Abstract
Background
To assess local control, survival, and conversion to resectability among locally advanced pancreatic cancer (LAPC) patients treated with induction chemotherapy (ICT) followed by chemoradiotherapy treatment using intensity-modulated radiation therapy (IMRT).
Material and Methods
Between 2007 and 2012, 134 LAPC patients were treated with ICT followed by IMRT. After chemoradiotherapy, 40 patients received maintenance chemotherapy.
Results
With a median follow-up of 20 months, median overall survival (OS) was 23 months. One-year and 2-year OS was 85% and 47%, respectively. On multivariate analysis, progression of disease after IMRT was associated with worse overall survival. Cumulative incidence of local failure was 10% at 1 year and 36% at 2 years. Twenty-six patients (19%) underwent resection after chemoradiotherapy including 22 patients (85%) with negative margins. On multivariate analysis, response to IMRT was associated with surgery (p=0.01). Acute grade 3–4 hematologic and non-hematologic toxicity rates were 26% and 4.5%, respectively.
Conclusion
IMRT is safe in patients with LAPC. Patients with non-progressive LAPC after ICT and who received IMRT had high rates of local control and prolonged survival.
Keywords: intensity-modulated radiation therapy, gemcitabine, locally advanced, pancreatic adenocarcinoma, chemoradiotherapy, FOLFIRINOX
Pancreatic adenocarcinoma is one of the leading causes of cancer-related mortality in the Western world [1]. The role of radiation therapy in locally advanced pancreatic cancer (LAPC) has been intensely debated over the past 30 years. Despite improved systemic agents and advances in chemoradiotherapy, patients who present with LAPC experience high rates of both distant metastatic failure and local progression, with a median survival time ranging from 5 to 11 months [2]. Induction chemotherapy (ICT) followed by chemoradiotherapy for patients without evidence of progressive disease potentially allows for selection of patients with a better prognosis [3–5]. This strategy has been evaluated in the GERCOR LAP07 phase III trial. Although overall survival was not improved in the chemoradiotherapy arm compared to the chemotherapy arm, patients with non-progressive LAPC after 4 months of ICT in the chemoradiotherapy arm had a longer time interval to second line therapy and significantly less local tumor progression [6].
Optimal methods of pancreatic irradiation are not clearly defined [7]. Delivery of adequate radiation dose to the pancreas is limited by the radiosensitivity of adjacent normal organs. It has been suggested that older radiotherapy protocols did not show a clear benefit due to insufficient dose and high toxicity. More conformal radiotherapy techniques, such as intensity-modulated radiation therapy (IMRT) are now commonly employed; however, its use is not well established in pancreatic cancer. IMRT allows dose escalation by reducing the dose received by surrounding organs at risk. A few groups have published retrospective series establishing the feasibility of IMRT in pancreatic cancer [8–19]. Given the controversies regarding the role of radiation therapy in the management of LAPC, the aim of this study was to report the toxicity and outcomes, in particular the conversion to resectability, for patients treated with ICT followed by IMRT.
Material and Methods
Patients
Between March 2007 and November 2012, 134 patients with LAPC received ICT followed by chemoradiotherapy using IMRT at our institution.
A waiver of authorization was obtained from the MSKCC Institutional Review Board and data were obtained from a prospectively maintained pancreatic cancer database. All patients had a tissue diagnosis of pancreatic adenocarcinoma.
Locally advanced unresectable disease was defined as superior mesenteric artery or celiac axis encasement greater than 180 degrees, or unreconstructible superior mesenteric vein / portal occlusion, or aortic invasion, according to National Comprehensive Cancer Network Practice Guidelines in Oncology starting in 2007. All patients included in the study had either 1) an unambiguous clinic note from a hepatobiliary surgeon or the multidisciplinary tumor board stating that the disease was locally advanced or 2) underwent an exploratory laparotomy and were found to have unresectable LAPC. For the patients who were ultimately converted to resectability, the pre-treatment CT scans were re-read at our institution by a radiologist (C.W.) with expertise in abdominal imaging to confirm locally advanced disease prior to chemoradiotherapy.
