Abstract
Introduction
The use of antipsychotic medications in Alzheimer's disease has been associated with an increased risk of mortality in clinical trials. However, an older postmortem literature suggests that those with schizophrenia treated in an era of exclusively conventional antipsychotic medications had a surprisingly low incidence of tau pathology. No previously published studies have investigated the impact of conventional antipsychotic exposure on tau outcomes in a tau mouse model of AD.
Methods
In two experiments, transgenic rTg (tauP301L) 4510 tau mice were treated with either haloperidol or vehicle and phosphotau epitopes were quantified using high-sensitivity tau ELISA.
Results
After treatments of 2 and 6 week's duration, mice treated with haloperidol evidenced a significant reduction in tau phosphorylation associated with an inactivation of the tau kinase AMPK.
Discussion
The data suggest that D2 receptor blockade reduces tau phosphorylation in vivo. Future studies are necessary to investigate the impact of this reduction on tau neuropathology.
Keywords: Alzheimer's disease, Antipsychotics, AMPK, Dopamine, Haloperidol, Tau
1. Introduction
Clinicians treating behavioral disturbances related to Alzheimer's disease (AD) face a well-publicized dilemma in the decision of whether to use antipsychotic medications associated with modest benefits [1], [2], [3] but a discomfiting elevation in the risk of mortality [4]. This class of agents, including both atypical and conventional antipsychotics, carries a black box warning from the US Food and Drug Administration (FDA) cautioning that the use of these medications in dementia with behavioral disturbances leads to an increased all-cause mortality [5]. Beyond the increased risk of death, there is evidence from an analysis of the large multi-centered Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer's Disease (CATIE-AD) study data that those with dementia treated with the newer atypical antipsychotics experience a more aggressive cognitive decline than those not exposed [6]. To date, there is no consensus regarding mechanisms that could explain the deleterious nonmotor outcomes associated with exposure to these agents, and it is not clear that the conventional antipsychotics carry the same risk of cognitive decline. Just as mouse models have been used systematically in AD research to facilitate a search for a cure, mouse models may be used in the opposite direction to evaluate the potential negative neuropathologic consequence of exposure to agents such as antipsychotics.
The neurofibrillary tangle, derived from paired helical filaments of hyper-phosphorylated tau protein, is the salient pathologic feature on which the postmortem Braak staging system of AD rests [7]. Tangles are the only pathologic finding in AD demonstrated to correlate topographically and quantitatively with clinical symptomatology [8], [9]. Neurophysiologically, microtubule-associated tau protein is thought to participate in the formation and stabilization of microtubules [10]. Tau exists as a phosphoprotein, and even in healthy adult brain tau is at least minimally phosphorylated [11]; however, what distinguishes AD is the scope and consistency of phosphorylation of 19 of 441 specific amino acid sequences along the tau protein resulting in a particular phosphorylation signature and a greater burden of phosphotau in AD brain [12]. Specifically, the magnitude of the difference in phosphotau burden between AD and healthy adult brain has been estimated to be between a 3–4X increase in the number of moles of phosphate per mole of tau [13]. Hyperphosphorylation of tau is believed to often—but not always—be an integral part of the process of paired helical filament formation and, ultimately, neurofibrillary tangle production, rendering phosphorylation, a critical step in disease pathogenesis and a potential target of intervention [14]. In support of studies focusing on pretangle tau phosphorylation as an outcome in evaluating therapeutics, there is growing speculation that intermediate hyperphosphorylated soluble oligomeric tau species may have the greatest clinical consequence, and that derivative neurofibrillary tangles may not play the primary role [15], [16]. The phosphorylation and dephosphorylation of tau are controlled by the equilibrium of activity of protein kinases such as GSK3β, cdK5, Akt/PKB, ERK1/2, AMPK, and phosphatases, such as PP1, PP2A and PP5 [17]. Modification of tau kinase or phosphatase activity may be one mechanism by which exposures may, depending on direction, either worsen or ameliorate tau phosphorylation and impact AD clinical pathology.
Haloperidol, a butyrophenone available at very low cost, is used commonly in the treatment of psychotic conditions, including schizophrenia [18], bipolar mania [19], and delirium [20], in addition to behavior disturbances in AD [21]. Its clinical efficacy has been attributed to its robust inhibition of dopamine D2 postsynaptic mesolimbic receptors, whereas side-effects including emotional blunting, cognitive problems, and extra-pyramidal motor side-effects are believed to be secondary to mesocortical and striatal postsynaptic D2 receptor blockade [22]. Among antipsychotics, there is evidence to suggest that haloperidol has the greatest negative impact on mortality in the dementia population [23], [24], and as a result of these studies, the agent has been decried as potentially “neurotoxic,” leading some to recommend avoiding its use altogether in the dementia population [25].
Existing evidence provides conflicting guidance in predicting the impact of haloperidol exposure on tau pathology in AD. From one perspective, haloperidol may be expected to worsen the disease. For instance, a study in neuronal cell culture suggests that haloperidol does induce tau hyperphosphorylation via oxidative stress [26]. If so, could an acceleration of disease leading to increased all-cause mortality be related to an exacerbation of tau pathology driven by increased tau phosphorylation in those exposed to haloperidol? From the opposite perspective, although controversial owing to the limitations of autopsy data derived from an institutional setting, an older literature has suggested that AD pathology including tangle density is reduced in schizophrenia [27], [28], [29], [30], [31], [32], and that the highly prescribed haloperidol may be responsible for this neuroprotection [33].
Taken together, although both alternatives to the null hypothesis stipulating higher or lower phosphotau burden would be viable hypotheses in an in vivo experiment, no previously published animal studies have investigated the impact of haloperidol exposure on tau pathology. As such, the present study was designed to evaluate the impact of haloperidol on tau phosphorylation in a tau mouse model of AD while entertaining either directional alternative to the null hypothesis.
