Abstract
Disease states such as neuropathic pain offer special challenges in drug design due to the system changes which accompany these diseases. In this manuscript we provide an example of a new approach to drug design in which we have modified a potent and selective peptide ligand for the CCK-2 receptor to a peptide which has potent agonist binding affinity and bioactivity at delta and mu opioid receptors, and simultaneous antagonist activity at CCK receptors. De novo design based on the concept of overlapping pharmacophores was a central hypothesis of this design, and led to compounds such as H-Tyr-DPhe-Gly-DTrp-NMeNle-Asp-Phe-NH2 (i.e., RSA 601) which have the designed properties.
Keywords: Peptide drug design, Opioid agonists, Cholecystokinin antagonists, Neuropathic pain
Introduction
There is a need for a new paradigm in drug discovery for pathological conditions including disease states such as neuropathic pain and conditions of opioid analgesic tolerance. We now know from multiple experimental approaches that many disease states lead to changes in expressed proteins (adaptation/neuroplasticity). Drug design based on normal states are inadequate or even possibly counter-indicated. Therefore the system changes that have occurred must be considered in any treatment for the disease. Such “systems changes” are clearly evident in neuropathic pain (Vanderah et al., 2001) and in conditions of opioid tolerance where opioids can actually heighten pain (Gardell et al., 2002). In these pain states there are increased levels and/or activity of pronociceptive neurotransmitters such as cholecystokinin (CCK) and their receptors. CCK and enkephalins and their receptors are co-localized in the CNS and, as a pronociceptive peptide, CCK acts as an “antiopioid” and alternate analgesic to diminish opioid antinociception.
In view of these and other findings we have decided to investigate a new paradigm for drug discovery aimed at treatment of pathological pain (such as neuropathic pain) and in conditions of opioid tolerance. In this approach we will design a single peptide or peptidomimetic molecule which can interact with opioid receptors as agonists and at CCK receptors as antagonists. Specifically, we suggest that compounds which are agonists at opioid mu or delta receptors and antagonists at CCK-1 or CCK-2 receptors (preferably both representing a “balanced” CCK receptor antagonist). We postulate that such a molecule should show superior efficacy to opioid agonists for the treatment of pathological pain states since it would block the antiopioid effects of CCK and be resistant to the development of paradoxical opioid-induced pain and antinociceptive tolerance. We report herein our progress toward these objectives using various approaches to rational peptide ligand based drug design we have developed in the past (Hruby, 1982, 2002; Hruby et al., 1990) though for single targets.
Methods
Peptide synthesis and purification
The peptide reported here were synthesized by standard solid phase peptide synthesis methods (Hruby and Meyer, 1998) on standard solid supports. The peptides generally were purified to greater than 95% purity using semi-preparation reversed phase high performance liquid chromatography (HPLC). Purity of the peptides generally was assessed using analytical HPLC (two systems), high resolution mass spectrometry and thin layer chromatography in two or three solvent systems.
Biological assays — binding affinity
Binding affinity of all synthetic peptide ligands were determined using stably transfected cell lines that expressed the human mu, delta, CCK-1 and CCK-2 receptors, respectively. Experiments were made using synthetic ligands and radiolabelled CTAP, deltorphin II, and sulfated CCK-8 respectively for the mu, delta, CCK-1 and CCK-2 ligands respectively. In vitro functional bioactivities were determined using the guinea pig ileum (GPI), mouse vas deference (GPI) and the unstimulated GPI /LLMP for determining respectively mu and delta agonist activity, and CCK-receptor antagonist activity (vs. the agonist sulfated CCK-8). Multiple assays were performed for each ligand reported and the results were analyzed statistically.
