Abstract
There is a consensus that there is no overt anterior joint capsule in the knee. However, other anterior structures act in lieu of a joint capsule: the quadriceps tendon and patellar retinacular fibres. In the absence of a capsule, the synovium forms the suprapatellar pouch. Other synovial structures, the plicae, are more controversial. They are often described as embryonic remnants with no function, despite surrounding the patella. We aimed to identify plical anatomy and histology on cadaveric dissection and to examine their embryology using the human virtual embryo website. Plicae were identified by two independent observers. Plical histology was examined using a panel of stains: H&E, Ab H&E, EVG and MSB trichrome. Embryonic knees were examined from Carnegie stages 20–23. Each knee had a suprapatellar plica and mediopatellar plica (MPP). The lateropatellar plica (LPP) appeared as a band in 5/10 cadavers, and as a ridge in the remainder. The overall impression, consistent across all specimens, was that the plicae formed a continuous band of synovial tissue around the proximal three‐quarters of the patella. The infrapatellar fat pad (IFP) surrounded the remainder. Histologically, the plicae and IFP consisted of three layers (in order): a synovial layer, an undulated collagenous layer, and an adipose or areoloadipose layer. The subsynovial collagenisation is normally associated with the synovio‐capsular boundary. Embryologically, plicae were not seen in either knee at any level for any given Carnegie stage. We suggest that plicae, along with the dynamic IFP, provide internal support to the patella mirroring the external support of retinacular fibres. Thus, the plicae complete the tissue complex acting in lieu of an anterior joint capsule. Evidence of plical functionality lends credence to the theory that the plicae are anatomical structures not functionless embryonic remnants.
Keywords: Knee, Synovial Plicae, Synovial Folds, Synovial Plica, Anterior Knee Pain
Introduction
There is a consensus that there is no overt anterior joint capsule in the knee (Reider et al. 1981). However, other anterior structures act in lieu of a joint capsule. Externally, these are the quadriceps tendon and patellar retinacular fibres. The situation within the joint space, however, is less well demonstrated. The infrapatellar fat pad (IFP) has been demonstrated to provide dynamic support to the patella (Bohnsack et al. 2005). The other main intra‐articular tissue, the synovium, forms the suprapatellar pouch in the absence of an anterior capsule. Other anterior synovial structures, the plicae, are more controversial. They are often described as functionless embryonic remnants (Ogata & Uhthoff, 1990), despite surrounding the patella.
There are four plicae named in anatomical relation to the patella: suprapatellar plica (SPP), infrapatellar plica (IPP), mediopatellar plica (MPP) and lateropatellar plica (LPP). The MPP and LPP course from their origins at the boundary between the patellofemoral joint (PFJ) and suprapatellar pouch, medially and laterally, to insert into the IFP (Iino, 1939; Sakakibara, 1976). The IPP is the most common plica (Dupont, 1997). It originates anterior to the anterior cruciate ligament within the intercondylar notch and inserts into the IFP (Demirag et al. 2006). However, given its anatomy it is unlikely to contribute to a tissue complex acting in lieu of an anterior joint capsule in the knee. The SPP lies to varying extents across the junction between the PFJ and the suprapatellar bursa (Iino, 1939; Zidorn, 1992).
The MPP is the most studied plica as it has been shown to be associated with anterior knee pain and cartilage damage (Hayashi et al. 2013). It is the only plica that has been histologically defined (Farkas et al. 2004). However, plicae are often described as consisting of ‘normal synovial tissue’, despite there being little histological evidence. Farkas et al. (2004) described asymptomatic MPP to consist of ‘undulated, regular collagen fibres under normal synovium’. An undulated appearance can indicate elastin rather than collagen (Ovalle et al. 2008). Indeed, Schindler (2014) describes plicae as ‘elastic’ and ‘pliable’. Farkas et al. (2004) did not use stains for elastin. It is therefore reasonable to question whether the MPP is purely collagenous or contains elastin, and whether the other plicae also consist of the same tissues. If they are purely collagenous it could indicate a level of functionality given that the fibrous form of synovium is only found at the synovio‐capsular border in the knee (Castor, 1960).
Papers examining plicae often comment that plicae are embryological remnants. This is based on research conducted prior to 1990 (Gray & Gardner, 1950; Ogata & Uhthoff, 1990). These papers do not take into account more recent studies showing that as fetuses mature, they demonstrate fewer plicae (Merida‐Velasco et al. 1997; Tena‐Arregui et al. 2003). This suggests intra‐articular structures of the knee are developing into the late stages of gestation (Tena‐Arregui et al. 2003).
This study aims to examine plical anatomy, histology and embryology to determine their origin and contribution to the tissue complex acting in lieu of an anterior joint capsule.
Materials and methods
There were three components to this study: (i) cadaveric knee dissection with observation of plicae in situ. (ii) plical histology, and (iii) interpretation of human knee embryology using the VHE website (Gasser et al. 2001–2015).
All cadavers were procured in accordance with the Human Tissue Act 2004 with prior consent given for research and photographs. Medical histories were taken from notes taken on acceptance of an anatomical bequest, which are not extensive. Notably, one cadaver had a history of osteoarthritis. However, none of the cadavers had any scars, either surgical or traumatic. Cadavers were assigned to the project by an independent person within the department by random allocation without prior knowledge of pathology.
Ten human cadaveric knees, harvested from embalmed cadavers, mid‐femur to mid‐tibia, were dissected. They were split evenly by sex and side: five left knees (three female, two male) and five right knees (two female, three male). Mean age of female cadavers was 81.6 years (range 49–90) and mean age of male cadavers was 81.2 years (range 65–91).
Coloured latex (60 mL) was injected into the suprapatellar pouch, simulating arthroscopic joint effusion, thereby replicating the potential distension of structures, and allowed to cure (set) for 48 h. With the knee in extension, a lateral incision was made from the proximal part of quadriceps tendon to the tibial tuberosity. Latex casts moulded to the joint space thus aiding identification and classification of plicae. Identification was performed by two independent observers.
If there was evidence of plical pathology (feathered appearance) and/or associated local joint pathology (e.g. chrondral damage, trochlear dysplasia) the plica was defined as ‘macroscopically affected’ and excluded from both anatomical description/photographs and histological analysis. ‘Macroscopically unaffected’ plicae did not have any of these features. All knees had some evidence of joint damage (chondral breakdown); however, joint damage is nearly ubiquitous in elderly knees (Chung, 1966). Therefore macroscopically unaffected plicae were included if there was distant plical pathology or unassociated joint damage (two knees had no plical pathology/associated joint damage but did have some unassociated joint damage).
Plicae were classified according to previously published grading systems: suprapatellar plica (SPP) – Zidorn (1992) (Table 1); mediopatellar plica (MPP) – Sakakibara (1976) (Table 2); infrapatellar plica (IPP) – Demirag et al. (2006) (Table 3). Lateropatellar plica (LPP) was described according to appearance and grouped into similar appearances as no grading system is currently published.
Table 1.
Grading of suprapatellar plicae (Zidorn, 1992)
| I | Complete septum |
| II | Septum with porta |
| III | Residual septum |
| IV | No septum |
Table 2.
Grading of mediopatellar plicae (Sakakibara, 1976)
| A | Cord‐like |
| B | Shelf‐like with nothing covering medial femoral condyle |
| C | Shelf‐like and covering medial femoral condyle |
| D | Double insertion (fenestrated) |
Table 3.
Grading of infrapatellar plica (Demirag et al. 2006)
| I | Separate |
| II | Split |
| III | Vertical septum |
| IV | Fenestrated |
| V | None of the above |
Histological sampling was undertaken in macroscopically unaffected plicae that could contribute to a tissue complex acting in lieu of an anterior join capsule. SPP was sampled from the middle of septum to medial edge (Fig. 1a). The medial edge is often retained, whereas the lateral edge is not (Zidorn, 1992). MPP and LPP were sampled across the width of plica (Fig. 1b). The suprapatellar part of the peripatellar retinaculum (SPPR) was sampled by punch biopsy (Fig. 1b). The infrapatellar fat pad (IFP) tag was sampled across the distal edge of tag to include a portion of IFP distally and macroscopically synovitic sections (if present) proximally (Fig. 1b). Given the unlikelihood of the IPP contributing to a tissue complex acting in lieu of an anterior joint capsule, it was excluded from histological analysis.