2. Methods and materials
2.1. Animals
Animal models of AD are not absolute replicas of the human disease process; they recapitulate particular neuropathologic features of the disease, most commonly amyloid-β or tau pathology. The rTg(tauP301L)rTg4510 mouse is a bigenic strain mouse model of taupathy comprising a transgenic tauP301L mutation associated with FTDP-17 in exon 10–placed downstream of a tetracycline-operon-responder construct [34]. To activate the responder gene requires co-expression of an activator construct. The activating tetracycline conditional gene expression system has been set downstream of a Ca2+/calmodulin kinase II (CaMKII) promoter that limits expression to forebrain structures [35]. While the derived transgenic tau mutation expressed in the rTg4510 mouse produces a frontotemporal tauopathy in humans, as a model of AD tauopathy, the mouse conditionally drives expression of the tau mutation in the entire forebrain. The rTg4510 mouse model manifests an aggressive phenotype of tau pathology in the forebrain structures in the form of neurofibrillary tangles with concomitant neuronal loss and cognitive deficits [35]. rTg4510 breeding pairs (Tg(tetO-MAPT*P301L), stock #015815 and Tg(Camk2a-tT stock #003010)) were procured from Jackson Laboratories and housed in the Center for Comparative physiology at the Feinstein Institute for Medical Research where all the experiments described in this report were conducted. Mice were housed three to four per cage, with access to food and water and maintained on a 12-h light/dark cycle (lights out at 8:30 AM). For each experiment, rTg4510 mice between 3 and 4.5 months were matched between treatment (haloperidol) and vehicle (sesame oil) cohorts for age and balanced for gender. All animal experiments were performed according to procedures approved by the Feinstein Institute for Medical Research Institutional Animal Care and Use Committee.
2.2. Long-acting haloperidol decanoate injections
For the treatment of schizophrenia, in which the rates of nonadherence to antipsychotic medications can be as high as 40% after an index inpatient psychiatric admission [36], long-acting injectable depot formulations have been developed to extend the half-life of medications, dramatically increasing dosing intervals and reducing relapse [37]. Haloperidol decanoate has emerged as the most widely prescribed conventional depot medication owing to its 4-week human dosing schedule and very convenient conversion formula from oral dosing, with depot monthly dosing generally around 20 times the daily oral dose, and perhaps for this reason is one of the long-acting agents associated with the lowest rate of relapse [37], [38]. The decanoate formulation of haloperidol exists as an ester with high-lipid solubility in a sesame oil suspension, and is gradually absorbed from the muscle injection site and then into plasma where esterases gradually convert it via hydrolysis to active drug [39]. Although the half-life is around 4 weeks in humans, the onset of action occurs within a couple of days [37].
In developing a strategy for the treatment of AD mouse models with antipsychotic, given the need for subacute or chronic exposure for pathological outcomes and the desire for adequate steady-state concentrations, conventional intraperitoneal (IP) delivery systems are unappealing owing to the need for at least daily dosing. Therefore, rather than using chemical grade haloperidol for IP injections, in the current report, pharmaceutical grade haloperidol decanoate was used for intramuscular (IM) injections. In designing the dosing regimen, approximating therapeutically relevant doses in the mouse model was enabled by previous work in rodents targeting 60%–70% D2 receptor occupancy with haloperidol decanoate with 21 mg/kg of drug given once weekly [40], [41], [42].
For the 6-week experiment, mice were injected IM in the hindquarter with haloperidol decanoate 50 mg/mL (Fresenius Kabi, Schaumburg, IL) at a dose of 21 mg/kg on day 1, then again at 1 week; 2 weeks; 3 weeks; 4 weeks; 5 weeks; and sacrificed at 6 weeks. For the 2 week replication, mice were treated on day 1 with an IM injection in the hindquarter at a dose of 21 mg/kg, then again at 1 week, then again at 2 weeks, and sacrificed at day 16. Mice were monitored twice daily by veterinary technicians for during the duration of the study for changes in motor behavior and food and water consumption. While Tmax is unknown for haloperidol decanoate in mice, as the half-life has been estimated at one week we used day 2 after injection as an estimate of the zenith of antipsychotic drug level for the 2 week replication.
2.3. Tau ELISA
Mice were euthanized via isoflurane overdose and the brain was removed, and hemisected sagitally. The forebrain region was dissected, and striatum, cortex, and hippocampus were separated. High-sensitivity ELISA using DA31 (Davies lab) (total tau), PHF-1 (pSer396/404) (Davies lab), CP13 (pSer202) (Davies lab), and RZ3 (pThr231) (Davies lab) for precise quantification of each phosphotau species was performed according to previously established protocols established in our laboratory [43]. After dissection, the brain was homogenized using appropriate volume of homogenizing buffer: a solution of Tris buffered saline, pH 7.4 containing 10-mM NaF, 1-mM NaVO3, and 2-mM EGTA, including a complete Mini protease inhibitor cocktail (Roche). Samples were stored at −80°C. Heat stable preparations were used to obtain soluble tau levels, by adding 5% ß-Mercaptoethanol and 200-mM NaCl to brain homogenate. Samples were then heated at 100°C for 10 minutes, cooled at 4°C for 30 minutes, and then centrifuged at 13,000 g at 4°C for 15 minutes, and supernatants were collected. Ninety six–well plates were respectively coated with DA31, PHF1, CP13, and RZ3 at a concentration of 6 μg/mL in coating buffer, for at least 48 hours at 4°C. Plates were washed 3 × in wash buffer and blocked for 1 hour at room temperature using StartingBlock (ThermoScientific) to avoid nonspecific binding. After the 1 hour block, each plate was washed 5 × and 50 μL of the appropriate sample was added to the wells, with 50 μL of DA9-HRP detection antibody. Plates were incubated O/N shaking at 4°C and washed 9 × in wash buffer. One-step ULTRA TMB-ELISA (Thermo Scientific) was added for 30′ at room temperature before stopping the reaction with 2M H2SO4. Plates were read with an Infinite m200 plate reader (Tecan) at 450 nm.