Treatment Plan
Chemotherapy
All patients received ICT. One hundred patients (75%) received gemcitabine-based chemotherapy and 34 patients (25%) received FOLFIRINOX. The patients received a median duration of 3.2 months of ICT prior to chemoradiation (range, 0.2–15.9).
All patients received chemotherapy concurrently with radiation therapy. Ninety-three patients (69%) received concurrent gemcitabine twice weekly (40 mg/m2 twice per week), with the 41 others (31%) receiving infusional 5-fluorouracil (200–225 mg/m2 daily) or oral capecitabine (1600 mg/m2 divided in twice-daily doses given from Monday to Friday).
Some patients received maintenance chemotherapy after completion of radiation therapy, at the discretion of the medical oncologist.
Radiation therapy
The gross tumor volume (GTV) included gross tumor and enlarged lymph nodes on CT simulation. The clinical target volume (CTV) encompassed the draining lymph node basins including celiac, superior mesenteric and retroperitoneal nodes. A first planning target volume (PTV1) including the GTV and CTV was generated to account for daily setup error. A second PTV (PTV2) included the GTV with a 3 to 5-mm margin. Doses of 50.4 Gy and 56 Gy in 28 daily fractions were prescribed to the PTV1 and PTV2, respectively. Fifty percent of the patients were treated using respiratory gating for motion management [20]. Respiratory gating used a RPM system software (Varian Medical Systems, Palo Alto, CA) that correlates chest wall motion to the respiration phase. Diagnostic quality x-ray images and cone-beam CT were obtained to allow for real-time assessment of tumor positioning before delivery while the patient was on the treatment table.
Toxicity assessment and follow-up
Acute radiation toxicity was evaluated by the clinician during each week of radiation therapy according to the Common Terminology Criteria for Adverse Events version 3.0. Tumor response was assessed by a CT scan in all patients using RECIST 1.0 (Response Evaluation Criteria in Solid Tumors) at three months after the start of induction chemotherapy, at 4–6 weeks after the completion of the chemoradiotherapy, at 3 months after completion of chemoradiotherapy then, at 3-month intervals. Patients were assessed by the surgeons or in our Pancreaticohepatobiliary Multidisciplinary Conference for resectability at each of those post-treatment time points.
Statistical Analysis
The endpoints assessed were overall survival (OS), local failure rate, subsequent surgery rate, and acute toxicity. Kaplan-Meier method was used to estimate the OS, which was calculated from the date of diagnosis till the date of death or last follow-up. The cumulative incidence of local failure was calculated from the date of diagnosis to date of local failure, death without local failure, or last follow-up. Univariate and multivariate analysis of OS were done using respectively a log-rank test and a stepwise Cox proportional hazards model. All factors whose p-values < 0.2 were considered as candidates for the stepwise multivariate logistic regression. Univariate analysis of local failure was done using Gray’s competing risks method. Univariate and multivariate analysis of relationship between treatment factors and surgery was done using respectively a Chi-square test and a stepwise logistic regression. In our series, surgery was not a baseline variable; therefore, we did not provide a Kaplan-Meier curve for overall survival for surgical versus non-surgical group to avoid estimation bias. Differences were assumed to be significant when P<0.05.
Results
Patient and Treatment Characteristics
Characteristics of the 134 LAPC patients are reported in Table 1.
Table 1.
Patient characteristics.
| Characteristic | Whole population (n, %) n=134 |
|---|---|
| Age (years) | |
| Mean ± SD | 64.1 ± 9.9 |
| Median | 64.5 |
| Range | 36.7 – 84.1 |
| Gender | |
| Male | 71 (53) |
| Female | 63 (47) |
| Karnofsky performance status | |
| 100–90% | 52 (39) |
| 80–70% | 79 (59) |
| <70% | 3 (2) |
| Location | |
| Head | 83 (62) |
| Body or tail | 51 (38) |
| Tumor size (cm) | |
| Mean ± SD | 3.8 ± 1.4 |
| Median | 3.5 |
| Range | 1.2 – 8.9 |
| Exploratory laparotomy | |
| no | 101 (75) |
| yes | 33 (25) |
| Level of CA19-9 at diagnosis | |
| Mean ± SD | 564 ± 1066.5 |
| Median | 185 |
| Range | 0 – 6806 |
| Not evaluated | 25 |
All patients first received ICT. Response to chemotherapy was evaluated on CT scan before the start of chemoradiotherapy. Fifty-one patients (38%) had partial response, 63 patients (47%) had stable disease, nine patients (7%) had local progression, and 11 were not assessed.
