Abstract
Objectives
The study aims to apply willingness-to-pay (WTP) values derived from the literature to inform decision-makers of the cost-effectiveness of the Tailored Activity Program (TAP), an intervention proven to reduce caregiver burden.
Methods
TAP and other caregiver interventions employ an individual perspective and non–quality-adjusted life-year (QALY) outcome measure where the primary objective is to determine caregiver burden from an individual perspective. Therefore, standard cost/QALY thresholds are not appropriate. To identify relevant WTP values, we searched for studies that: 1) were published in the past 5 years and used contingent valuation methodology to identify WTP; 2) assessed WTP for a dementia-related intervention requiring out-of-pocket expenditure; and 3) asked caregivers their WTP for an outcome related to reducing caregiver burden. Three studies were identified utilizing four WTP values. We also assessed potential financial savings that caregivers could achieve from purchasing TAP. To assess the probability of TAP being cost-effective, we built a Monte Carlo simulation to test the four WTP values applied to two TAP outcome measures: reduction in caregiver hours “on duty;” and “doing things.”
Results
For outcome measure “on duty,” WTP varied between $1.06/hour and $4.58/hour. For outcome measure “doing things,” WTP varied between $2.21/hour and $9.57/hour. Applying the four identified WTP values from the literature to TAP outcomes resulted in TAP cost-effectiveness varying between 50% and 80% for both outcome measures.
Conclusions
When WTP data are not collected prospectively or conventional metrics cannot be applied, retrospectively assessing literature-derived WTP may be acceptable for informing decision-makers of potential cost-effectiveness of a proven program. Application of WTP to TAP shows potential cost-effectiveness that can be expected under the tested WTP scenarios.
Keywords: Alzheimer’s disease, contingent valuation method, outcomes of mental health care, willingness-to-pay
Introduction
Dementia is one of the most costly diseases in the United States with a total annual cost to society estimated to be $152 billion [1]. Dementia is also financially debilitating for patients and their family caregivers. The largest portion of dementia costs is associated with time spent caregiving by family members, reflecting an estimated aggregate cost of $97 billion. This equates to $40,000 a year that an individual family spends in caring for a relative with dementia [2]. Costs incurred by family caregivers include direct out-of-pocket expenses and/or, informal costs (unpaid help or lost income by family members) [1] and lost productivity. Neuropsychiatric behaviors are a common clinical symptom of dementia that increase the amount of time required to provide care and hence caregiver burden and cost [3,4].
Although the optimal treatment to address behavioral symptoms and reduce caregiver burden is unclear, emerging nonpharmacological approaches appear promising [5–7]. Yet, for nonpharmacologic interventions to become part of the standard of care for dementia patients, economic evaluations are needed to determine their value to society and individuals [8]. At the individual level, economic evaluations are needed to determine the willingness-to-pay (WTP) for different effective caregiver interventions. Although there are now many proven caregiver interventions, to date, few incorporate an economic evaluation [9,10]. Of those studies that have evaluated cost-effectiveness, most have not determined caregiver WTP, making it difficult to assess how individuals value these interventions [8]. The need to offer proven interventions to reduce the burden of family caregivers and understand their economic value is underscored by it becoming an explicit priority goal of Health People 2020 [11].
Lack of WTP data also makes it difficult to compare economic outcomes across caregiver programs or with other health services. Thus, identifying WTP levels appropriate for economic analyses of these proven programs that are from an individual caregiver perspective is an important methodological step. Such an approach may help to advance economic evaluations and the translation of dementia caregiver programs for delivery in diverse service settings.
Most economic evaluations of health services utilize a standard societal threshold of less then $50,000/quality-adjusted life-year (QALY) as a measure of cost-effectiveness [12]. Nevertheless, when a QALY is not employed as the outcome measure, it is not possible to compare cost-effectiveness to the $50,000 threshold. There have also been several studies that question the validity of applying a standard $50,000 threshold. These studies suggest that WTP should be tailored to individual preferences [13,14].