Figure 1.
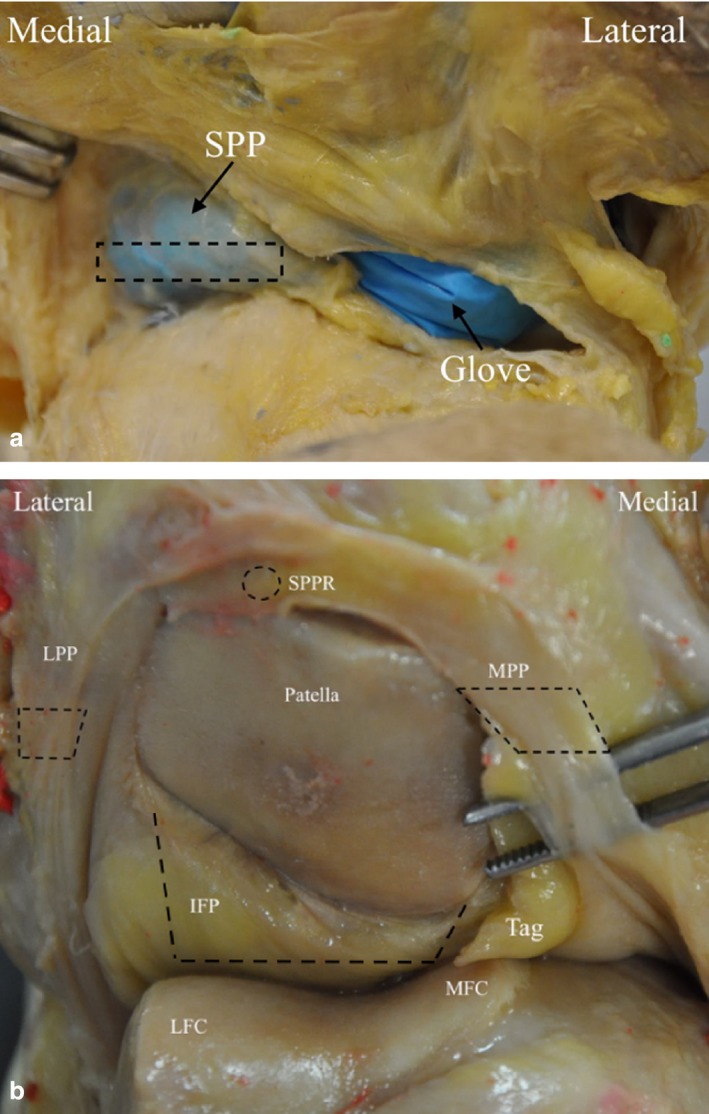
Sample points of (a) suprapatellar plica (SPP), (b) suprapatellar part of the peripatellar retinaculum (SPPR), lateropatellar plica (LPP) and mediopatellar plica (MPP), infrapatellar fat pad (IFP).
Cut edges were marked with tissue dye for histological orientation. Each sample was individually labelled, with thin samples blocked ‘on edge’ to ensure that a full section was visualised.
Plical samples were embedded in paraffin wax. Samples were cut using a microtome to a depth of 5 μm. Sections were floated on a 40 °C water‐bath and mounted onto Thermoscientific Superfrost Plus® slides, as plical tissue is very fine and friable.
Sections were stained using a panel of four stains: H&E (standard histological stain); Alcian blue (Ab) H&E (chrondocytes); Martius, Scarlet and Blue (MSB) stain (collagen, fibrin and muscle); Elastic Van Gieson (EVG) (elastin). For full staining procedures see Carleton et al. (1967). Control tissues were as follows: Ab H&E – cartilage, MSB – muscle, EVG – elastin within arterioles.
Knee embryology was examined using the virtual human embryo (VHE) website (Gasser et al. 2001–2015). The entire knee from Carnegie stages 20–23 (O'Rahilly, 1973) was examined on both sides. The specific stains used were identified from the website as follows: chromatin, periodic acid‐Schiff and Ab H&E.
Statistical analysis was performed using spss version 21. Dichotomous data was tested using chi‐square test. Continuous parametric data was tested with an independent t‐test or one‐way anova if there were more than two samples.
Results
There was no difference in plical presence between the sexes [P = 0.95, 95% confidence interval (CI) −0.18 to 0.92] or between left and right knees (P = 0.855, 95% CI −1.74 to 2.09). As there were no differences, data were pooled. Eleven plicae were macroscopically affected and were excluded from anatomical description (Table 4). The affected plicae were: suprapatellar plica (SPP) (n = 1), mediopatellar plica (MPP) (n = 7) and lateropatellar plica (LPP) (n = 3).
Table 4.
Presence of each plica for each of the cadaveric specimens
| Sex | Plica Grade | Presence of LPP as band (I) or ridge (II) | ||
|---|---|---|---|---|
| SPP | IPP | MPP | ||
| M | I | I | C | I |
| M | II | I | C | I |
| M | I | II | A | II |
| M | I | I | C | II |
| aM | IV | I | B | I |
| F | I | II | B | I |
| F | III | III | B | II |
| F | III | I | C | II |
| F | II | II | C | I |
| F | I | II | B | I |
M, male; F, female.
Bold, macroscopically affected.
Cadaver with osteoarthritis.
The SPP had the appearance of a complete (type I, n = 5) or partial (types II–IV, n = 5) perpendicular septum between the suprapatellar pouch and the patellofemoral joint (PFJ) (Fig. 2). The IPP had the appearance of a separate (type 1, n = 5) or split (type II, n = 4) band, or a vertical septum (type III, n = 1) originating anterior to the anterior cruciate ligament within the intercondylar notch and inserting into the infrapatellar fat pad (IFP) (Fig. 3).The MPP had the appearance of a cord (type A, n = 1) or band originating at the medial edge of the suprapatellar pouch/PFJ border, which courses to the medial side of the patella to insert onto the IFP (Fig 4). The MPP variably covered (type C, n = 5) (Fig. 4b) or did not cover (type B, n = 4) (Fig. 4a) the medial femoral condyle (MFC) (Table 4).
Figure 2.
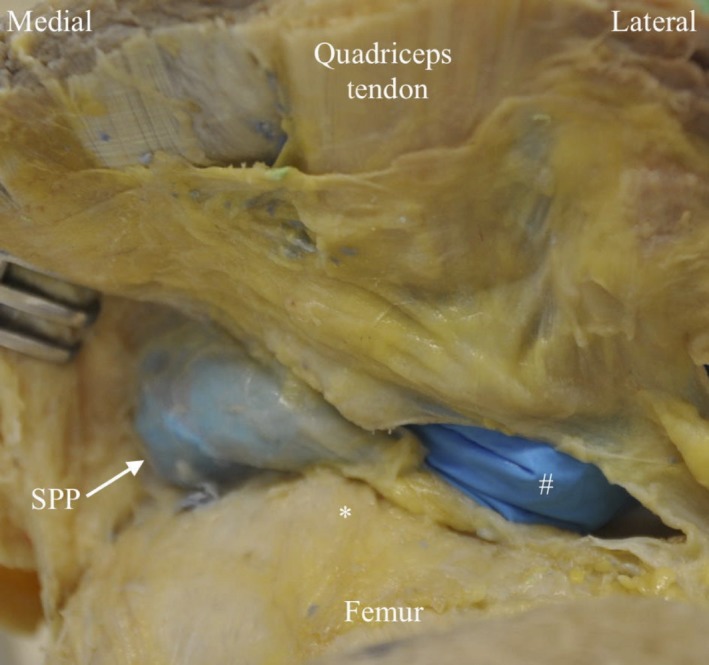
Proximo ‐distal photograph of cadaveric right knee demonstrating a Grade III SPP. #Glove inside. PFJ to demonstrate extent of SPP. *Suprapatellar pouch location.