Response to chemoradiotherapy
The objective tumor response to chemoradiotherapy was assessed on a CT scan one month after the completion of chemoradiotherapy. Three patients (2%) achieved a complete response, 38 patients (28%) a partial response, 74 patients (55%) had stable disease, 13 patients (10%) experienced progressive disease, and six were not assessed.
Forty patients (30%) received maintenance chemotherapy after the completion of radiation therapy. Thirty-two patients (80%) received as maintenance chemotherapy the same regimen as ICT. Median duration of maintenance chemotherapy was 3.8 months (range, 0.5–19.4 months). The reasons for stopping maintenance chemotherapy were mainly surgery, disease progression, and toxicity.
Survival
Median follow-up time was 20 months (range, 12.9–81.8 months). At the time of analysis, 98 deaths occurred. Median OS was 23 months. The 1- and 2-year actuarial OS rates were 86% and 48%, respectively (Figure 1a). On univariate analysis, response to ICT, response to IMRT (Figure 1b), and surgery were significantly associated with a better OS. On multivariate analysis, progression of disease after IMRT (PD vs. CR/PR, HR=4.42; 95%CI=1.96–9.98; p=0.0004) was associated with worse OS, and absence of surgery (HR=1.84; 95%CI=0.99–3.39;p=0.052) was marginally associated with worse OS (Table 2).
Figure 1a.
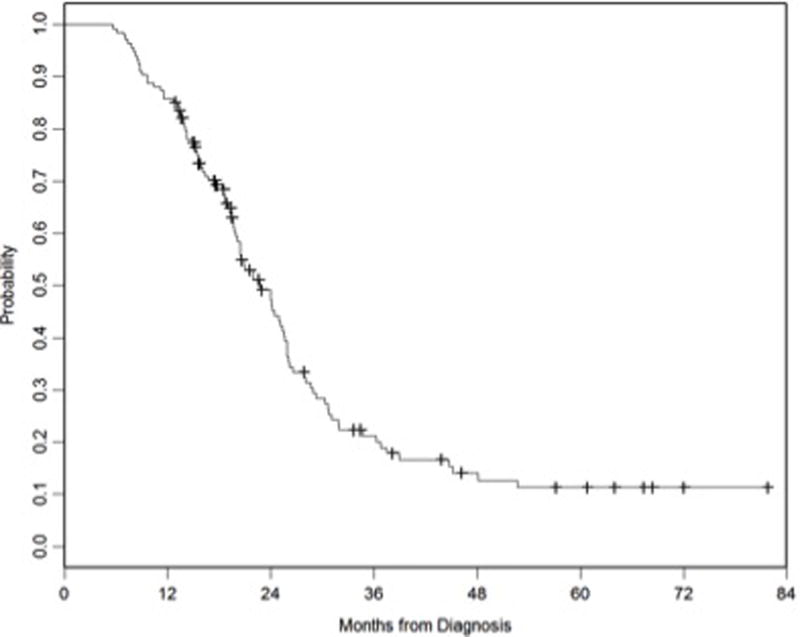
Kaplan Meier overall survival curve for all patients.
Figure 1b.