Moreover, when assessing interventions for dementia caregivers, a societal perspective that employs QALYs may not be appropriate. Traditional methodologies to capture QALYs focus on mobility and physical and emotional functioning. Nevertheless, they do not address the quality of life challenges experienced by families such as the increasing amount of time required to attend to dementia patients [8,15]. Guidelines for health economic methods suggest that studies should select outcome measures and perspectives that are most relevant to the patient population rather than apply a standard metric [16]. As respite or time to self is a highly valued commodity by caregivers, time spent caregiving serves as an appropriate, targeted outcome [9].
Currently, caregiver interventions are not supported by third party payers and thus may need to be purchased out-of-pocket. Thus, applying an individual perspective using contingent valuation to evaluate the Tailored Activity Program (TAP) is appropriate [17]. Determining a caregiver’s WTP based on the clinical and economic metrics which are important to this population is vital for informing decision-makers [18–20].
The purpose of this exploratory study was to apply different caregiver-based WTP values to evaluate retrospectively the cost-effectiveness and financial savings of a proven nonpharmacologic intervention, the TAP.
TAP, an 8-session, 4-month structured occupational therapy intervention, provides dementia patients with activities tailored to their capabilities, and trains family caregivers in their use. Specifically, occupational therapists assess the cognitive functioning and preserved capabilities of individuals with dementia including their previous and current interests and daily activities, caregiver communication style, and the physical home environment. Based on this assessment, therapists develop activities to match patient interests and capabilities and then instruct caregivers in their use, including how to set up the environment, introduce and supervise the activity, and communicate and cue effectively. As reported elsewhere, TAP was shown to reduce behavioral occurrences and increase activity engagement of dementia patients. It also benefited caregivers by enhancing their sense of confidence using activities in daily routines and reducing the time required in daily oversight or vigilance [21,22]. In a previous cost-effectiveness study, we showed that the cost to reduce 1 hour of the caregiver “doing things” for the patient per day was $2.37, and the cost to reduce 1 hour of the caregiver having to be “on duty” was $1.10 [23]. This exploratory study extends our cost-effectiveness analysis by evaluating four different WTP scenarios and examining the potential financial savings caregivers may achieve by purchasing the TAP intervention.
Methods
Study Sample
The WTP analysis was applied to the original TAP trial and cost-effectiveness analysis. The original TAP trial consisted of a two-group randomized parallel design in which 60 dyads (dementia patients/caregivers) were recruited between 2005 and 2006 and randomly assigned to treatment or wait-list control [21,22]. At 4-months (main trial end point) from baseline, all dyads were interviewed and reassessed on study outcomes.
The characteristics of study participants have been previously reported [21,22]. Briefly, to participate in TAP, dementia patients had to be English speaking, have a physician diagnosis or Mini-Mental State Examination score less than 24 [24], and have one or more behavioral symptoms as reported by caregivers. Caregivers had to be English-speaking, 21 years of age or older, living with patients, and providing four or more hours of care daily.
Outcome Measures
We used two caregiver-time related items as outcomes (hours spent “on duty” and hours spent “doing things”) from the 4-item Caregiver Vigilance Scale [25]. The two other items (how long patient can be left at home alone in an emergency, and how long patient can be left in a room alone if someone is home) were not used in the original TAP trial as they do not measure caregiver time in daily care. Each item on the scale can be used independently and has been shown to have content validity [25]. Additionally, two recent studies provide further evidence of the construct validity of the two independent vigilance items used in this study [9,26]. Nichols and colleagues found a treatment effect for “doing things,” and Gitlin and colleagues found a treatment effect for “on duty.”
Data Sources for WTP Values
To determine different WTP levels to apply to TAP, we identified three published studies involving four WTP values that were conducted in the past 5 years and that fit these criteria: 1) used contingent valuation methodology to identify WTP; 2) assessed WTP for a dementia-related intervention that required an out-of-pocket expenditure; and 3) asked caregivers what they would be willing to pay for an outcome of either stabilizing dementia or reducing caregiver burden.
We only included studies specific to dementia caregivers versus studies of informal caregiving in general for several reasons. Caring for an individual with dementia is one of the most profoundly disturbing and time-consuming forms of caregiving. Compared to caregivers of individuals with nondementing conditions, dementia caregivers are more distressed and pay higher out-of-pocket expenses [27]. Thus, we reasoned that WTP values derived from informal caregiving studies in general would not adequately reflect valuations of dementia caregivers.