2.4. Immunoblotting
Lysates samples were run on 12.5% tris-HCl precast gel (Biorad). Briefly, samples were boiled for 5 minutes, in 2% SDS and 4% mercaptoethanol, and equal volumes were loaded, then separated using SDS-PAGE, and transferred to nitrocellulose membranes. Once transferred, membranes were probed with antibodies to glycogen synthase kinase (GSK)-3β (cell signaling #9315); Phospho GSK-3β (Ser9) (cell signaling #5558); Phospho-AMPKα (Thr172); and AMPKα (cell signaling #2603). Bands were then detected with either enhanced chemiluminsecence (ECL Millipore) or 4-chloronaphthol with 1% hydrogen peroxide (4CN) depending on their intensity. Densitometry was performed using ImageJ image processing and analysis (nih.gov).
2.5. Statistical analysis
All statistical calculations were performed with GraphPad Prism 5 software. For quantification of phosphotau, results of ELISA for each antibody (PHF-1, CP13, RZ3) measured in ug/mg protein was converted to a ratio of phosphotau/total tau by dividing individual phosphotau antibody concentrations by the concentration of DA31 (ptau/total tau). These measurements were the continuous dependent variable in statistical analysis, with treatment group (haloperidol, sesame oil vehicle) establishing the categorical independent variable. The means ± standard error of mean values of the ptau/total tau was compared for each forebrain region (cortex, striatum, hippocampus) between haloperidol-treated and vehicle-treated mice, and the Student t test was calculated for the difference in means to evaluate statistical significance. For each statistically significant difference (P < .05), a Cohen's d was calculated to estimate the effect size of the difference. For Western Blot, ImageJ was used to quantify density of the bands for each antibody, and this density was converted within the software to a continuous variable for comparison between groups using a Student t test.
3. Results
3.1. Haloperidol reduces tau phosphorylation
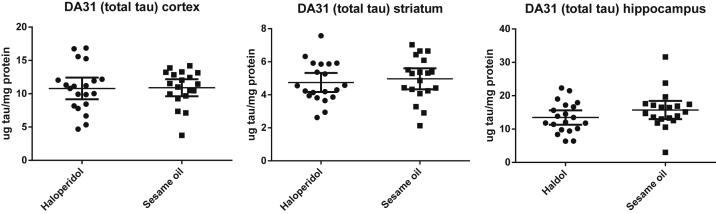
In the first experiment, rTg4510 tau mice were treated for 6 weeks with haloperidol (N = 20) or sesame oil vehicle (N = 19). Injections of haloperidol decanoate were very well tolerated by the mice, and there were no gross motor abnormalities or neuroleptic malignant syndrome in any of the treated mice. Total tau was measured with DA31 and did not differ in any brain region between cohort of haloperidol and vehicle-treated mice (Fig. 1). To quantify a range of tau pathology, we used a panel of highly specific monoclonal antibodies to total and phosphorylated tau developed in our laboratory [43]. Ser396/404, an epitope known to be phosphorylated in early to mature tau aggregates [44], was measured with PHF-1 antibody; pSer202 was quantified with CP13, an antibody that identifies early neuritic tau pathology [45]; pThr231, a conformational epitope phosphorylated early in the development of disease and present even in pre-tangles was quantified with RZ3 [12]. In each brain region, at each phosphorylation site, ptau/total tau was decreased in the haloperidol-treated cohort (Table 1). This difference achieved statistical significance in the cortex and striatum with robust effect sizes. To replicate this finding and to determine whether even briefer treatment with haloperidol might reduce levels of phosphotau, rTg4510 tau mice were again treated with haloperidol (N = 11) or sesame oil vehicle (N = 9), this time for only 2 weeks. The mice-tolerated haloperidol decanoate without any untoward effect. The results were essentially unchanged, with no significant differences in total tau (data not shown) but with robust reductions of phosphotau in the cortex and striatum and more modest changes in the hippocampus (Table 2). The discrepancy in the magnitude of the reduction in phosphotau observed in the cortex and striatum versus the hippocampus in the haloperidol-treated mice may be the result of the relative strength of dopaminergic innervation to these regions and D2 receptor expression [46] or may be a consequence of regional tau expression in this forebrain expressed model [35] or an interaction between the two.
Fig. 1.
Total tau quantified in the cortex, striatum, and hippocampus with DA31 monoclonal antibody in rTg4510 mice treated for 6 weeks with haloperidol (N = 20) or sesame oil vehicle (N = 19). Levels of total tau did not differ significantly between groups.
Table 1.
rTg4510 mice were treated with either haloperidol at a dose of 21 mg/kg/week or vehicle for 6 weeks
| Region | Vehicle, N = 19, age = 20 ± 3 weeks | Haloperidol, N = 20, age = 20 ± 3 weeks | Δ ptau/total | P | Effect size (95% CI) |
|---|---|---|---|---|---|
| Cortex | |||||
| PHF-1/DA31 | 43.6 ± 3.21 | 33.0 ± 1.56 | −10.6 ± 3.51 | .005 | 0.963 (0.299–1.63) |
| RZ3/DA31 | 16.3 ± 1.44 | 12.3 ± 1.01 | −3.96 ± 1.74 | .029 | 0.727 (0.079–1.37) |
| CP13/DA31 | 27.7 ± 1.50 | 24.9 ± 1.30 | −2.85 ± 1.98 | .160 | NS |
| Striatum | |||||
| PHF-1/DA31 | 31.8 ± 3.42 | 22.62 ± 2.21 | −9.16 ± 4.04 | .029 | 0.726 (0.078–1.37) |
| RZ3/DA31 | 13.7 ± 1.69 | 9.05 ± 1.29 | −4.67 ± 2.12 | .033 | 0.707 (0.06–1.35) |
| CP13/DA31 | 26.8 ± 1.84 | 19.5 ± 0.886 | −7.29 ± 2.01 | .0009 | 1.16 (0.484–1.84) |
| Hippocampus | |||||
| PHF-1/DA31 | 30.4 ± 2.62 | 28.4 ± 2.84 | −1.95 ± 3.87 | .618 | NS |
| RZ3/DA31 | 19.3 ± 1.29 | 18.2 ± 1.07 | −1.13 ± 1.67 | .502 | NS |
| CP13/DA31 | 26.4 ± 1.64 | 25.3 ± 1.95 | −1.51 ± 2.56 | .655 | NS |
Abbreviation: NS, nonsignificant.