Figure 3.
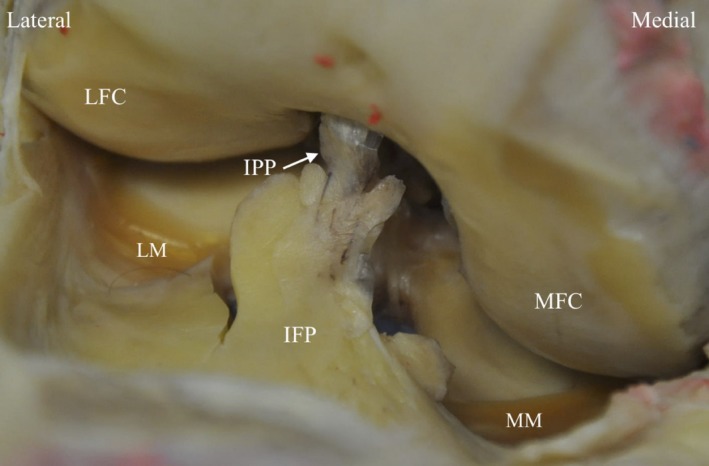
Photograph of cadaveric right knee demonstrating attachment of IPP (grade I) into IFP. LFC, lateral femoral condyle; LM, lateral meniscus; MFC, medial femoral condyle; MM, medial meniscus.
Figure 4.
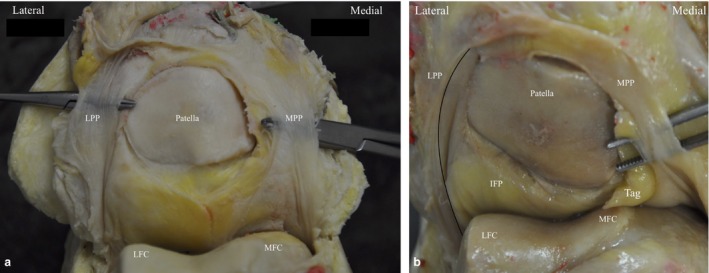
(a) Photograph of cadaveric left knee demonstrating MPP (grade C) and band‐like LPP either side of the patella. (b) Photograph of Left cadaveric knee demonstrating MPP type B and ridge‐like LPP. LFC, lateral femoral condyle; MFC, medial femoral condyle.
The LPP originates at the lateral edge of the boundary between the suprapatellar pouch and the PFJ. It then courses distally along the lateral edge of the patella to insert into the lateral edge of the IFP. The LPP did not cross the lateral femoral condyle (LFC) in any specimens. A band‐like LPP (classified as type I) was present in half of the cadavers at the lateral edge of the patella (Fig. 4a) and as a ridge‐like structure (classified as type II) in the remainder (Fig. 4b).
The overall appearance was of a continuous band surrounding the proximal three‐quarters of the patella, which consisted of the MPP, LPP and suprapatellar part of the peripatellar retinaculum (SPPR). The infrapatellar fat pad surrounded the remaining distal one‐third (Fig. 4).
The SPP consists of predominantly subsynovial adipose tissue, with some dense collagenous fibres immediately adjacent to the synovial layer (Figs 5, 6, 7, 8); capillaries were noted in the synovial layer (Fig. 5). Special stains did not identify any chondrocytes (Ab H&E) (Fig. 6) and little elastin (EVG) (Figs 7 and 9). However, both EVG and MSB demonstrated subsynovial undulated collagen fibres (Figs 7 and 8). Skeletal muscle was noted on the medial edge of the section (Figs 6, 7, 8).
Figure 5.
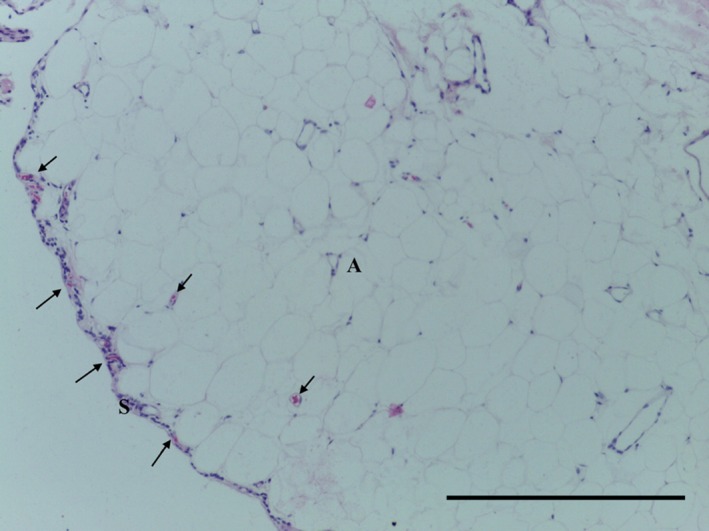
Transverse section of SPP demonstrating synovium (S), adipose tissue (A) and capillaries (arrows). H&E, 10× magnification. Scale bar: 100 m.
Figure 6.
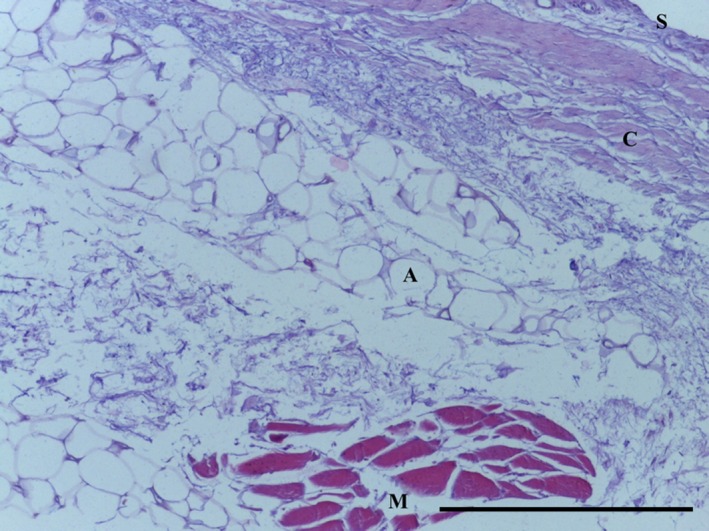
Transverse section of SPP demonstrating subsynovial (S) adipose tissue (A) underneath dense bands of collagen (C) and skeletal muscle (M). Ab H&E, 10× magnification. Scale bar: 100 m.
Figure 7.
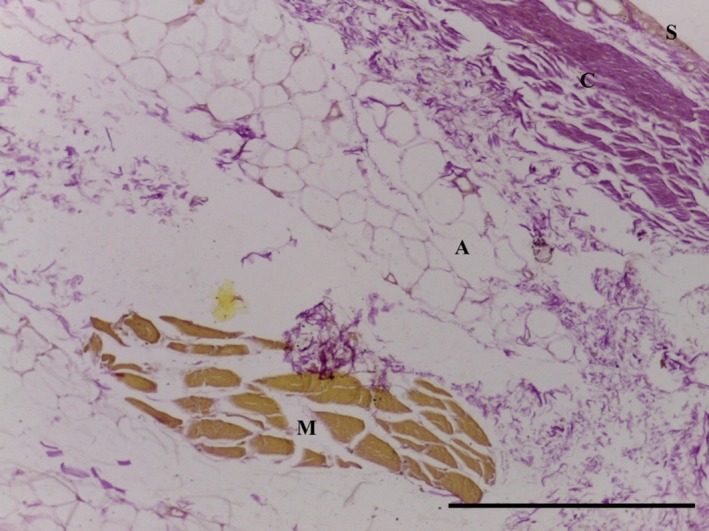
Transverse section of SPP demonstrating subsynovial (S) adipose tissue (A) underlying dense bands of undulated collagen (C) and skeletal muscle (M). No elastin identified. EVG, 10× magnification. Scale bar: 100 m.
Figure 8.