One study, conducted by Chiu et al., interviewed 136 family caregivers of patients with dementia to determine the maximum amount they would be willing to pay in a month for nursing home services [28]. A second study by Werner et al. used an open-ended question to determine the amount of money 220 caregivers would be willing to pay in a month to purchase a medication that controls dementia [29]. The third identified study by Wu et al. sought to determine the WTP of 28 caregivers for a cholinesterase inhibitor for mild to moderate dementia [30]. Although Wu et al. presented four outcome scenarios, only two were relevant and used here. The two relevant scenarios sought to determine WTP for stabilizing dementia, similar to the purpose of the TAP intervention, whereas the other two scenarios not used here addressed curative interventions for dementia (Table 1).
Table 1.
Demographics of study samples included in cost analyses
| Study | N (dyads) | Caregivers
|
Patients with dementia
|
|||
|---|---|---|---|---|---|---|
| Age Mean (SD) |
Sex % Female |
Age Mean (SD) |
Sex % Female |
Cognitive status Mean MMSE (SD) |
||
| Gitlin et al. [22] | 60 | 65 (11.1) | 88 | 79 (9.4) | 43 | 12 (8.1) |
| Chiu et al. [28] | 136 | <65 = 67.6%* | 73.5 | <65 = 20% | 44.9 | 65.5% Mild-Moderate MMSE† |
| Werner et al. [29] | 220 | 62* | 68.2 | 73.4* | 50.9 | 17.1* |
| Wu et al. [30] | 28 | 66 (14.32) | 75 | 78.29 (4.31) | 32 | Mild-Moderate MMSE† |
Study did not publish SD.
Numeric MMSE scores not published.
MMSE, Mini Mental State Examination; SD, Standard Deviation.
All WTP monetary values taken from the literature were adjusted to 2008 US dollars (USD) [31]. For studies which presented results in foreign currency, currency conversion (foreign currency to USD) rates were taken from the first of the month of the publication date of the study [32].
Data Analysis
Adjusted WTP Values
Because the original TAP trial evaluated the two outcome measures of caregiver time on a per hour basis, we adjusted WTP levels used in the selected studies to reflect a unit value per hour. To adjust WTP values on a per hour basis for each measure, we derived a daily WTP estimate (based on the time frame in the published study) and then divided the per day estimate by the average benefit the TAP intervention delivered in a day for each outcome measure. The mean amount of benefit purchased in a day for the outcome measure “on duty” was 6.9 hours and for the outcome measure time spent “doing things” was 3.3 hours [21,22]. We derived the mean benefit in a day that a caregiver could purchase based on the original TAP study outcomes as they equate to the average benefit the intervention group derived over the control group for each outcome measure. Finally, we assumed that an individual would be able to purchase caregiver time in increments of 1 hour.
Financial Savings as a Measure of WTP
We also evaluated WTP from the perspective of the potential financial savings caregivers may achieve from purchasing the TAP intervention. When evaluating WTP values of TAP based on financial savings a caregiver could achieve by participating in the intervention, we assumed that the value of 1 hour of time for a family caregiver was equal to the federal minimum wage of $7.25 [33]. We assumed an individual would be willing to purchase TAP as long as the cost of the intervention did not exceed the value of time saved per hour.
Monte Carlo Simulation
To evaluate the probability of TAP being cost-effective given varying WTP thresholds, we constructed a decision tree using TreeAge Pro 2009 (Williamstown, MA). Our model inputs for the TAP decision tree were taken from our previously published cost-effectiveness study of TAP [23] and included seven model inputs (training, caregiver time, assessment materials, intervention supplies, interventionist time, travel time, mileage reimbursement), accounting for the cost of delivering and implementing TAP. To account for variability in the TAP decision tree model and assess probability of TAP being cost-effective based on varying WTP values and financial savings analysis, we conducted a second order Monte Carlo simulation. All model inputs needed for the Monte Carlo sensitivity analysis were derived from the previous TAP cost-effectiveness study. Mean and standard deviations were used to account for variability.