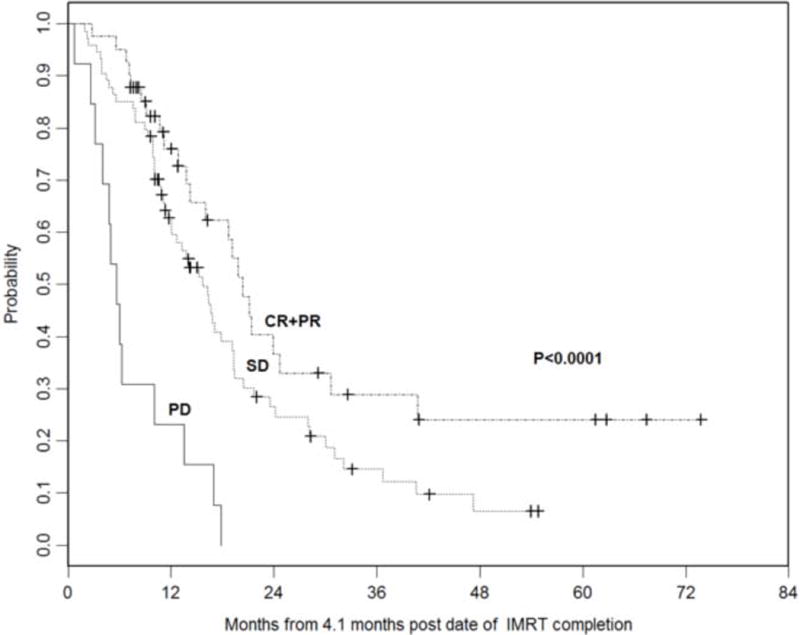
Kaplan Meier overall survival curves stratified by response to IMRT. (PD, progressive disease; SD, stable disease; CR+PR, complete response + partial response)
Table 2.
Univariate and multivariate analysis of overall survival.
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Number of death / censored, n | P-value1 | P-value1 | ||
| Radiographic arterial involvement | 0.30 | |||
| No | 36 / 6 | |||
| Yes | 62 / 30 | |||
| Response to ICT | 0.0009 | |||
| PR | 33 / 18 | |||
| SD | 47 / 16 | |||
| PD | 8 / 1 | |||
| Response to IMRT1 | <0.0001 | |||
| CR + PR | 23 / 18 | 1.00 | ||
| SD | 57 / 17 | 1.37 (0.82 – 2.28) | 0.23 | |
| PD | 13 / 0 | 4.42 (1.96 – 9.98) | 0.0004 | |
| Maintenance chemotherapy | 0.58 | |||
| No | 29 / 21 | |||
| Yes | 66 / 15 | |||
| Duration of induction chemotherapy | 0.47 | |||
| < 3.2 months | 46 / 19 | |||
| ≥ 3.2 months | 51 / 17 | |||
| Surgery1 | 0.0041 | |||
| Yes | 13 / 13 | 1.00 | ||
| No | 69 / 23 | 1.84 (0.99 – 3.39) | 0.052 | |
Time to overall survival (OS) was calculated from 4.1 months post RT end date for response to IMRT and surgery on univariate analysis (UVA), and for stepwise multivariate analysis; Time to OS was calculated from RT end date for other factors on UVA.
At the time of analysis, 93 patients (69%) presented with a relapse: 16 patients (17%) had isolated local relapse, 33 patients (36%) had both local and distant relapse, and 44 patients (47%) had distant metastases without local relapse.
The cumulative incidence of local failure at 1 year and 2 years were 10% (95%CI=5–15%) and 36% (95%CI=27–45%), respectively (Figure 2). None of the factors analyzed was significantly associated with local failure.
Figure 2.
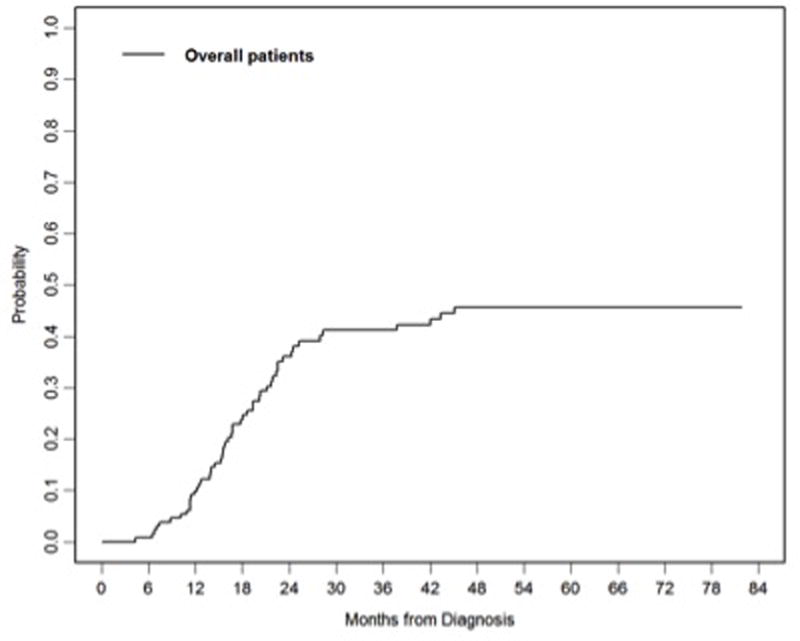
Cumulative incidence of local failure for all patients.
