, hippocampus and cerebellum by monoclonal antibody and sensitive staining method. Histochemistry 90, 427–445. [DOI] [PubMed] [Google Scholar]
- Marín O, Smeets WJ, González A (1998) Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci 21, 487–494. [DOI] [PubMed] [Google Scholar]
- Mendez JA, Bourque MJ, Dal Bo G, et al. (2008) Developmental and target‐dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci 28, 6309–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Chuhma N, Kusnoor SV, et al. (2015) Functional connectome analysis of dopamine neuron glutamatergic connections in forebrain regions. J Neurosci 35, 16259–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience 282C, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Fernández J, Stephenson‐Jones M, Suryanarayana SM, et al. (2014) Evolutionarily conserved organization of the dopaminergic system in lamprey: SNc/VTA afferent and efferent connectivity and D2 receptor expression. J Comp Neurol 522, 3775–3794. [DOI] [PubMed] [Google Scholar]
- Pierre J, Mahouche M, Suderevskaya EI, et al. (1997) Immunocytochemical localization of dopamine and its synthetic enzymes in the central nervous system of the lamprey Lampetra fluviatilis . J Comp Neurol 380, 119–135. [PubMed] [Google Scholar]
- Pombal MA, El Manira A, Grillner S (1997a) Afferents of the lamprey striatum with special reference to the dopaminergic system: a combined tracing and immunohistochemical study. J Comp Neurol 386, 71–91. [PubMed] [Google Scholar]
- Pombal MA, El Manira A, Grillner S (1997b) Organization of the lamprey striatum – transmitters and projections. Brain Res 766, 249–254. [DOI] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, et al. (2006) Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res 168, 152–156. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF (2001) The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res 889, 316–330. [DOI] [PubMed] [Google Scholar]
- Rodicio MC, Villar‐Cerviño V, Barreiro‐Iglesias A, et al. (2008) Colocalization of dopamine and GABA in spinal cord neurones in the sea lamprey. Brain Res Bull 76, 45–49. [DOI] [PubMed] [Google Scholar]
- Ryczko D, Grätsch S, Auclair F, et al. (2013) Forebrain dopamine neurons project down to a brainstem region controlling locomotion. Proc Natl Acad Sci U S A 110, E3235–E3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJ, González A (2000) Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Brain Res Rev 33, 308–379. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM, Van Vliet SP, Bol JGJM, et al. (1991) Development and application of antibodies to primary (DA, L‐Dopa) and secondary (cGMP) messengers: a technical report In: Neurocytochemical Methods, NATO ASI Series, Vol. 58. (eds Calas A, Eugène D.), pp. 1–27. Berlin: Springer‐Verlag. [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, et al. (2010) Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30, 8229–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, et al. (1998) Dopamine neurons make glutamatergic synapses in vitro. J Neurosci 18, 4588–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar‐Cerviño V, Barreiro‐Iglesias A, Anadón R, et al. (2008) Aspartate immunoreactivity in the telencephalon of the adult sea lamprey: comparison with GABA immunoreactivity. Brain Res Bull 75, 246–250. [DOI] [PubMed] [Google Scholar]
- Villar‐Cerviño V, Barreiro‐Iglesias A, Anadón R, et al. (2009) Development of glycine immunoreactivity in the brain of the sea lamprey: comparison with gamma‐aminobutyric acid immunoreactivity. J Comp Neurol 512, 747–767. [DOI] [PubMed] [Google Scholar]
- Villar‐Cerviño V, Barreiro‐Iglesias A, Mazan S, et al. (2011) Glutamatergic neuronal populations in the forebrain of the sea lamprey, Petromyzon marinus: an in situ hybridization and immunocytochemical study. J Comp Neurol 519, 1712–1735. [DOI] [PubMed] [Google Scholar]
- Villar‐Cerviño V, Barreiro‐Iglesias A, Fernández‐López B, et al. (2013) Glutamatergic neuronal populations in the brainstem of the sea lamprey, Petromyzon marinus: an in situ hybridization and immunocytochemical study. J Comp Neurol 521, 522–557. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang HL, et al. (2015) Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci 41, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Vernier P (2011) The evolution of dopamine systems in chordates. Front Neuroanat 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]