We ran 10,000 iterations through the Monte Carlo simulation for both outcome measures. Acceptability curves were derived for each outcome measure and used to asses the probability of TAP being cost-effective based on varying WTP levels.
Results
Sample Characteristics
Table 1 details the demographic characteristics of each study including the TAP study sample. In the TAP study, most dementia patients were male (57%) and white (77%) with a mean age of 79 (SD 9.4) years and MMSE score of 11.6 (SD 8.1) [24]. The majority of caregivers were female (88%) and white (77%) with a high school diploma (56%), and average age of 65 (SD 11.1) years. Caregivers reported managing many behavioral symptoms (Mean = 8; SD 3.8) and a high number of functional dependencies (Mean = 7.8; SD 0.5). On average, caregivers spent 6.25 (SD 3.81) hours of their time doing things for patients and 16.85 (SD 7.54) hours providing direct oversight or “being on duty.” At baseline, there were no statistically significant differences between the wait-list control and TAP intervention groups except for caregiver age. Caregivers in the TAP group were on average 5 years younger than those in the control group.
Table 1 also shows the basic demographic characteristics of patients in those studies which were used to derive the WTP comparisons. Similar to the TAP sample, the majority of caregivers in the three studies were female and less than 65 years old. Most dementia patients were men and greater than 65 years old.
Adjusted WTP Values
Table 2 details the estimated per hour WTP of each study for both outcome measures. Adjusted WTP values from Chiu et al. [28] were $4.58 (“on duty”) and $9.57 (“doing things”). For Werner et al. [29], WTP values were adjusted to $1.06 (“on duty”) and $2.21 (“doing things”). Finally, for Wu et al. [30], WTP was adjusted to $1.68 (“on duty”) and $3.51 (“doing things”) for a cholinesterase inhibitor.
Table 2.
Adjusted willingness to pay values and probability of cost-effectiveness
| Study | Intervention | WTP per intervention ($) | Estimated WTP per hour ($)
|
% of time TAP cost-effective
|
||
|---|---|---|---|---|---|---|
| On duty | Doing things | On duty | Doing things | |||
| Chiu et al. [28] | Caregivers asked amount willing to pay in a month to receive nursing home services. | 978 | 4.58 | 9.57 | 80 | 70 |
| Werner et al. [29] | Caregivers asked amount willing to pay in a month for a drug treatment. | 225 | 1.06 | 2.21 | 50 | 50 |
| Wu et al. [30] | Caregivers asked amount willing to pay to stabilize patient over 1 year in both cognition and behavior with a cholinesterase inhibitor treatment. | 4224 | 1.68 | 3.51 | 60 | 60 |
| Wu et al. [30] | Caregivers asked amount willing to pay to stabilize over 1 year in both cognition and behavior with a cholinesterase inhibitor treatment; however, with adverse events. | 3788 | 1.50 | 3.14 | 60 | 60 |
All studies used contingent valuation methodology.
WTP, willingness-to-pay.
Financial Savings as a Measure of WTP
For the outcome measure “on duty,” we estimated that the TAP intervention saved on average $50 a day or $6003 over the course of the intervention ($7.25 × 6.9 hours of time saved on average a day × 120 days). Similarly, for the outcome measure “doing things,” we estimated that TAP saved on average $24 a day or $2872 over the course of the intervention ($7.25 × 3.3 hours of time saved on average a day from TAP trial × 120 days).
Monte Carlo Simulation
Figure 1 shows the acceptability curves from the Monte Carlo simulation for each outcome measure, “on duty” and “doing things.” For both outcome measures, WTP was assessed on a per hour basis. The acceptability curve serves as a visual representation of the Monte Carlo simulation. The x-axis represents a range of WTP values per hour. The y-axis represents the probability of TAP being cost-effective for intervention group caregivers.
Figure 1.