The cumulative incidence of distant metastasis at 1 year and 2 years were 30% (95%CI=22–38%) and 64% (95%CI=55–72%), respectively. On univariate analysis, absence of response to IMRT was associated significantly with the development of distant metastases (p=0.03). This was confirmed on multivariate analysis (HR=2.75; 95%CI=1.20–6.34; p=0.02).
Conversion to resectability
Conversion to resectability was achieved in 26 patients (19%) who were initially judged to have unresectable locally advanced disease by imaging and multidisciplinary board assessment (n=21) or prior laparoscopy (n=5). The median time from the end of the chemoradiotherapy to surgery was 3.3 months (range, 1.1–11.6 months). The decision regarding resectability was based on whether there was any improvement in the involvement of the involved vessel(s). While a clear fat-plane was not always achieved prior to surgery, there was generally decreased soft-tissue around the vessel, but there could still be residual stranding that might have been related to fibrosis. Twenty-five patients underwent definitive pancreaticoduodenectomy and one a total pancreatectomy. Twenty-two patients (85%) had negative surgical margins (R0). One patient (4%) had a complete pathologic response (ypT0). The median number of lymph nodes examined was 13 (range, 6–24). Twenty-three patients (88%) had no involved lymph nodes (ypN0). Fifteen of the resected patients (58%) received adjuvant chemotherapy for a median period of 2 months (range, 1.2–3.8 months).
On univariate analysis, response to IMRT was associated with subsequent surgery (p=0.01). This was confirmed on multivariate analysis (HR=3.25; 95%CI=1.31–8.04; p=0.01).
Tolerance
One hundred twenty-eight patients (96%) completed the prescribed course of radiation therapy in a median of 38 days. Treatment was discontinued prematurely in six patients (four patients had disease progression while on treatment, one patient had severe treatment-related toxicity, and one patient passed away at an outside hospital due to bleeding from unknown source). Seventeen patients (13%) had an interruption in radiation course due to toxicity (n=16) or social issues (n=1). Acute toxicities are summarized in Table 3. Thirty-five patients (26%) developed grade 3–4 hematological toxicity, primarily thrombocytopenia, and six patients (4.5%) developed grade 3–4 non-hematological toxicity. There was no difference in terms of grade 3–4 toxicity between patients who received concurrent gemcitabine versus fluoropyrimidine (p=0.7).
Table 3.
Acute Treatment-Related Toxicity According to NCI CTCAE Version 3.0.
| Toxicity (CTCAE) |
Grade 2 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
|---|---|---|---|
| Hematologic toxicity | |||
| Anemia | 47 (35) | 12 (9) | 0 |
| Neutropenia | 13 (10) | 4 (3) | 1 (1) |
| Thrombocytopenia | 20 (15) | 22 (16) | 4 (3) |
| Non hematologic toxicity | |||
| Fatigue | 55 (41) | 3 (2) | 0 |
| Nausea | 18 (13) | 1 (1) | 0 |
| Vomiting | 3 (2) | 0 | 1 (1) |
| Diarrhea | 8 (6) | 3 (2) | 0 |
| Rash | 1 (1) | 0 | 0 |
| Mucositis | 2 (1) | 0 | 0 |
Discussion
Management of patients with LAPC has been actively debated over the past decade and the optimal therapeutic approach remains unclear [2]. Although distant metastatic disease remains the primary cause of mortality, local progression also poses a significant problem. In a recent autopsy series, the death cause in one-third of patients was locally destructive pancreatic cancer [21]. The strategy combining ICT followed by chemoradiotherapy for LAPC aims to control both local and distant disease [3–5].