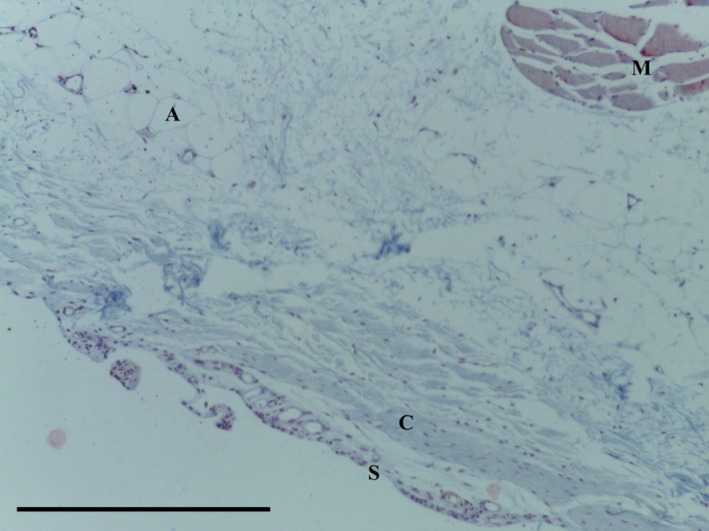
Transverse section of SPP demonstrating adipose tissue (A) with undulated collagen fibres (C) underlying the synovial layer (S) and skeletal muscle (M). MSB trichrome, 10× magnification. Scale bar: 100 m.
Figure 9.
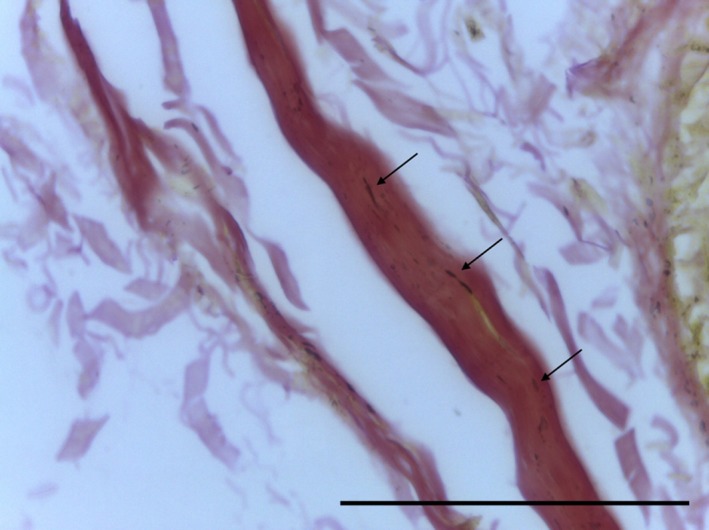
Transverse section of SPP demonstrating undulated collagen strand with elastin staining (arrows). EVG, 63× magnification. Scale bar: 10 m.
The MPP consisted of adipose tissue surrounded by undulated regular collagen fibres underlying synovium (Figs 10, 11, 12). Special stains failed to identify chondrocytes (Ab H&E) or elastin (EVG). Collagen was demonstrated with MSB trichrome stain (Fig. 12).
Figure 10.
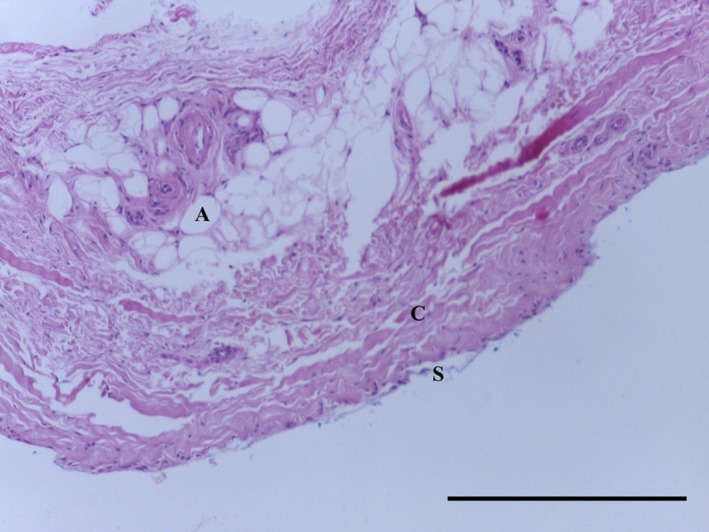
Transverse section of MPP demonstrating adipose tissue (A) surrounded by undulated regular collagen fibres (C) underlying synovium (S). H&E, 10× magnification. Scale bar: 100 m.
Figure 11.
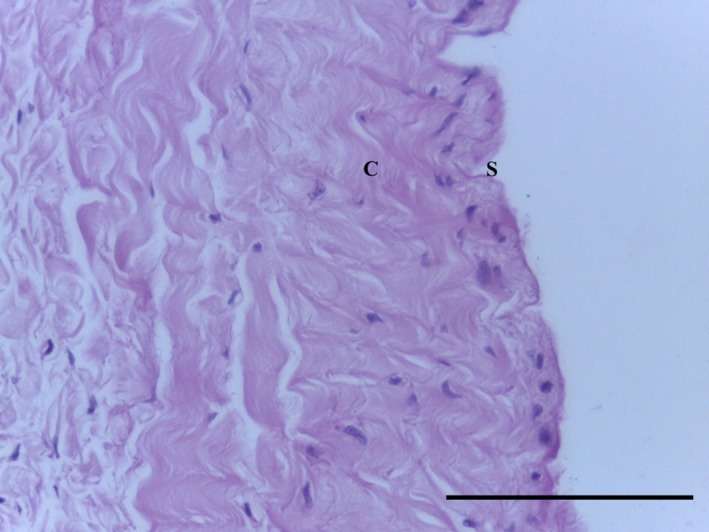
Transverse section of MPP demonstrating regular undulated collagen fibres (C) underlying synovium (S). H&E 40× magnification. Scale bar: 10 m.
Figure 12.
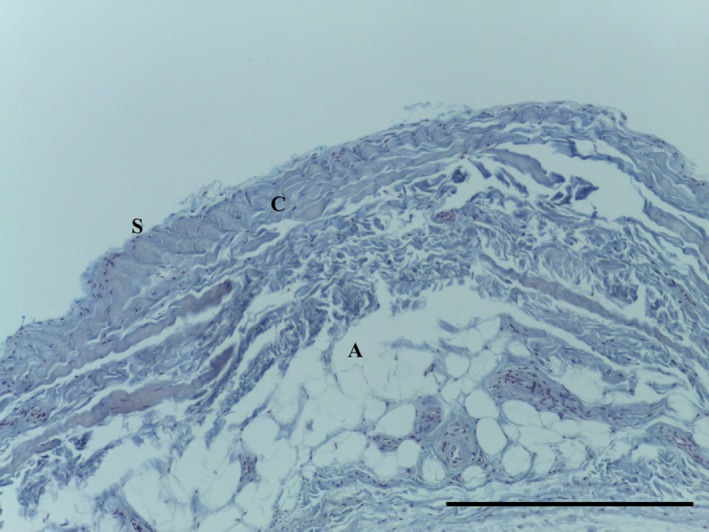
Transverse section of MPP demonstrating adipose tissue (A) surrounded by regular undulated collagen fibres (C) underlying synovium (S). MSB 10× magnification. Scale bar: 100 m.
The LPP consists of a subsynovial layer of areoloadipose tissue with collagen fibres coursing through (Figs 13, 14, 15, 16). Special stains did not identify any chondrocytes (Ab H&E) (Fig. 14) or elastin (Fig. 15). Collagen was demonstrated with EVG (Fig. 15) and MSB stains (Fig. 16).
Figure 13.