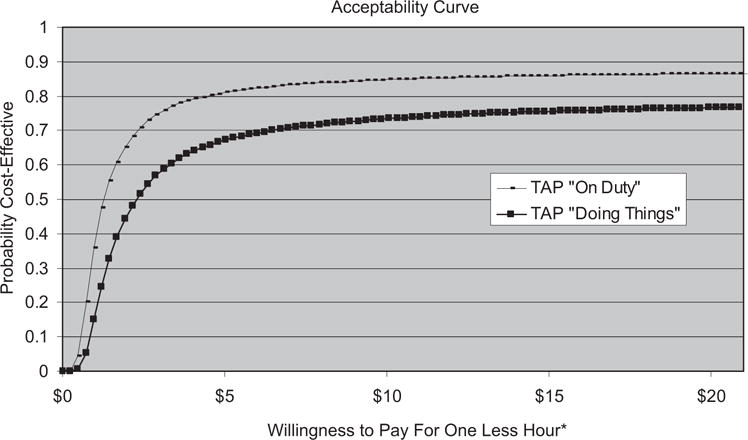
Acceptability curves for outcome measure “On Duty” and “Doing Things.” *One less hour is equivalent to one more hour of free time.
For the outcome measure “on duty,” the point at which TAP had a 50% probability of being cost-effective is equal to a WTP of $1.20 an hour. At any WTP level greater than $1.20 for the outcome measure “on duty,” the probability of TAP being cost-effective was greater than 50%. Similarly, for the outcome measure “doing things,” TAP had a 50% probability of being cost-effective given a WTP of $2.40 an hour. At any WTP value greater than $2.40 for the outcome measure “on duty,” the probability of TAP being cost-effective was greater than 50%.
WTP Comparisons
Table 2 also details the probability of TAP being cost-effective for each outcome measure based on the four estimated per hour WTP values. Using the WTP levels derived from Chiu et al. [28], we found that TAP had an 80% probability of being cost-effective for the measure “on duty” and 70% probability for the measure “doing things.” Based on the WTP values derived by Werner et al. [29], we estimated that TAP had a 50% probability of being cost-effective for both outcome measures. Finally, based on the WTP values from Wu et al. [30], we estimated that for both outcome measures, TAP had a probability of being cost-effective 60% of the time. Based on our assumptions for the financial savings analysis, we found that for both outcome measures (“on duty” and “doing things”), TAP had an 80% probability of being cost-effective.
Discussion
There is a dearth of published literature on WTP thresholds and cost-effectiveness for nonpharmacologic dementia interventions from an individual caregiver perspective. Generally, cost-effectiveness analyses employ a societal threshold of $50,000/QALY; however, when studies employ non-QALY outcome measures and use nonsocietal perspectives, standard conventions cannot be applied [12,13]. Others have argued that traditional cost-effectiveness thresholds such as the $50,000/QALY are arbitrary and as such do not allow for a true valuation of an intervention [14,17]. In our analysis, we adopted WTP thresholds that were not arbitrary but rather represented a composite of WTP values derived via contingent valuation from previously published studies. Contingent valuation methodology has been validated in several studies [34,35] and is an acceptable approach to value informal care. As such, the WTP values derived from the literature on dementia caregivers serve as the best proxy for the market price and subsequently, the value of TAP.
To date, there is no consensus in the health economic literature as to how to value informal care in economic analyses [36]. The analyses presented here are novel in that we incorporated time spent caregiving as the outcome measures rather than as a cost. TAP uses an individual perspective and employs two measures (“on duty” and “doing things”) of caregiver time which is a highly salient commodity to families. Nevertheless, typically, when valuing interventions in health economics, elements of an intervention pertaining to time are considered part of the cost variables. By incorporating time as an outcome measure rather than in the cost component of the analysis, we are able to avoid ambiguities with valuing time [36]. Instead, time is valued in our analysis via WTP values derived from contingent valuation.
We believe that these time-based outcome measures, also used in previous caregiver cost analysis studies [9,25,27], are the most clinically relevant and as such should be used as the primary outcome measures when determining WTP for caregiver programs. In addition, we believe that if individuals were to purchase the TAP intervention on their own, they would do so on the merits of it reducing time spent caregiving and not on their QALY benefits.