NOTE. Total tau (DA31) and phosphotau were quantified with ELISA. Phosphotau (ptau) was quantified using an ensemble of antibodies: PHF-1 (pSer396/404); CP13 (pSer202); and RZ3 (pThr231); and a ratio of ptau/DA31 was calculated for each antibody in each region. Changes (Δ ptau/total) in the ratio of ptau/DA31 (arbitrary units) represent the difference between mean ptau/DA31 of vehicle-treated mice and mean ptau/DA31 of haloperidol-treated mice.
NOTE. P < .05 was considered significant, and for each significant difference, effect sizes were calculated with 95% confidence intervals.
Table 2.
rTg4510 mice were treated with either haloperidol at a dose of 21 mg/kg/week or vehicle for 2 weeks
| Region | Vehicle, N = 9, age = 17 ± 0.4 weeks | Haloperidol, N = 11, age = 17 ± 0.3 weeks | Δ ptau/total | P value | Effect size (95% CI) |
|---|---|---|---|---|---|
| Cortex | |||||
| PHF-1/DA31 | 42.1 ± 3.14 | 33.6 ± 2.36 | −8.53 ± 3.86 | .040 | 0.994 (0.061–1.928) |
| RZ3/DA31 | 24.4 ± 1.6 | 16.9 ± 1.06 | −7.55 ± 1.86 | .0007 | 1.824 (0.777–2.87) |
| CP13/DA31 | 47.4 ± 3.45 | 28.9 ± 1.58 | −18.5 ± 3.56 | <.0001 | 2.33 (1.29–3.47) |
| Striatum | |||||
| PHF-1/DA31 | 32.1 ± 1.79 | 26.0 ± 1.58 | −6.13 ± 2.38 | .019 | 1.16 (0.207–2.11) |
| RZ3/DA31 | 14.4 ± 0.632 | 12.28 ± 1.46 | −2.12 ± 1.17 | .231 | NS |
| CP13/DA31 | 28.8 ± 2.37 | 22.1 ± 1.38 | −6.72 ± 2.63 | .0198 | 1.15 (0.199–2.10) |
| Hippocampus | |||||
| PHF-1/DA31 | 108.2 ± 7.53 | 93.9 ± 5.72 | −14.3 ± 9.3 | .141 | NS |
| RZ3/DA31 | 22.99 ± 2.61 | 18.4 ± 1.06 | −4.60 ± 2.62 | .0965 | NS |
| CP13/DA31 | 101.3 ± 6.078 | 104.1 ± 3.82 | +2.79 ± 6.92 | .692 | NS |
Abbreviation: NS, nonsignificant.
NOTE. Total tau (DA31) and phosphotau were quantified with ELISA. Phosphotau (ptau) was quantified using an ensemble of antibodies: PHF-1 (pSer396/404); CP13 (pSer202); and RZ3 (pThr231); and a ratio of ptau/DA31 was calculated for each antibody in each region. Changes (Δ ptau/total) in the ratio of ptau/DA31 (arbitrary units) represent the difference between mean ptau/DA31 of vehicle treated mice and mean ptau/DA31 of haloperidol treated mice.
NOTE. P < .05 was considered significant, and for each significant difference effect sizes were calculated with 95% confidence intervals.