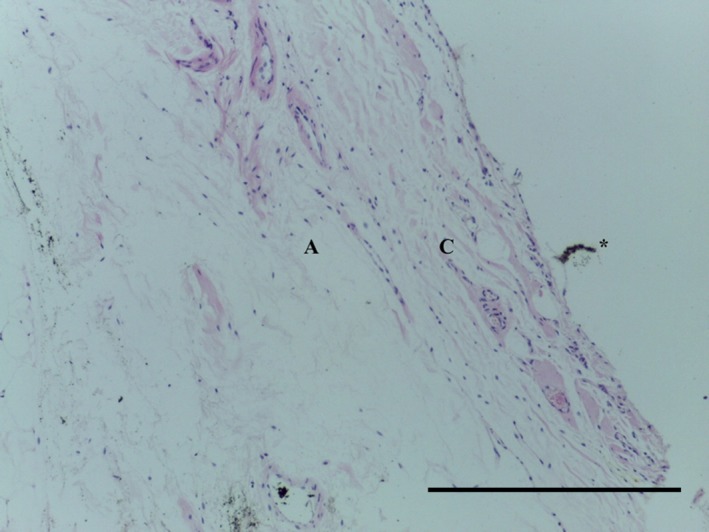
Transverse section of LPP demonstrating mature collagenous subsynovial layer (C) overlying the normal areoloadipose tissue (A). *Artefact. H&E, 10× magnification. Scale bar: 100 m.
Figure 14.
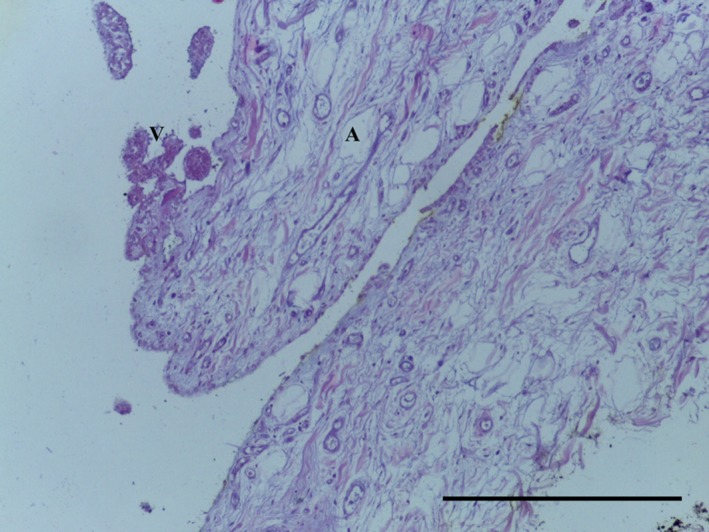
Transverse section of LPP demonstrating areoloadipose subsynovial tissue (A) with villi (V). No chondrocytes noted. Ab H&E, 10× magnification. Scale bar: 100 m.
Figure 15.

Transverse section of LPP demonstrating distribution of collagen fibres (red). No elastin identified. EVG, 10× magnification. Scale bar: 100 m.
Figure 16.
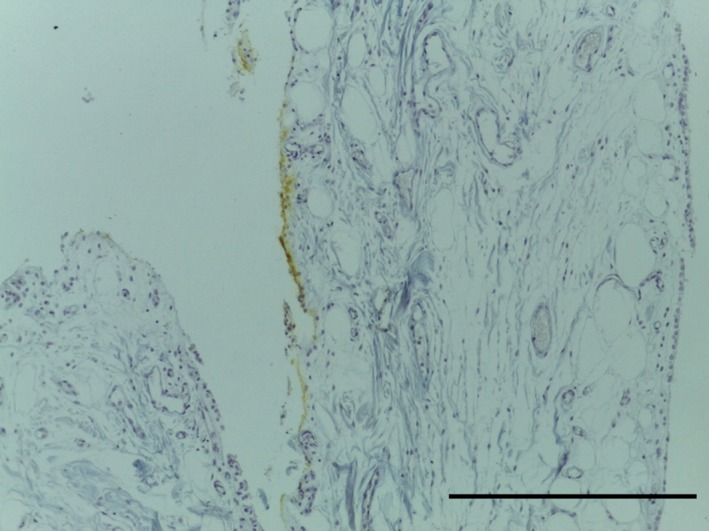
Transverse section of LPP demonstrating distribution of collagen fibres (blue). Plical surface, yellow dye. MSB, 10× magnification. Scale bar: 100 m.
The SPPR consisted of areoloadipose tissue with adjacent dense undulated collagen fibres (Fig. 17).
Figure 17.
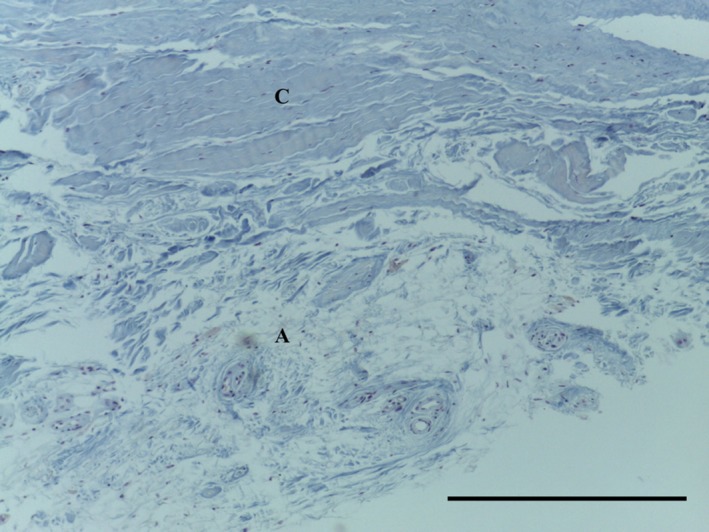
Transverse section of punch biopsy of SPPR demonstrating areoloadipose tissue (A) and dense undula ted collagen fibres (C) MSB 10× magnification. Scale bar: 100 m.
The IFP tag consisted of adipose tissue, with subsynovial collagenisation (Fig. 18). Special stains did not identify any chondrocytes (Ab H&E) (Fig. 19) or elastin (Fig. 20). Collagen was demonstrated with EVG (Fig. 20) and MSB stains (Fig. 21).
Figure 18.
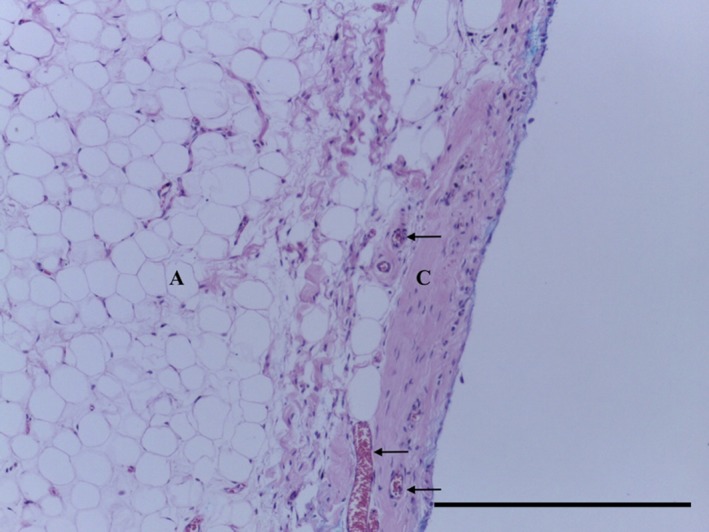
Transverse section of IFP demonstrating adipose tissue (A) with a vascularised (arrows) subsynovial dense undulated collagenisation (C). H&E, 10× magnification. Scale bar: 100 m.
Figure 19.
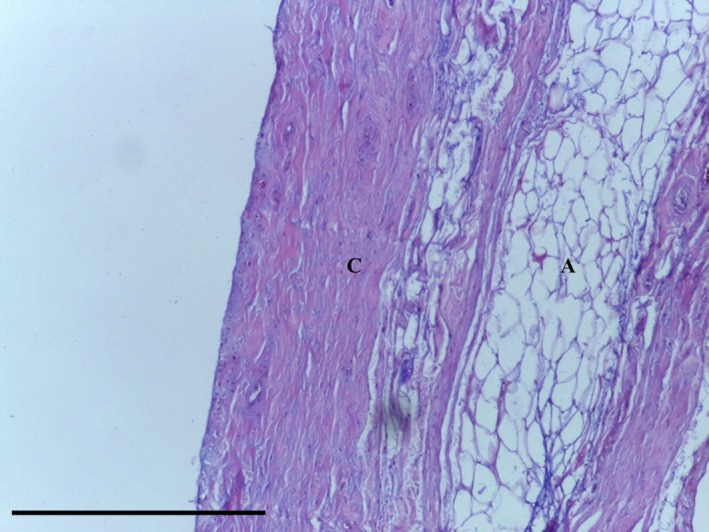
Transverse section of IFP demonstrating adipose tissue (A) underlying collagen fibres (C). Ab H&E, 10× magnification. Scale bar: 100 m.