Results and discussion
Design considerations
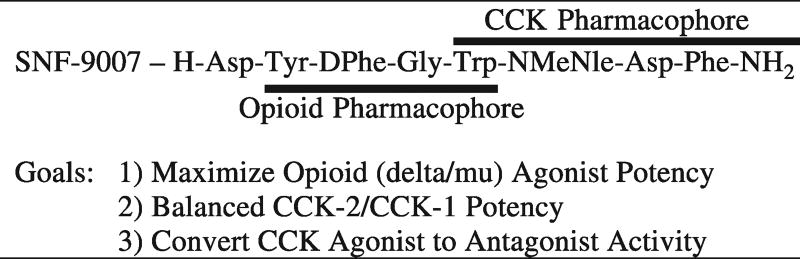
The major initial goal in this research was to obtain a small peptide ligand with mixed mu/delta agonist activity based on opioid peptides (Fig. 1), and balanced CCK-1/CCK-2 antagonist activity based on the CCK-8 structure (Fig. 1). In previous studies in our laboratory we had developed three dimensional pharmacophore models for the delta opioid receptor (Nikiforovich et al., 1991; Collins et al., 1996; Shenderovich et al., 2000) and for the CCK-8 receptor (Nikiforovich and Hruby, 1993). Some time ago we had noted (Hruby et al., 1994) that there were some interesting topographical three dimensional similarities between the opioid receptor pharmacophore and the cholecystokinin receptor pharmacophore. On further examination we postulated that it should be possible to design a small linear peptide ligand in which we could have overlapping pharmacophores (Fig. 2). The basic concept in this design is that key side chain groups of aromatic residues could serve for molecular recognition and even for transduction in both opioid (delta and mu receptors) and CCK (CCK-1 and CCK-2 receptors) receptors. Interestingly, we already found by accident some time ago (Slaninová et al., 1991) a ligand, SNF9007, that had potent selective agonist activity at the CCK-2 receptor, and weakly potent binding but robust biological opioid agonist activity at the delta opioid receptor. Therefore we set as an initial goal a redesign of this ligand, to have mixed mu/delta binding affinity for the opioid receptors and more balanced binding affinity for the CCK-1 and CCK-2 receptors (Fig. 2 outlines one of the original approaches we have taken). Ultimately, of course, the goal was to have exclusively potent agonist activity at the opioid receptors, and potent antagonist activity at both the CCK-2 and CCK-1 receptors in vivo.
Fig. 1.
Opioid Peptide and Cholecystokinin Structures Used in the Design of a Mixed Opioid/CCK Peptide Ligand.
Fig. 2.
Drug Design Based on Lead Compound.
Structure-activity studies
To obtain the goals outlined in Fig. 2, we began by redesigning the structure of SNF 9007 to increase the potency of the ligand at the opioid receptors. As shown in Table 1, this could be done by making several modest changes. First we truncated the amino terminal aspartic acid residue. Previous structure-activity studies in many laboratories have demonstrated that a free amino-terminal tyrosine residue was important for potent opioid receptor activity. Thus the N-terminal Asp residue from SNF-9007 was removed. In addition it has been recognized for many years that the second amino acid residue in an opioid ligand should either be a glycine residue or a D-amino acid residue. Hence both Gly and D-amino acids were substituted into this position. A few key examples are given in Table 1 (1 and 2).
Table 1.
Binding affinity of CCK/Opioid analogues at opioid and CCK receptors
| Compound | Opioid Binding (nM) | CCK Binding (nM) | ||
|---|---|---|---|---|
|
|
|
|||
| δa | μb | CCK-1c | CCK-2c | |
| SNF-9007 | 250 | 5,200 | 3,300 | 2.1 |
| 1. H-Tyr-Gly-Gly-Trp-NMeNle-Asp-Phe-NH2 | 20 | 600 | 870 | 3.0 |
| 2. H-Tyr-DPhe-Gly-Trp-NMeNle-Asp-Phe-NH2 | 0.42 | 86 | >10,000 | 2.1 |
| 3. H-Tyr-DAla-Gly-Trp-NMeNle-Asp-Phe-NH2 | 13 | 46 | 5,600 | 2.1 |
| 4. H-Tyr-DPhe-Gly-Trp-Nle-Asp-Phe-NH2 | 0.42 | 79 | 120 | 8.1 |
| 5. H-Tyr-DAla-Gly-Trp-Nle-Asp-Phe-NH2 | 39 | 3.9 | 5,700 | 150 |
| 6. H-Tyr-DPhe-Gly-DTrp-NMeNle-Asp-Phe-NH2 | 0.55 | 5.7 | 1,100 | 1.6 |
| 7. H-Tyr-DPhe-Gly-DTrp-Nle-Asp-Phe-NH2 | 130 | 560 | 6,500 | 2,700 |
vs. [3H]DPDPE.
vs. [3H]DAMGO.
vs. [3H]CCK-8.