The present study is one of the largest to report the results of a strategy of ICT followed by IMRT for LAPC. The regimen was well tolerated with a low rate of grade 3–4 gastrointestinal toxicity (4.5%), indicating that IMRT with an integrated boost to a dose of 56 Gy is safe and feasible when combined with concurrent chemotherapy. Our short-term local control and survival rates are promising and appear better than historical standards. Perhaps the most promising sign of the efficacy of this combined-modality approach is a significant rate (19%) of patients rendered resectable after IMRT and the achievement of margin- and node-negative resections in 85% and 88% of these patients respectively.
Although the recent phase III randomized LAP07 trial did not demonstrate a survival benefit with the use of ICT followed by chemoradiotherapy compared to chemotherapy, there is still evidence that this is a promising therapeutic strategy. Indeed, in the chemoradiotherapy arm, the disease free survival rate was improved with a longer time off therapy prior to initiation of second line therapy [6]. The patterns of failure were also impacted by the use of radiotherapy, with significantly fewer patients developing local progression. Taken together, these results suggest that chemoradiotherapy could confer an advantage for these patients in terms of quality of life.
When compared to patients treated on the LAP07 trial for whom the median OS times ranged from 15 to 16 months, our results appear better, with a median OS of 23 months [6]. Our results compared favorably with those of a recent phase II study including patients with LAPC and borderline resectable pancreatic cancer treated with ICT combining gemcitabine, oxaliplatin and cetuximab, followed by radiation therapy at a dose of 50.4 Gy with concurrent capecitabine and cetuximab [22]. In this phase II trial, none of the patients with LAPC went on to a surgical resection whereas 19% of our patients underwent surgical resection, potentially signifying a benefit of dose escalation to 56 Gy used in our cohort. Interestingly, partial response to IMRT was associated with conversion to resectability, better overall survival, and less distant metastasis on both univariate and multivariate analysis. These observations underscore the therapeutic advantage of combining induction chemotherapy and chemoradiotherapy for LAPC and the role of response to IMRT as a predictive factor of outcome after combined modality treatment.
Several groups have demonstrated the feasibility of IMRT in the treatment of pancreatic tumors (Table 4) [8–19]. Milano et al. reported on 25 patients with pancreatic and bile duct carcinomas, comparing IMRT with three-dimensional conformal radiotherapy plans [11]. The dose received by critical structures such as kidneys, liver, and small bowel was significantly reduced with the use of IMRT plans. Yovino et al. reported on 46 patients with pancreatic/ampullary cancer treated with concurrent 5-fluorouracil based chemoradiotherapy using IMRT [13]. When compared to patients who had three-dimensional treatment planning in the RTOG 97–04 trial, IMRT significantly reduced the incidence of grade 3–4 nausea and vomiting (0% vs. 11%, p=0.024) and diarrhea (3% vs. 18%, p=0.017). One limitation of these studies is that they included both post-operative and LAPC patients even though the treatment intent, prognosis, and potential toxicities are different in these two settings. Ben-Josef et al. published the results of a phase I/II trial of IMRT dose escalation with concurrent fixed-dose gemcitabine in 50 patients with LAPC [14]. The recommended dose was 55 Gy. The median and 2-year OS were 14.8 months and 30%, respectively. Similar to our cohort, 12 (24%) patients underwent resection (10 R0, 2 R1). In another recent phase II trial, patients were treated with ICT followed by chemoradiotherapy with IMRT at a dose of 54 Gy and concurrent capecitabine [18]. This regimen was well tolerated with 9.5% of grade 3–4 gastrointestinal toxicity. Among the 24 LAPC included in this trial, the median OS was 9.3 months and the resection rate 8%. Interestingly, Krishnan et al. have shown in a retrospective study of MDACC cohort that patients who received focal dose escalation with IMRT had an improved OS and locoregional relapse free survival without additional toxicity [23]. This has to be confirmed prospectively.