Figure 20.
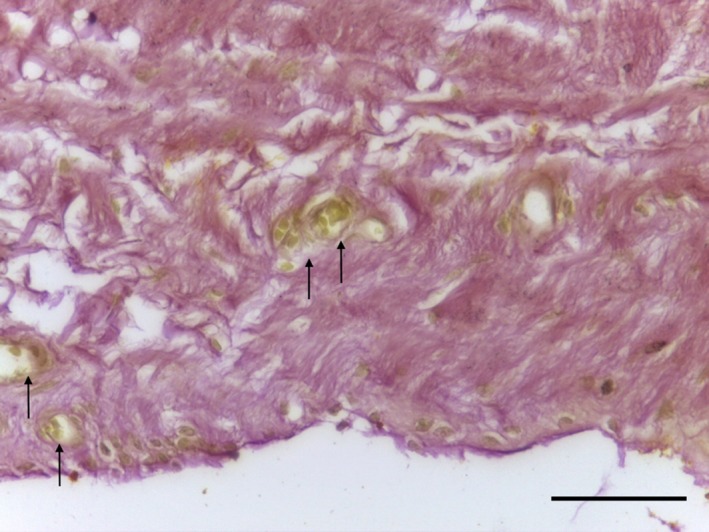
Transverse section of IFP demonstrating dense undulated regular collagen fibres (red) and capillaries (arrows). EVG 40× magnification. Scale bar: 10 m.
Figure 21.
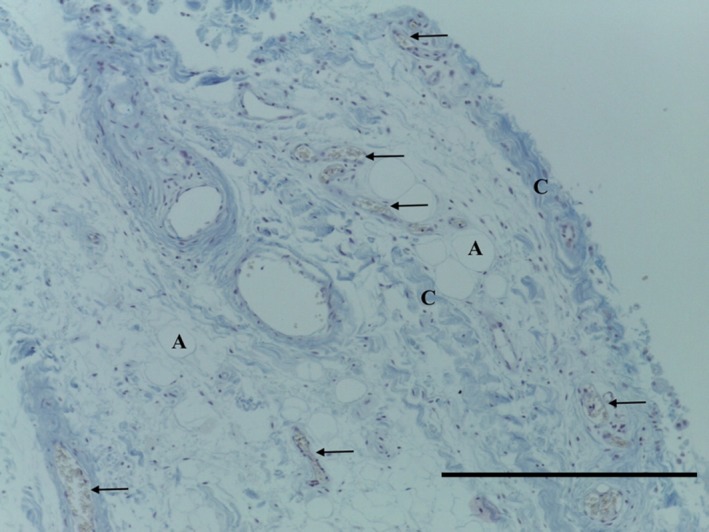
Transverse section of IFP demonstrating subsynovial adipose tissue (A), regular undulated collagen fibres (C) and capillaries (arrows). MSB, 10× magnification. Scale bar: 100 m.
Examination of knee embryology using the virtual human embryo website (Gasser et al. 2001–2015) did not demonstrate any plicae from Carnegie stages 20–23 on examination of the entire knee on both left and right knees (Figs 22, 23, 24, 25). Menisci and ligaments appear as darker concentrations (of synovial mesenchyme) at the same location as in the adult knee.
Figure 22.
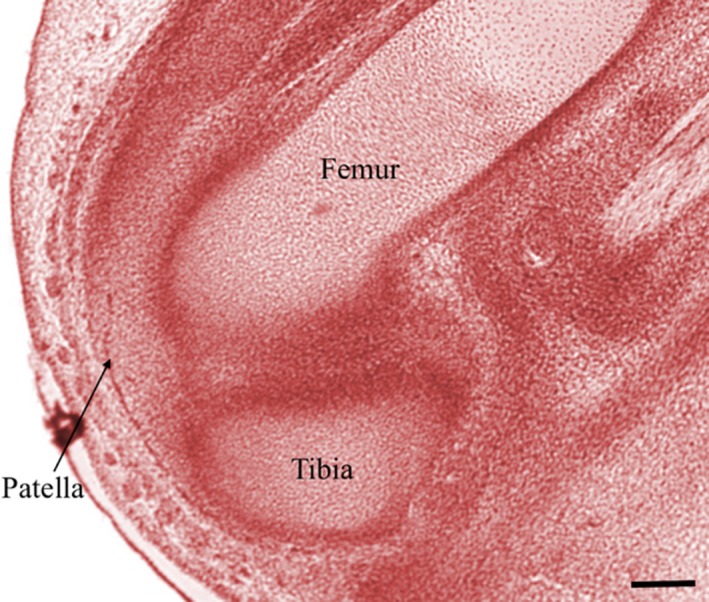
Right knee, Carnegie stage 20. Chromatin. Scale bar: 100 m (Gasser et al. 2001–2015).
Figure 23.
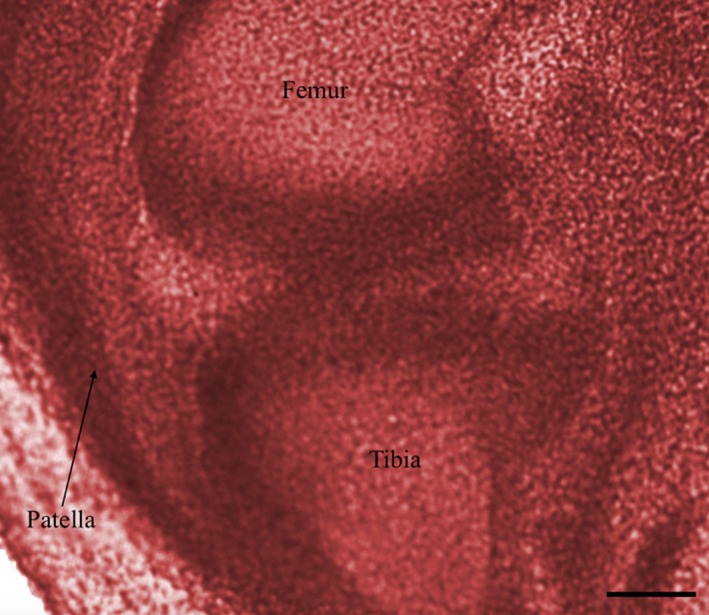
Right knee, Carnegie stage 21. Chromatin. Scale bar: 100 m (Gasser et al. 2001–2015).
Figure 24.
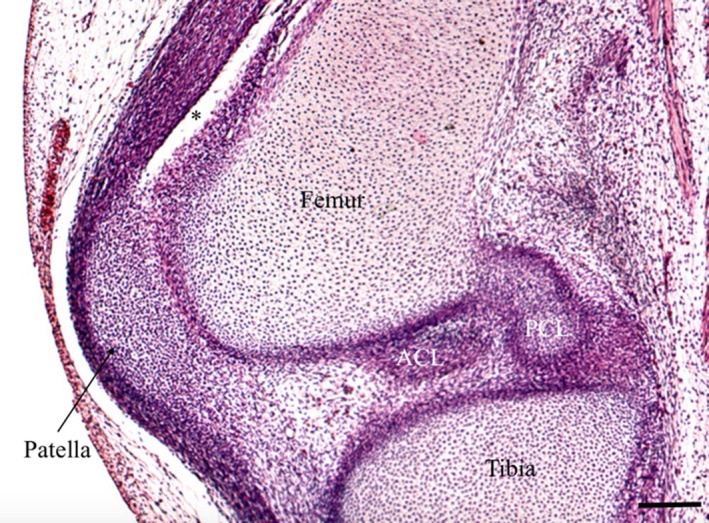
Right knee, Carnegie stage 22. *Suprapatellar pouch, ACL, anterior cruciate ligament. Periodic acid‐Schiff. Scale bar: 100 m (Gasser et al. 2001–2015).