Using WTP thresholds previously reported in research on caregiver preferences, we were able to estimate the probability of TAP being cost-effective [28–30]. All WTP values from the literature were greater than $1.06 for the outcome measure “on duty” and greater than $2.20 for the outcome measure “doing things.” As such, given the baseline cut points for cost-effectiveness, TAP compares favorably to the WTP values from the literature. For the outcome measure “on duty,” we found that for any WTP greater than $1.20 an hour, TAP has a greater than 50% chance of being cost-effective. Similarly for the outcome measure “doing things,” we found that for any WTP greater than $2.40 an hour, TAP is cost-effective greater than 50% of the time. Although this appears to be a positive result, it is not possible to compare this to other studies because of a lack of published literature on WTP for caregiver interventions.
Some health economic researchers have argued that WTP metrics are static and do not provide adequate information over time [14]. Nevertheless, the use of acceptability curves can accommodate this shortcoming [14,37]. Given the individual perspective of our study, the acceptability curve can be used by the individual decision-maker who is able to account for his/her own WTP as it may change over time.
The financial savings analysis is comparable to an opportunity cost analysis [34]. Although opportunity cost analyses have been critiqued when presented on their own, when part of an overall valuation such as our analysis, it provides additional information for the decision-maker. The financial savings analysis showed that TAP was cost-effective 80% of the time. This analysis assumes that caregivers would be able to replace time spent caregiving with a wage-earning activity. This may be a limitation of our study as it places a monetary value on time, even when caregivers may choose to spend their newfound time differently than earning real cash wages. Nevertheless, for many caregivers, the opportunity for respite during the day is an invaluable commodity which is not easy to achieve, particularly caring for individuals at the moderate and severe disease stages when hands-on care and heightened vigilance are required [38,39]. As such, we believe that the financial savings analysis may understate the value of time gained given the assumptions in our model and the moderate hourly wage we employed. It should be noted that TAP resulted in caregivers having extra time by meaningfully engaging the individual with dementia in safe and pleasant activities. Thus, TAP enhanced the quality of life of the individual with dementia, and the caregiver’s time away from caregiving did not compromise the safety or well-being of the dementia patient. Also, it should be noted that at baseline there was a 5-year age difference with those assigned to TAP being younger than those in the control group. Younger caregivers may be more likely to work and thus value their time more as it might represent lost income. Thus, TAP may have been more cost-effective than the data show.
Several limitations should be noted. As we did not capture WTP values prospectively in the initial TAP trial, we necessarily had to rely on WTP values derived from studies that evaluated different although similar treatments. There are numerous factors that can lead to inconsistency when applying different WTP thresholds derived from other studies including social norms/values, and different methodologies used to elicit WTP. Nevertheless, the studies we chose had comparable treatments in that they valued outcomes of dementia-related interventions similarly. In addition, using multiple studies render our WTP analysis more robust than selecting one study and applying a single value from the literature.
Another potential study limitation is the adjustment of the WTP thresholds to reflect a per hour basis so that a comparison could be made to TAP. We assumed that when adjusting WTP on a per hour basis, there would be consistency and reliability between published values and adjusted values. Again, we also addressed this limitation by using four different WTP thresholds derived from the literature. Finally, another limitation may be our assumption that TAP can be purchased in increments of 1 hour. For example, individual caregivers cannot deliberately choose to purchase 6.9 hours of less time spent being “on duty.” Although we recognize this argument, we selected 1-hour increments because of ease of use when conducting such a micro economic analysis. Furthermore, this level of analysis also benefits decision-makers who can then use this most basic unit of measurement and adjust to a level they believe to be appropriate.
Despite these limitations, we believe that WTP analyses are at the forefront of evaluating cost-effectiveness of existing caregiver programs from an individual perspective. In addition, our findings help to demonstrate the value of informal caregiving and offer insight in understanding the needs of caregivers [8,11]. Most importantly, our findings from this exploratory study illustrate a method to evaluate cost-effectiveness when WTP data are not collected prospectively and standard WTP metrics cannot be used. To our knowledge, this is the first study which shows that it is possible to use previously reported relevant WTP levels and apply these values to a similar study in an effort to inform decision-makers of potential cost-effectiveness. We show the feasibility of assessing WTP retrospectively via two approaches. First, by using comparable WTP thresholds in the literature, and secondly, by assessing the potential financial savings one may achieve from partaking in the intervention [34,35]. As such, this study adds incrementally to the health economic literature and highlights the value of evaluating caregiver interventions to substantiate their translational potential.