Table 4.
Summary of IMRT Studies in Locally Advanced Pancreatic Adenocarcinoma.
| Author | Year | N | Treatment | Grade 3–4 GI Acute Toxicity (%) | Median OS (months) | 1-year OS (%) | Rate of resection (%) |
|---|---|---|---|---|---|---|---|
| Current study | 2016 | 134 | 56 Gy 5-FU or Gem |
4.5 | 23 | 85 | 19 |
| Krishnan | 2016 | 200 | 50–67,5 Gy Capecitabine or 5-FU or Gem |
2 | 15.3 | 60 | – |
| Ke | 2014 | 32 | 54 Gy S-1 |
25 | 15.2 | 75 | – |
| Esnaola | 2014 | 37 (24 LA) | 54 Gy Capecitabine |
9.5 | 9.3 | 50 | 8 |
| Badiyan | 2013 | 32 (25 LA) | 55 Gy Gem |
22 | 23.1 | – | 24 |
| Abelson | 2012 | 47 (18 LA) | 50.4–54 Gy 5-FU or capecitabine |
9 | 7.7 | 24 | – |
| Tunceroglu | 2012 | 20 | 50.4–54 Gy 5-FU or Gem |
20 | 11.6 | 55 | – |
| Ben-Josef | 2012 | 50 | 50–60 Gy Gem |
24 | 14.8 | 73 | 24 |
| Yovino | 2010 | 46 (15 LA) | 50.4–59.4 Gy 5-FU or capecitabine |
4 | 9.7 | – | – |
| Fuss | 2007 | 41 (24 LA) | 54 Gy Concurrent CT |
7 | 10 | 33 | – |
| Ben-Josef | 2004 | 15 (8 LA) | 45–55 Gy Capecitabine or Gem |
7 | – | 69 | 25 |
| Milano | 2004 | 20 (14 LA) | 50.4–59.4 Gy 5-FU |
20 | 9.3 | – | 32 |
| Bai | 2003 | 16 | 30 Gy then 21–30 Gy 5-FU or Gem |
– | – | 35 | – |
| Crane | 2001 | 5 | 33 Gy Gem |
50 | – | – | 20 |
Abbreviations: GI, gastrointestinal; OS, overall survival; LA, locally advanced; 5-FU, 5-fluorouracil; Gem, gemcitabine.
Although all patients in our study were treated with the same therapeutic strategy, our study is retrospective and thus hypothesis-generating. While the IMRT was homogeneously administered, the chemotherapy approach was more heterogeneous. Patients were treated with different induction chemotherapeutic regimens, concurrent chemotherapy was either gemcitabine or 5-FU based, and only one third of patients received maintenance chemotherapy. The efficacy of maintenance chemotherapy needs to be prospectively evaluated.
Advances in both systemic and local therapies may be contributing to incremental improvement in outcome for patients with LAPC. Intensified chemotherapy regimen such as FOLFIRINOX allows a higher rate of resection when followed by chemoradiotherapy, ranging from 23% to 41% [17, 24–27]. However, in our study, the number of patients treated with FOLFIRINOX was insufficient to assess its impact on outcome compared to gemcitabine-based chemotherapy. In a recent publication evaluating LAPC patients who received FOLFIRINOX with or without chemoradiation, the median OS was 25 months [27]. In view of the good tolerance of IMRT, the next step is to assess the influence of dose escalation on conversion to resectability and survival. Two ongoing randomized phase II trials are investigating this approach, SCALOP2 (NCT02024009) and RTOG 1201 (NCT01921751).
Finally, identifying both clinical and genomic predictors of response to chemoradiotherapy may help in selecting a subgroup of patients with LAPC who could benefit from chemoradiotherapy with the goal of allowing for surgical resection and improving survival.
Acknowledgments
The authors would like to thank the Nuovo-Soldati Foundation for Cancer Research for its support and Lawrence A. Herman for editorial assistance.
Funding information
The study was supported by a grant from Nuovo-Soldati Foundation for Cancer Research (FH).