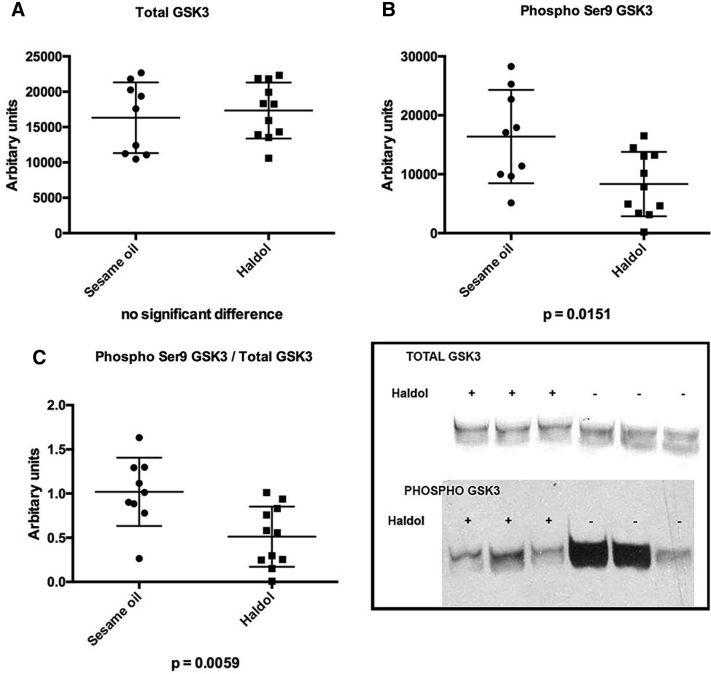
3.2. Haloperidol activates the tau kinase GSK-3β and inhibits AMPK
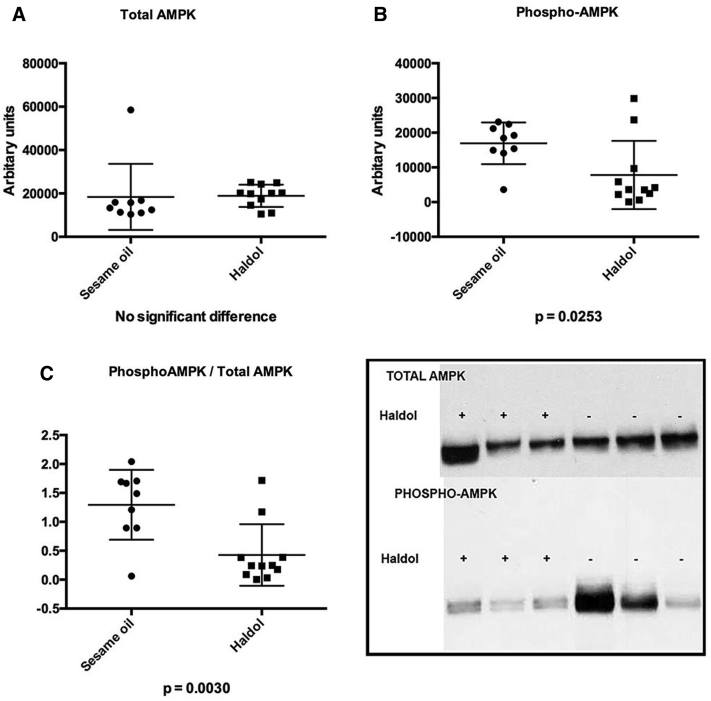
To explore mechanism, we tested the hypothesis that reductions in phosphotau were driven by GSK-3β phosphorylation and inactivation. GSK-3β, among other cellular functions, is a tau kinase whose inhibition in wild-type mice happens to have been previously associated with haloperidol treatment [47]. Contrary to expectation, we did not observe an increase in pGSK-3β/total GSK-3β immunoblot density that would suggest inactivation of the kinase rather a significant reduction in pGSK-3β was observed that suggests an activation of the kinase (Fig. 2). We next tested the hypothesis that the observed effects may be driven by a decrease in phosphorylated AMP-activated protein kinase (pAMPK), a tau kinase implicated in the regulation of cellular metabolic pathways that are known to be affected by antipsychotic treatment and coupled to downstream signaling pathways that include GSK-3β (see Discussion). Results of immunoblot analysis provide evidence that the ratio of activated kinase to total protein, pAMPK/AMPK, is significantly diminished in haloperidol-treated mice in the cortex and striatum (Fig. 3), whereas immunoblot density analysis revealed no significant differences in pAMPK/AMPK in the hippocampus in the same mice (haloperidol density 1.4+/−0.14 versus sesame oil 1.2+/−0.17). These data are consistent with ptau/total regional effects and suggest that inactivation of AMPK is a potential explanation for the observed D2 mediated reduction in phosphotau.
Fig. 2.
Western blots (WB) performed on frontal cortex of mice treated with haloperidol decanoate (N = 11) or vehicle (N = 9) for 2 weeks. (A) No differences were observed in total GSK-3β. (B) Inactivated pGSK-3β was reduced in haloperidol treated mice. (C) The ratio of inactive/total pGSK-3β/total GSK-3β was reduced in haloperidol treated mice, suggesting an activation of the kinase. The insert displays typical gel lanes from immunoblots used for quantitative analyses with ImageJ.
Fig. 3.
Western blots (WB) performed on frontal cortex of mice treated with haloperidol decanoate (N = 11) or vehicle (N = 9) for 2 weeks. (A) No differences were observed in total AMPK. (B) Activated pGSK-3β was reduced in haloperidol treated mice. (C) The ratio of active/total pAMPK-3β/total AMPK was reduced in haloperidol treated mice, suggesting an inactivation of the kinase. The insert displays typical gel lanes from immunoblots used for quantitative analyses with ImageJ.
4. Discussion
The observation made in the current report that haloperidol suppresses the phosphorylation of tau protein in an AD mouse model, does cohere with a previous report of the impact of D2 blockade on the more primitive transgenic Caenorhabditis elegans model of tauopathy. In this model, over expression of human tau results in a phenotype of worsening motility in addition to the accumulation of insoluble tau and neurodegeneration [48]. In a study designed to identify drugs that target tau-induced neurotoxicity, the Prestwick chemical library, comprising 1120 generally off-patent compounds approved by FDA, was screened for the ability of these compounds to suppress motility defects and tau pathology in tau transgenic C. elegans. [49] Of all of the compounds screened, six evidenced a significant rescue of motility defect manifested in reduced tau-induced lethargy and improved swimming. Of note, among the six was azaperone, a butyrophenone antipsychotic drug structurally similar to haloperidol. Azaperone also reduced tau-aggregation and tau-induced neuronal degeneration in this model [48]. In an exploration of mechanism, worms generated that lacked both dopamine D2-like receptors exhibited improved motility and a decrease in insoluble tau, pointing to a D2-mediated pathway of tau aggregation [48]. If so, haloperidol exposure resulting in D2 blockade may be expected to reduce tau pathology in vertebrates, including tau mouse models.