Figure 25.
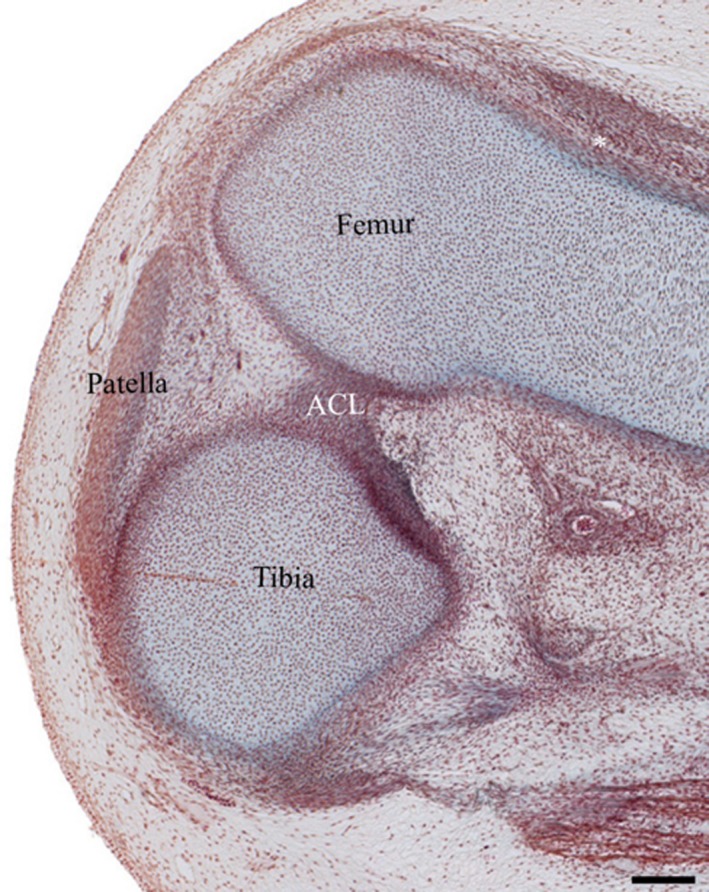
Right knee at Carnegie stage 23. *Suprapatellar pouch, ACL, anterior cruciate ligament. Ab H&E. Scale bar: 100 m (Gasser et al. 2001–2015).
Discussion
This study has demonstrated the plical relationship to the patella via a novel dissection technique not used in previous studies (Dupont, 1997; Gurbuz et al. 2006). Structures were observed in situ without the need for further dissection. Inferences about position in vivo could be made, as the embalming fluid used does not shrink or expand the tissue (Coleman & Kogan, 1998). Plical presence in this study was higher than previous cadaveric studies (Dupont, 1997; Gurbuz et al. 2006). This may be due to the novel technique utilising latex and a proximodistal approach, which enables plical preservation and thus more accurate plical identification. The MPP was noted to be most commonly macroscopically affected, i.e. feathered appearance with evidence of associated joint damage, which is in keeping with the existing literature (Hayashi et al. 2013). Notably the LPP was also affected in three cadavers. However, demonstrating anything other than a cursory association is beyond the scope of this study.
The anatomical appearance of the SPP, MPP, IPP and IFP was consistent with the previous studies by Zidorn (1992), Sakakibara (1976), Demirag et al. (2006) and Gallagher et al. (2005), respectively. The LPP has not been well investigated but is said to appear as a band originating from the above popliteus hiatus and coursing lateral to the patella to insert into the IFP (García‐Valtuille et al. 2002). This band was demonstrated along with the previously unreported ridge‐like appearance. Unlike the MPP, the LPP has not been demonstrated to cross the lateral femoral condyle, although anecdotally this appearance does exist. A larger cadaveric or arthroscopic study is warranted to investigate this phenomenon.
The overall macroscopic appearance was that the MPP and LPP connect in the suprapatellar part of the peripatellar tissue to form a continuous band of synovial tissue. This band surrounds the proximal three‐quarters of the patella, with the IFP supporting the remainder (Fig. 26). This suggests a functional role, which perhaps contributes to the tissue complex acting in lieu of an anterior joint capsule.
Figure 26.
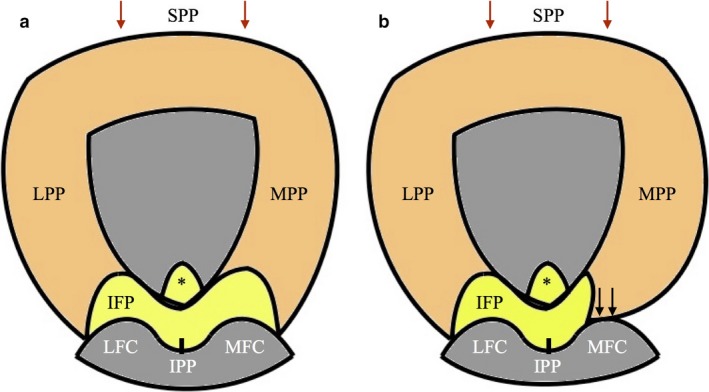
Proximo‐distal diagram (patella reflected distally) demonstrating continuous band of plical synovial tissue acting in lieu of an anterior joint capsule. Grade II MPP (a) and Grade III MPP (b). Arrows, contact between MPP and MFC; *IFP tag.
This is the first study to examine systematically the histology of the SPP, LPP and IFP. The MPP has been examined when symptomatic and asymptomatic by Farkas et al. (2004). This study has demonstrated that plicae are indeed synovial. Plicae also demonstrated subsynovial collagenisation similar to that demonstrated by Farkas et al. (2004) in asymptomatic plicae (sampled from plicae with no associated pathological processes in operations for unrelated pathology). Few fibroblasts were identified in collagenised sections, suggesting the collagenous tissue is mature (Rougraff & Shelbourne, 1999). The plical histological appearances described in this study are in keeping with the histological appearance of established intra‐articular structures in the knee (Castor, 1960).
Differences between the band surrounding the patella (MPP, LPP and SPPR) and the SPP were noted. Whereas the SPP from different specimens demonstrated variable amounts of collagen, the MPP and LPP had consistent dense subsynovial collagenisation with no elastin. There is a similar collagenisation to that seen in the MPP and LPP at the synovio‐capsular border in other intraarticular structures adjacent to the posterior joint capsule in the knee (Castor, 1960). However, there is no anterior joint capsule (Reider et al. 1981) and thus no anterior synovio‐capsular border. Therefore, the collagenisation seen in plicae suggests they may have a structural role within the knee.
Notably, the histology of the IFP had the same subsynovial collagenous appearance as the plicae. This microscopic analysis supports the macroscopic evidence that the IFP has a dynamic role in patellar support (Bohnsack et al. 2005). Furthermore, it is evidence that plicae may have a similar supportive role in light of the macroscopic appearance of a band surrounding the patella.
Although the plicae are demonstrably synovial, their embryological origin is uncertain. In the embryo, knee mesenchymal tissues condense where future intra‐articular structures will form. The condensed mesenchyme then becomes fibrous before responding to specific signalling molecules (Koyama et al. 2008; Mundy et al. 2011) to differentiate into specific tissues. The source of the signalling molecules may be intra‐cellular, as exposure to a joint space or cavity induces synovial tissue in limbs (Edwards et al. 1981). Therefore, plical exposure to the joint space may induce synovial differentiation. Thus, both embryonic remnants and normal structures become synovial when exposed to the joint space.