Footnotes
Presented at the 53rd American Society for Therapeutic Radiology and Oncology (ASTRO) Meeting, Miami, October 2011.
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 3.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 5.Ko AH, Quivey JM, Venook AP, et al. A Phase II Study of Fixed-Dose Rate Gemcitabine Plus Low-Dose Cisplatin Followed by Consolidative Chemoradiation for Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2007;68:809–816. doi: 10.1016/j.ijrobp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–53. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 7.Huguet F, Goodman KA, Azria D, Racadot S, Abrams RA. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: american-French consensus recommendations. Int J Radiat Oncol Biol Phys. 2012;83:1355–64. doi: 10.1016/j.ijrobp.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Crane CH, Antolak JA, Rosen II, et al. Phase I study of concomitant gemcitabine and IMRT for patients with unresectable adenocarcinoma of the pancreatic head. Int J Gastrointest Cancer. 2001;30:123–132. doi: 10.1385/IJGC:30:3:123. [DOI] [PubMed] [Google Scholar]
- 9.Bai YR, Wu GH, Guo WJ, et al. Intensity modulated radiation therapy and chemotherapy for locally advanced pancreatic cancer: results of feasibility study. World J Gastroenterol. 2003;9:2561–2564. doi: 10.3748/wjg.v9.i11.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Fuss M, Wong A, Fuller CD, et al. Image-guided intensity-modulated radiotherapy for pancreatic carcinoma. Gastrointest Cancer Res. 2007;1:2–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys. 2011;79:158–162. doi: 10.1016/j.ijrobp.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Josef E, Schipper M, Francis IR, et al. A Phase I/II Trial of Intensity Modulated Radiation (IMRT) Dose Escalation With Concurrent Fixed-dose Rate Gemcitabine (FDR-G) in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–71. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunceroglu A, Park JH, Balasubramanian S, et al. Dose-painted intensity modulated radiation therapy improves local control for locally advanced pancreas cancer. ISRN Oncol. 2012;2012:572342. doi: 10.5402/2012/572342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abelson JA, Murphy JD, Minn AY, et al. Intensity-modulated radiotherapy for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;82:e595–601. doi: 10.1016/j.ijrobp.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Badiyan SN, Olsen JR, Lee AY, et al. Induction Chemotherapy Followed by Concurrent Full-dose Gemcitabine and Intensity-modulated Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2016;39:1–7. doi: 10.1097/COC.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 18.Esnaola NF, Chaudhary UB, O’Brien P, et al. Phase 2 trial of induction gemcitabine, oxaliplatin, and cetuximab followed by selective capecitabine-based chemoradiation in patients with borderline resectable or unresectable locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2014;88:837–44. doi: 10.1016/j.ijrobp.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ke QH, Zhou SQ, Yang JY, et al. S-1 plus gemcitabine chemotherapy followed by concurrent radiotherapy and maintenance therapy with S-1 for unresectable pancreatic cancer. World J Gastroenterol. 2014;20:13987–92. doi: 10.3748/wjg.v20.i38.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huguet F, Yorke ED, Davidson M, et al. Modeling pancreatic tumor motion using 4-dimensional computed tomography and surrogate markers. Int J Radiat Oncol Biol Phys. 2015;91:579–87. doi: 10.1016/j.ijrobp.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–43. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–65. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–8. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marthey L, Sa-Cunha A, Blanc JF, et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann Surg Oncol. 2015;22:295–301. doi: 10.1245/s10434-014-3898-9. [DOI] [PubMed] [Google Scholar]
- 26.Pietrasz D, Marthey L, Wagner M, et al. Pathologic Major Response After FOLFIRINOX is Prognostic for Patients Secondary Resected for Borderline or Locally Advanced Pancreatic Adenocarcinoma: An AGEO-FRENCH, Prospective, Multicentric Cohort. Ann Surg Oncol. 2015;22:S1196–205. doi: 10.1245/s10434-015-4783-x. [DOI] [PubMed] [Google Scholar]
- 27.Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol. 2015;22:3512–21. doi: 10.1245/s10434-015-4647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