The observed reduction in phosphotau resulting from haloperidol exposure in rTg4510 mice suggests tau kinase inhibition, and previous research suggests that GSK-3β could be responsible. GSK-3β is a highly expressed serine/threonine kinase, described as a “cellular nexus,” [50] enmeshed in cellular signal transduction of wide-reaching scope that includes phosphatidylinositol-3-kinase (PI3-K)/Akt and Wnt cascades [51]. The best evidence for the therapeutic potential of GSK-3β inhibition in the modification of tau pathology comes from studies of another psychotropic medication, lithium. Targeted inhibition of GSK-3β in mouse models of AD with lithium has been shown to reduce phosphorylation of tau in studies ranging from 4 weeks [52], [53] to 32 weeks [54]. Our initial hypothesis was that haloperidol was similarly interrupting GSK-3β activity. Support for this comes from a study focused on the exploration of the AKT-GSK3β signaling pathway in schizophrenia that evaluated the effect of haloperidol injections on this pathway in C57Bl/6 wild-type mice [47]. In that study, haloperidol injections of 1-mg/kg IP given for 12 days significantly increased the phosphorylation of GSK-3β at Ser9. Inhibition of GSK3β is under the control of AKT1-dependent phosphorylation at Ser9, and increased phosphorylation and deactivation of GSK3β driven by haloperidol would potentially predict a reduction of phosphotau [55].
In the current report, we observed a reduction in the inactive pGSK-3β, all the more surprising as this would predict an increase rather than a decrease in phosphotau. However, the direction of haloperidol's effect on the phosphorylation of Ser9 may be conditional, and the drug may not always function as an inhibitor of GSK-3β. Evidence for this comes from a higher dose and longer duration study of antipsychotic injections. Haloperidol injections given daily IP at 10 mg/kg to adult Sprague–Dawley rats for 21 days resulted in a 30% reduction of pGSK-3β in the frontal cortex, a finding similar to our own results [56]. It was suggested by the authors of the latter report that this discrepancy in the influence of haloperidol on GSK-3β may reflect either a biphasic dose response or perhaps a biphasic time-course response to haloperidol. In any event, an alternate explanation for the observed reduction in phosphotau is required.
AMPK, a serine/threonine kinase straddling a cellular metabolic regulatory role with a concurrent influence on tau-driven AD pathology, has been reported to be activated in the hypothalamus of mice as a consequence of atypical antipsychotic exposure [57]. AMPK plays a central role in cellular energy homeostasis, and is activated by metabolic stress (hypoxia, ischemia, poor nutrient availability) [58]. The activation of AMPK is associated with increased food intake; the use of antipsychotic medications (including haloperidol but more dramatically the atypicals) [59] has been associated with weight gain and metabolic syndrome, including an atherogenic lipid profile and emergent diabetes mellitus [60]. AMPK activity has been implicated as a potential medication-induced mediator of the syndrome. In support of this, the atypical antipsychotics, including clozapine, olanzapine, and quetiapine, but not haloperidol, have been shown to induce AMPK phosphorylation in mice (activating the enzyme), whereas polymorphisms in AMPK subunit genes have been implicated in human antipsychotic-induced weight gain [57], [61]. Only one previous study has investigated the influence of antipsychotics on AMPK in the brains of a mouse model of AD. Consistent with expected outcomes, in an amyloid mouse model of AD, clozapine exposure was shown to increase AMPK phosphorylation activating the enzyme [62]. No previously published studies have reported on AMPK inactivation after haloperidol exposure.
In addition to contributing to metabolic homeostasis, AMPK is a known tau kinase, and the effect of different classes of antipsychotic drugs on this kinase may have relevance for disease prevention or may confer risk depending on the direction of influence. Work done by two of the authors of the current report (P.D. and P.M.) has demonstrated that AMPK phosphorylates tau at multiple sites in and around the microtubule binding domain, and that in human AD brain and other tauopathies phosphorylated AMPK was over-represented in tangle and pre-tangle bearing neurons [63]. The current report builds on this finding. Most of the pre-clinical literature on kinase inhibition from the re-purposing of existing medications in the service of phosphotau reduction has been focused on GSK-3β [50], [64], and no previous published studies have reported on medication-induced inhibition of AMPK activity as a potential method of phosphotau reduction in vivo. In a manner congruous with the effect of haloperidol on phosphorylation of GSKβ but with the opposite outcome, at the dose given in the current report haloperidol appears to inhibit the tau kinase AMPK. These may not be unrelated findings. It has been demonstrated that PI3K/Akt signaling pathways are downstream targets of AMPK activity [58]; it may be that inactivation of AMPK results in reduced downstream phosphorylation and activation of GSKβ. This is important, as combining haloperidol (an AMPK inhibitor) with a GSK-3β phosphorylating agent such as lithium would be expected to produce dramatic reductions in phosphotau.
The reported data suggest that haloperidol reduces tau phosphorylation events. This reduction may eventuate in a reduction in neurofibrillary tangles, an outcome not investigated in the present study. However, the relationship between tau phosphorylation events and the development of aggregated tau and neurofibrillary tangles is complex. While tangles are comprised of hyperphosphorylated tau [65], and phosphorylation reduces the affinity of tau for microtubules [66] whether phosphorylation ineluctably leads to increased tau aggregation is not yet clear. For instance, evidence from studies investigating lithium in the prevention of tangle formation in tau mice suggests that inhibition of tau phosphorylation via GSK3β ameliorates tau pathology [52]. Nonetheless, there is evidence that phosphorylation of tau at certain sites may reduce affinity for microtubules while still inhibiting paired helical filament formation, suggesting that phosphorylation can be protective [67]. Recently, chronic treatment with the hypoglycemic metformin was shown to reduce tau phosphorylation in P301S tau mice while promoting tau aggregation [68]. Tau dephosphorylation was demonstrated to be the result of induction of the tau phosphatase protein phosphatase 2A (PP2A) expression as a consequence of AMPK/mammalian target of rapamycin (mTOR) signaling, an effect that seemed to foster rather than reduce insoluble tau aggregates [68]. It is unknown whether the reduction in tau phosphorylation associated with haloperidol, seemingly mediated by interruption of AMPK activity, will promote or inhibit tau aggregation and tangle formation. Longer term studies are required to evaluate these potentialities.