In summary, this study lends further support to the economic value of TAP. It also shows the value of applying WTP scenarios retrospectively, offering an exploratory methodology to evaluate and compare existing proven caregiver programs.
Acknowledgments
Source of financial support: Research reported in this article was sup-ported in part by National Institute of Mental Health Grant # R21 MH069425 and RC1 MH090770.
References
- 1.Wimo A, Winblad B, Jonsson L. An estimate of the total world-wide societal costs of dementia in 2005. Alzheimers Dement. 2007;3:81–91. doi: 10.1016/j.jalz.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Zhu CW, Clipp EC. Informal costs of dementia care: estimates from the national longitudinal caregiver study. J Gerontol B Psychol Sci Soc Sci. 2001;56(Suppl):S219–28. doi: 10.1093/geronb/56.4.s219. [DOI] [PubMed] [Google Scholar]
- 3.Zhu CW, Leibman C, McLaughlin T, et al. The effects of patient function and dependence on costs of care in Alzheimer’s disease. J Am Geriatr Soc. 2008;56:1497–503. doi: 10.1111/j.1532-5415.2008.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murman DL, Chen Q, Powell MC, et al. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59:1721–9. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 5.CohenMansfield JPD. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry. 2001;9:361–81. [PubMed] [Google Scholar]
- 6.Cohen-Mansfield JP. Nonpharmacological interventions for persons with dementia. Am J Geriatr Psychiatry. 2005;6:129–45. [Google Scholar]
- 7.Lyketsos CG, Colenda CC, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14:561–73. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 8.McDaid D. Estimating the costs of informal care for people with Alzheimer’s disease: methodological and practical challenges. Int J Geriatr Psychiatry. 2001;16:400–5. doi: 10.1002/gps.353. [DOI] [PubMed] [Google Scholar]
- 9.Nichols LO, Chang C, Lummus A, et al. The cost-effectiveness of a behavior intervention with caregivers of patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:413–20. doi: 10.1111/j.1532-5415.2007.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff MJL, Adang EMM, Vernooij-Dassen MJM, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. BMJ. 2008;336:134–8. doi: 10.1136/bmj.39408.481898.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthy People 2020. (Developmental) Reduce the proportion of unpaid caregivers of older adults who report an unmet need for caregiver support services (OA HP2020-2) Available from: http://www.healthypeople.gov/HP2020/Objectives/ [Accessed February 23, 2010]
- 12.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Exp Rev Pharmacoeconom Res. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 13.Gyrd-Hansen D. Willingness to pay for a QALY: theoretical and methodological issues. Pharmacoeconomics. 2005;23:423–32. doi: 10.2165/00019053-200523050-00002. [DOI] [PubMed] [Google Scholar]
- 14.Gafni A, Birch S. Incremental cost-effectiveness ratios (ICERs): the silence of the lambda. Soc Sci Med. 2006;62:2091–100. doi: 10.1016/j.socscimed.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm D, Healey A, Knapp M. QALYs and mental health care. Soc Psychiatry Psychiatr Epidemiol. 1997;32:68–75. doi: 10.1007/BF00788923. [DOI] [PubMed] [Google Scholar]
- 16.Hjelmgren J, Berggren F, Andersson F. Health economic guidelines—similarities, differences and some implications. Value Health. 2001;4:225–50. doi: 10.1046/j.1524-4733.2001.43040.x. [DOI] [PubMed] [Google Scholar]
- 17.Owens D. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13:716–17. doi: 10.1046/j.1525-1497.1998.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brian B, Gafni A. When do the “dollars” make sense?: toward a conceptual framework for contingent valuation studies in health care. Med Decis Making. 1996;16:288. doi: 10.1177/0272989X9601600314. [DOI] [PubMed] [Google Scholar]