There are three points of evidence supporting plicae not being embryonic remnants. The first is that the plane of the ‘SPP’ demonstrated by Ogata & Uhthoff (1990) is not the same as in adults (Fig. 27). The SPP is a perpendicular, complete or partial septum (Zidorn, 1992). Ogata & Uhthoff (1990) showed the SPP running longitudinally within the suprapatellar pouch, parallel to the femur. If this structure is retained, then it could be an embryological remnant; however, it is in the wrong plane to be the SPP. Secondly, this study has demonstrated plicae form a continuous band around the proximal three‐quarters of the patella, which has demonstrable subsynovial collagenisation suggestive of functionality as discussed above. Thirdly, plicae exist in other joints (Clarke, 1988; Atlihan et al. 2003; Funk et al. 2006) that undergo different embryological processes (Sadler & Langman, 2010), which one would expect not to have plicae. The combination of evidence from this study and other studies suggests plicae are more likely to be true anatomical features, not embryological remnants.
Figure 27.
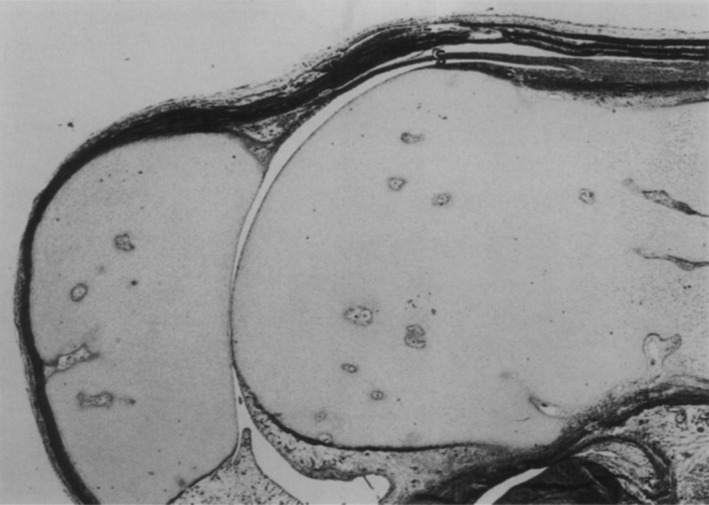
Sagittal section of 17‐week fetal knee showing suprapatellar plica (S). Goldner, ×8 magnification (Ogata & Uhthoff, 1990).
Future studies should examine larger numbers of cadavers using the techniques used in this study to demonstrate the true incidence of plicae.
Conclusion
Plicae are common, forming a continuous band of collagenous synovial tissue around the proximal part of the patella. This band may support the patella internally, reflecting the external support of patella retinacular fibres. The evidence presented here suggests that plicae have functionality and are therefore anatomical structures, not functionless embryonic remnants.
References
- Atlihan D, Jones DC, Guanche CA (2003) Arthroscopic treatment of a symptomatic hip plica. Clin Orthop Relat Res 411, 174–177. [DOI] [PubMed] [Google Scholar]
- Bohnsack M, Hurschler C, Demirtas T, et al. (2005) Infrapatellar fat pad pressure and volume changes of the anterior compartment during knee motion: Possible clinical consequences to the anterior knee pain syndrome. Knee Surg Sports Traumatol Arthrosc 13, 135–141. [DOI] [PubMed] [Google Scholar]
- Carleton HM, Drury RAB, Wallington EA (1967) Carleton's Histological Technique. New York: Oxford University Press. [Google Scholar]
- Castor C (1960) The microscopic structure of normal human synovial tissue. Arthritis Rheum 3, 140–151. [DOI] [PubMed] [Google Scholar]
- Chung EB (1966) Aging in human joints. J Natl Med Assoc 58(2), 87. [PMC free article] [PubMed] [Google Scholar]
- Clarke RP (1988) Symptomatic, lateral synovial fringe (plica) of the elbow joint. Arthroscopy 4, 112–116. [DOI] [PubMed] [Google Scholar]
- Coleman R, Kogan I (1998) An improved low‐formaldehyde embalming fluid to preserve cadavers for anatomy teaching. J Anat 192, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirag B, Ozturk C, Karakayali M (2006) Symptomatic infrapatellar plica. Knee Surg Sports Traumatol Arthrosc 14, 156–160. [DOI] [PubMed] [Google Scholar]
- Dupont JY (1997) Synovial plicae of the knee. Controversies and review. Clin Sports Med 16, 87–122. [DOI] [PubMed] [Google Scholar]
- Edwards JCW, Sedgwick AD, Willoughby DA (1981) The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol 134, 147–156. [DOI] [PubMed] [Google Scholar]
- Farkas C, Hargitai Z, Gáspár L, et al. (2004) Histological changes in the symptomatic mediopatellar plica. Knee 11, 103–108. [DOI] [PubMed] [Google Scholar]
- Funk L, Levy O, Even T, et al. (2006) Subacromial plica as a cause of impingement in the shoulder. J Shoulder Elbow Surg 15, 697–700. [DOI] [PubMed] [Google Scholar]
- Gallagher J, Tierney P, Murray P, et al. (2005) The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc 13, 268–272. [DOI] [PubMed] [Google Scholar]
- García‐Valtuille R, Abascal F, Cerezal L, et al. (2002) Anatomy and MR imaging appearances of synovial plicae of the knee. Radiographics 22, 775–784. [DOI] [PubMed] [Google Scholar]
- Gasser R, Cork R, Noe A (2001. –2015) Virtual Human Embryo Project. http://virtualhumanembryo.lsuhsc.edu (date accessed 10/10/15)
- Gray D, Gardner E (1950) Prenatal development of the human knee and superior tibiofibular joints. Am J Anat 86, 235–287. [DOI] [PubMed] [Google Scholar]
- Gurbuz H, Calpur OU, Ozcan M, et al. (2006) The synovial plicae in the knee joint. Saudi Med J 27, 1839–1842. [PubMed] [Google Scholar]
- Hayashi D, Xu L, Guermazi A, et al. (2013) Prevalence of MRI‐detected mediopatellar plica in subjects with knee pain and the association with MRI‐detected patellofemoral cartilage damage and bone marrow lesions: data from the Joints on Glucosamine study. BMC Musculoskelet Disord 14, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S (1939) Normal arthroscopic findings in the knee joint in adult cadavers. 14, 467–523. [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, et al. (2008) A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol 316, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida‐Velasco J, Sanchez‐Montesinos I, Espin‐Ferra J, et al. (1997) Development of the human knee. Anat Rec 248, 269–278. [DOI] [PubMed] [Google Scholar]
- Mundy C, Yasuda T, Kinumatsu T, et al. (2011) Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol 351, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S, Uhthoff HK (1990) The development of synovial plicae in human knee joints: an embryologic study. Arthroscopy 6, 315–321. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R (1973) Developmental stages in human embryos, including a survey of the Carnegie collection, Vol 1 Washington, DC: Carnegie Institution of Washington. [Google Scholar]
- Ovalle WK, Nahirney PC, Netter FH (2008) Netter's Essential Histology. London/Philadelphia: Elsevier/Saunders. [Google Scholar]
- Reider B, Marshall JL, Koslin B, et al. (1981) The anterior aspect of the knee joint. J Bone Joint Surg Am 63(3), 351–356. [PubMed] [Google Scholar]
- Rougraff BT, Shelbourne KD (1999) Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 7, 9–14. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Langman JM (2010) Langman's Medical Embryology. Philadelphia: Wouters Kluwer/Lippincott Williams & Wilkins. [Google Scholar]
- Sakakibara J (1976) Arthroscopic study on Iino's band (plica synovialis mediopatellaris). J Jpn Orthop Assoc 50, 513–522. [Google Scholar]
- Schindler OS (2014) ‘The Sneaky Plica’ revisited: morphology, pathophysiology and treatment of synovial plicae of the knee. Knee Surg Sports Traumatol Arthrosc 22, 247–262. [DOI] [PubMed] [Google Scholar]
- Tena‐Arregui J, Barrio‐Asensio C, Viejo‐Tirado F, et al. (2003) Arthroscopic study of the knee joint in fetuses. Arthroscopy 19, 862–868. [DOI] [PubMed] [Google Scholar]
- Zidorn T (1992) Classification of the suprapatellar septum considering ontogenetic development. Arthroscopy 8, 459–464. [DOI] [PubMed] [Google Scholar]