The potential reduction in tau pathology mediated by haloperidol may explain some of the early neuropathologic reports of decreased AD pathology in schizophrenia, emerging in an era before the introduction of atypical antipsychotics. However, these data should be interpreted with some caution. A sapient critic of the notion of disease prevention with haloperidol in AD would note that haloperidol is widely prescribed for agitation and psychosis related to AD, a condition that affects nearly half of those suffering with disease, and there has been no suggestion of improvement in cognition as a consequence [69], [70]. Studies of human disease are certainly required before concluding that haloperidol has any impact on pathology or cognition. However, data derived from studies of individuals with AD treated with haloperidol may not be informative. It may be that those treated with haloperidol in studies focused on agitation as an outcome are, in general, at a stage of disease beyond which the salutary clinical effects of reduction in phosphorylation of tau are obtained.
It has not previously been reported that D2 receptor signaling pathways regulate AMPK, a kinase that is typically activated by cellular conditions of metabolic stress [71]. AMPK is activated upstream by three kinases—one of them, Ca/calmodulin-dependent protein kinase kinase β, is highly expressed in neurons—[72] and inhibited by one of several phosphatases [73]. Further exploration of D2 receptor mediated regulation of AMPK activation is necessary to determine whether D2 blockade is indeed responsible for the observations made in the current report, or whether there may be other direct or indirect effects of haloperidol that are responsible. As the AMPK/mTOR pathway has been shown to regulate PP2A activity, and as tau dephosphorylation has been shown recently to be driven by AMPK activation, future studies should focus on the downstream consequence of haloperidol-driven inactivation of AMPK on mTOR and the tau phosphatase PP2A.
Acknowledgments
None of the authors have any direct or indirect financial or personal relationships, interests, or affiliations relevant to the subject matter of the manuscript.
Footnotes
None of the authors have any financial conflicts to disclose.
References
- 1.Schneider L.S., Dagerman K., Insel P.S. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 2.Sultzer D.L., Davis S.M., Tariot P.N., Dagerman K.S., Lebowitz B.D., Lyketsos C.G. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165:844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher A.R., Maglione M., Bagley S., Suttorp M., Hu J.H., Ewing B. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- 4.Schneider L.S., Dagerman K.S., Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 5.Public Health Advisory; Montgomery, Maryland: 2005. FDA, Deaths with antipsychotics in elderly patients with behavioral disturbances. [Google Scholar]
- 6.Vigen C.L., Mack W.J., Keefe R.S., Sano M., Sultzer D.L., Stroup T.S. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer's disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168:831–839. doi: 10.1176/appi.ajp.2011.08121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H., Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278-84. [DOI] [PubMed] [Google Scholar]
- 8.Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 9.Braak H., Braak E. Neuropahological staging of Alzheimer-related changes. Acta Neuropath Appl Neurobiol. 1991;14:39–44. [Google Scholar]
- 10.Spillantini M.G., Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 11.Seubert P., Mawal-Dewan M., Barbour R., Jakes R., Goedert M., Johnson G.V. Detection of phosphorylated Ser262 in fetal tau, adult tau, and paired helical filament tau. J Biol Chem. 1995;270:18917–18922. doi: 10.1074/jbc.270.32.18917. [DOI] [PubMed] [Google Scholar]
- 12.Augustinack J.C., Schneider A., Mandelkow E.M., Hyman B.T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 13.Ksiezak-Reding H., Liu W.K., Yen S.H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992;597:209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- 14.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal K., Wang X., Blanchard J., Liu F., Gong C.X., Grundke-Iqbal I. Alzheimer's disease neurofibrillary degeneration: pivotal and multifactorial. Biochem Soc Trans. 2010;38:962–966. doi: 10.1042/BST0380962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina M., Avila J. Further understanding of tau phosphorylation: implications for therapy. Expert Rev Neurother. 2015;15:115–122. doi: 10.1586/14737175.2015.1000864. [DOI] [PubMed] [Google Scholar]
- 17.Chung S.H. Aberrant phosphorylation in the pathogenesis of Alzheimer's disease. BMB Rep. 2009;42:467–474. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- 18.Adams C.E., Bergman H., Irving C.B., Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2013:CD003082. doi: 10.1002/14651858.CD003082.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipriani A., Rendell J.M., Geddes J.R. Haloperidol alone or in combination for acute mania. Cochrane Database Syst Rev. 2006:CD004362. doi: 10.1002/14651858.CD004362.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Lonergan E., Britton A.M., Luxenberg J., Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007:CD005594. doi: 10.1002/14651858.CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Lonergan E., Luxenberg J., Colford J. Haloperidol for agitation in dementia. Cochrane Database Syst Rev. 2002:CD002852. doi: 10.1002/14651858.CD002852. [DOI] [PubMed] [Google Scholar]
- 22.Schotte A., Janssen P.F., Megens A.A., Leysen J.E. Occupancy of central neurotransmitter receptors by risperidone, clozapine and haloperidol, measured ex vivo by quantitative autoradiography. Brain Res. 1993;631:191–202. doi: 10.1016/0006-8993(93)91535-z. [DOI] [PubMed] [Google Scholar]