As can be seen in Table 1 removal of the N-terminal Asp and placing a Gly in the 2-position gave 1 (Table 1). When only the N-terminal Asp was removed from SNF-9007 2 (Table 1) was obtained which is a nanomolar agonist at the delta opioid receptor and which still retains high selectivity at the CCK-2 receptor. In order to get a better balanced ligand for the CCK receptors than SNF-9007 we replaced the NMe-Nle residue with Nle to give 4, Table 1. This compound retains its potent CCK-2 binding affinity, but now has increased CCK-1 potency, resulting in much more balanced CCK-1 and CCK-2 binding affinity at the CCK receptors. It also retains good affinity for the mu and delta opioid receptor. Interestingly when the DPhe2 residue in 4 was replaced with a DAla residue to give 5 (Table 1) selectivity for the opioid receptor switched from delta selectivity to mu selectivity, but binding affinities at the CCK-2 and CCK-1 receptor dropped off by a factor of greater than 10 at both receptors. It appears that the binding affinities are not additive between opioid vs. CCK receptors. This is further illustrated when the Trp4 residue in 3 is replaced by a DTrp4 residue, a modification made to convert the ligand into a CCK receptor antagonist (6, Table 1). As can be seen the analogue has very much the same bioactivity profile as 2. However when the NMeNle5 residue was replaced by Nle5 to give analogue 7 (Table 1) to give a more balanced CCK-1/CCK-2 receptor ligand, the analogue lost potency (up to over two orders of magnitude) at all of the receptors tested.
When these analogues were tested in functional assays very interesting results were obtained (Table 2). As expected analogue 1, which is SNF-9007, has very weak activity in both the MVD (δ) and GPI (μ) assays, and also has very weak agonist activity in CCK assay using the unstimulated GPI/LLMP assay. When the Gly2 residue was replaced with a DAla2 bioactivity at the MVD and GPI improved somewhat (3, Table 2) and interestingly and unexpectedly the compound showed weak antagonist activity in the CCK assay. Further improvement in all three assays occurred when the DAla2 residue was replaced with DPhe2, and when the NMeNle5 residue was replaced with Nle to give 4 (Table 2). Analogue 4 had MVD IC50 of 12 nM, GPI IC50 of 420 nM and antagonist potency Ke = 40 nM at the GPI/LLMP. The DAla2,Nle5 analogue 5 (Table 2) was somewhat less potent as expected based on the binding data, but the DPhe2,DTrp4,NMeNle5 analogue 6 (Table 2) had good bioactivity in all three bioassays with IC50 = 24 nM (MVD), IC50 = 71 nM (GPI) and Ke = 6.9 nM (GPI/LLMP) as in the binding assays the DPhe2,DTrp4,Nle5 analogue 7 (Table 2) had very weak bioactivity in all three assays. Currently we are examining in vivo biological activity of several analogues for their antinociception bioactivity in acute pain models, and their bioactivity in neuropathic pain models. Our preliminary results are very encouraging.
Table 2.
Functional assay results for designed ligands at opioid and CCK receptor
| Compound | Opioid Activity (nM) | CCK Agonist Unstimulated GPI |
CCK Antagonist Unstimulated GPI/LLMP |
|
|---|---|---|---|---|
|
| ||||
| MVD | GPI | |||
| 1. H-Tyr-Gly-Gly-Trp-NMeNle-Asp-Phe-NH2 | 250 | 4,200 | 3.5% at 1 µM | No Antag. Act. |
| 3. H-Tyr-DAla-Gly-Trp-NMeNle-Asp-Phe-NH2 | 66 | 470 | 0% at 1 µM | Ke = 104 nM |
| 4. H-Tyr-DPhe-Gly-Trp-Nle-Asp-Phe-NH2 | 12 | 420 | 0% at 1 µM | Ke = 40 nM |
| 5. H-Tyr-DAla-Gly-Trp-Nle-Asp-Phe-NH2 | 45 | 160 | 0% at 1 µM | Ke = 190 nM |
| 6. H-Tyr-DPhe-Gly-DTrp-NMeNle-Asp-Phe-NH2 | 24 | 71 | 0% at 1 µM | Ke = 6.9 nM |
| 7. H-Tyr-DPhe-Gly-DTrp-Nle-Asp-Phe-NH2 | 170 | 2,700 | 0% at 1 µM | Ke = 528 nM |
Concluding comments
It is increasingly evident from current molecular and functional studies that many disease states involve changes in the normal patterns of genome control and expression that require careful reevaluation of the proper approach to drug design and treatment. Neuropathic pain certainly is one such disease state. From our evaluation of the current difficulties in treating neuropathic pain we have hypothesized that current treatments of pain especially current opioid drugs are in fact counter-indicated. We have hypothesized that treatment for neuropathic pain will require the development of a drug that can interact with delta and mu opioid receptors as an agonist and at CCK-2/CCK-1 receptors as an antagonist. We have demonstrated herein that such a design is quite reasonable in a small ligand (7 amino acid residues — molecular weight < 1000). The availability of three dimension pharmacophore models for the 4 receptors involved and the use of the concept of overlapping pharmacophores were critical to the success we have experienced thus far. Clearly we still have a lot to learn in order to develop this approach into a robust strategy, but our results suggest that the development of such a strategy will become a critical component of modern drug design and development.