- 19.Bayoumia A. The measurement of contingent valuation for health economics. Pharmacoeconomics. 2004;22:691. doi: 10.2165/00019053-200422110-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bala M, Mauskopf J, Wood L. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9. doi: 10.2165/00019053-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Gitlin LN, Winter L, Vause Earland T, et al. The tailored activity program to reduce behavioral symptoms in individuals with dementia: feasibility, acceptability, and replication potential. Gerontologist. 2009;49:428–39. doi: 10.1093/geront/gnp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitlin LN, Winter L, Burke J, et al. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. J Am Geriatr Soc. 2008;16:229–39. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gitlin LN, Hodgson N, Jutkowitz E, Pizzi LT. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. Am J Geriatr Psychiatry. 2009 Dec 16; doi: 10.1097/JGP.0b013e3181c37d13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney DF, Jones RN, Coon DW, et al. The caregiver vigilance scale: application and validation in the resources for enhancing Alzheimer’s caregiver health (REACH) project. Am J Alzheimers Dis Other Demen. 2003;18:39–48. doi: 10.1177/153331750301800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gitlin L, Winter L, Corcoran M, et al. Effects of the home environmental skill building program on the caregiver-care recipient dyad: 6-month outcomes from the Philadelphia REACH initiative. Gerontologist. 2003;43:532–46. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- 27.Ory MG, Hoffman RR, III, Yee JL, et al. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177–86. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 28.Chiu L, Tang K, Liu Y, et al. Willingness of families caring for victims of dementia to pay for nursing home care: results of a pilot study in Taiwan. J Manag Med. 1998;12:349. doi: 10.1108/02689239810234571. [DOI] [PubMed] [Google Scholar]
- 29.Werner P, Schnaider-Beeri M, Aharon J, et al. Family caregivers’ willingness to pay for drugs indicated for the treatment of Alzheimer’s disease: an economic or psychological model? Dementia. 2002;1:59–74. [Google Scholar]
- 30.Wu G, Lanctot KL, Herrmann N, et al. The cost-benefit of cholinesterase inhibitors in mild to moderate dementia: a willingness-to-pay approach. CNS Drugs. 2003;17:1045–57. doi: 10.2165/00023210-200317140-00004. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Bureau of Labor and Statistics. CPI inflation calculator. 2009 Available from: http://data.bls.gov/cgi-bin/cpicalc.pl [Accessed November 9, 2009]
- 32.Currency converter for 164 currencies. 2009 Available from: http://www.oanda.com/convert/classic [Accessed November 9, 2009]
- 33.U.S. Department of Labor. U.S. Department of Labor—Wage and Hour Division (WHD)—Compliance Assistance-Fair Labor Standards Act (FLSA) 2009 Available from: http://www.dol.gov/whd/Flsa/index.htm [Accessed November 9, 2009]
- 34.Van den Berg B, Bleichrodt H, Eeckhoudt L. The economic value of informal care: a study of informal caregivers’ and patients’ willingness to pay and willingness to accept for informal care. Health Econ. 2005;14:363–76. doi: 10.1002/hec.980. [DOI] [PubMed] [Google Scholar]
- 35.Van denBerg B, Brouwer W, van Exel J, Koopmanschap M. Economic valuation of informal care: the contingent valuation method applied to informal caregiving. Health Econ. 2005;14:169–83. doi: 10.1002/hec.893. [DOI] [PubMed] [Google Scholar]
- 36.Van den Berg B, Spauwen P. Measurement of informal care: an empirical study into the valid measurement of time spent on informal caregiving. Health Econ. 2006;15:447–60. doi: 10.1002/hec.1075. [DOI] [PubMed] [Google Scholar]
- 37.Birch S, Gafni A. Cost effectiveness/utility analysis: do current decision rules lead us to where we want to be? J Health Econ. 1992;12:469–76. doi: 10.1016/0167-6296(92)90004-k. [DOI] [PubMed] [Google Scholar]
- 38.Tranmer JE, Guerriere DN, Ungar WJ, Coyte PC. Valuing patient and caregiver time: a review of the literature. Pharmacoeconomics. 2005;23:449–59. doi: 10.2165/00019053-200523050-00005. [DOI] [PubMed] [Google Scholar]
- 39.Russell LB. Completing costs: patients’ time. Med Care. 2009;47(Suppl):S89–93. doi: 10.1097/MLR.0b013e31819bc077. [DOI] [PubMed] [Google Scholar]