Acknowledgments
The support of the National Institute of Drug Abuse, U.S. Public Health Service, is gratefully acknowledged. The opinions expressed in this paper are those of the authors and not necessarily those of the USPHS.
References
- Collins N, Flippen-Anderson JL, Haaseth RC, Deschamps JR, George C, Kövér K, Hruby VJ. Conformational determinants of agonist versus antagonist properties of [D-Pen2,D-Pen5]enkephalin (DPDPE) analogs at opioid receptors. Comparison of X-ray crystallographic structure, solution1H NMR data, and molecular dynamic simulations of [LAla2]DPDPE and [D-Ala3]DPDPE. J. Am. Chem. Soc. 1996;118:2143–2152. [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, Lai J, Porreca F. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J. Neuroscience. 2002;22(15):6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ. Conformational restrictions of biologically active peptides via amino acid side chain groups. Life Sciences. 1982;31:189–199. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- Hruby VJ. Designing peptide receptor agonists and antagonists. Nature Reviews Drug Discovery. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Al-Obeidi F, Kazmierski WM. Emerging approaches in the molecular design of receptor selective peptide ligands: Conformational, topographical and dynamic considerations. Biochem. J. 1990;268:249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby VJ, Fang SN, Kramer TH, Davis P, Parkhurst D, Nikiforovich GV, Boteju LW, Slaninová J, Yamamura HI, Burks TF. Analogues of cholecystokinin26 – 33 selective for B-type CCK receptors possess delta opioid receptor agonist activity in vitro and in vivo: Evidence for similarities in CCK-B and δ opioid receptor requirements. In: Hodges RS, Smith JA, editors. Peptides: Chemistry, Structure and Biology. ESCOM Publishers; Leiden: 1994. pp. 669–671. [Google Scholar]
- Hruby VJ, Meyer J-P. Chemical Synthesis of Peptides. In: Hecht SM, editor. Bioorganic Chemistry: Peptides and Proteins. Oxford University Press; New York: 1998. pp. 27–64. [Google Scholar]
- Nikiforovich GV, Hruby VJ. Models for the A- and B- receptor-bound conformations of CCK-8. Biochem. Biophys. Res. Commun. 1993;194:9–16. doi: 10.1006/bbrc.1993.1777. [DOI] [PubMed] [Google Scholar]
- Nikiforovich GV, Hruby VJ, Prakash O, Gehrig CA. Topographical requirements for δ-selective opioid peptides. Biopolymers. 1991;31:941–955. doi: 10.1002/bip.360310804. [DOI] [PubMed] [Google Scholar]
- Shenderovich MD, Liao S, Qian X, Hruby VJ. A three dimensional model of the opioid pharmacophore: Comparative molecular modeling of peptide and non-peptide ligands. Biopolymers. 2000;53:565–580. doi: 10.1002/(SICI)1097-0282(200006)53:7<565::AID-BIP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Slaninová J, Knapp RJ, Wu J, Fang SN, Kramer T, Hruby VJ, Yamamura HI. Opioid receptor binding properties of analgesic analogues of cholecystokinin octapeptide. Eur. J. Pharmacol. 1991;200:195–198. doi: 10.1016/0014-2999(91)90688-m. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]