- 23.Kales H.C., Kim H.M., Zivin K., Valenstein M., Seyfried L.S., Chiang C. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71–79. doi: 10.1176/appi.ajp.2011.11030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maust D.T., Kim H.M., Seyfried L.S., Chiang C., Kavanagh J., Schneider L.S. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasrallah H.A. Does the neurotoxicity of haloperidol explain the higher mortality in dementia patients compared with the second generation agents? Am J Psychiatry. 2012;169:663–664. doi: 10.1176/appi.ajp.2012.12020279. author reply 664–5. [DOI] [PubMed] [Google Scholar]
- 26.Benitez-King G., Ortiz-Lopez L., Jimenez-Rubio G., Ramirez-Rodriguez G. Haloperidol causes cytoskeletal collapse in N1E-115 cells through tau hyperphosphorylation induced by oxidative stress: Implications for neurodevelopment. Eur J Pharmacol. 2010;644:24–31. doi: 10.1016/j.ejphar.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 27.Casanova M.F., Carosella N.W., Gold J.M., Kleinman J.E., Weinberger D.R., Powers R.E. A topographical study of senile plaques and neurofibrillary tangles in the hippocampi of patients with Alzheimer's disease and cognitively impaired patients with schizophrenia. Psychiatry Res. 1993;49:41–62. doi: 10.1016/0165-1781(93)90029-g. [DOI] [PubMed] [Google Scholar]
- 28.Powchik P., Davidson M., Nemeroff C.B., Haroutunian V., Purohit D., Losonczy M. Alzheimer's-disease-related protein in geriatric schizophrenic patients with cognitive impairment. Am J Psychiatry. 1993;150:1726–1727. doi: 10.1176/ajp.150.11.1726. [DOI] [PubMed] [Google Scholar]
- 29.Purohit D.P., Davidson M., Perl D.P., Powchik P., Haroutunian V.H., Bierer L.M. Severe cognitive impairment in elderly schizophrenic patients: a clinicopathological study. Biol Psychiatry. 1993;33:255–260. doi: 10.1016/0006-3223(93)90291-k. [DOI] [PubMed] [Google Scholar]
- 30.Arnold S.E., Franz B.R., Trojanowski J.Q. Elderly patients with schizophrenia exhibit infrequent neurodegenerative lesions. Neurobiol Aging. 1994;15:299–303. doi: 10.1016/0197-4580(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 31.Arnold S.E., Gur R.E., Shapiro R.M., Fisher K.R., Moberg P.J., Gibney M.R. Prospective clinicopathologic studies of schizophrenia: accrual and assessment of patients. Am J Psychiatry. 1995;152:731–737. doi: 10.1176/ajp.152.5.731. [DOI] [PubMed] [Google Scholar]
- 32.Arnold S.E., Trojanowski J.Q. Cognitive impairment in elderly schizophrenia: a dementia (still) lacking distinctive histopathology. Schizophr Bull. 1996;22:5–9. doi: 10.1093/schbul/22.1.5. [DOI] [PubMed] [Google Scholar]
- 33.Higaki J., Murphy G.M., Jr., Cordell B. Inhibition of beta-amyloid formation by haloperidol: a possible mechanism for reduced frequency of Alzheimer's disease pathology in schizophrenia. J Neurochem. 1997;68:333–336. doi: 10.1046/j.1471-4159.1997.68010333.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramsden M., Kotilinek L., Forster C., Paulson J., McGowan E., SantaCruz K. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson D., Woerner M.G., Alvir J.M., Bilder R., Goldman R., Geisler S. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- 37.Meyer J.M. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18 Suppl 1:58–67. doi: 10.1017/S1092852913000783. quiz 68. [DOI] [PubMed] [Google Scholar]
- 38.Tiihonen J., Haukka J., Taylor M., Haddad P.M., Patel M.X., Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- 39.Spanarello S., La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9:310–317. doi: 10.2174/15748847113089990051. [DOI] [PubMed] [Google Scholar]
- 40.El-Mallakh R.S., Decker S., Morris M., Li X.P., Huff M.O., El-Masri M.A. Efficacy of olanzapine and haloperidol in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1261–1264. doi: 10.1016/j.pnpbp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Barth V.N., Chernet E., Martin L.J., Need A.B., Rash K.S., Morin M. Comparison of rat dopamine D2 receptor occupancy for a series of antipsychotic drugs measured using radiolabeled or nonlabeled raclopride tracer. Life Sci. 2006;78:3007–3012. doi: 10.1016/j.lfs.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Girgenti M.J., Nisenbaum L.K., Bymaster F., Terwilliger R., Duman R.S., Newton S.S. Antipsychotic-induced gene regulation in multiple brain regions. J Neurochem. 2010;113:175–187. doi: 10.1111/j.1471-4159.2010.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acker C.M., Forest S.K., Zinkowski R., Davies P., d'Abramo C. Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models. Neurobiol Aging. 2013;34:338–350. doi: 10.1016/j.neurobiolaging.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondragon-Rodriguez S., Perry G., Luna-Munoz J., Acevedo-Aquino M., Williams S. Phosphorylation of tau protein at sites Ser is one of the earliest events in Alzheimer's disease and Down syndrome. Neuropathol Appl Neurobiol. 2014;40:121–135. doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- 45.Janocko N.J., Brodersen K.A., Soto-Ortolaza A.I., Ross O.A., Liesinger A.M., Duara R. Neuropathologically defined subtypes of Alzheimer's disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol. 2012;124:681–692. doi: 10.1007/s00401-012-1044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozzi Y., Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–174. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Emamian E.S., Hall D., Birnbaum M.J., Karayiorgou M., Gogos J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer B.C., Zhang B., Leverenz J.B., Thomas J.H., Trojanowski J.Q., Schellenberg G.D. Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proc Natl Acad Sci U S A. 2003;100:9980–9985. doi: 10.1073/pnas.1533448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormick A.V., Wheeler J.M., Guthrie C.R., Liachko N.F., Kraemer B.C. Dopamine D2 receptor antagonism suppresses tau aggregation and neurotoxicity. Biol Psychiatry. 2013;73:464–471. doi: 10.1016/j.biopsych.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina M., Avila J. Understanding the relationship between GSK-3 and Alzheimer's disease: a focus on how GSK-3 can modulate synaptic plasticity processes. Expert Rev Neurother. 2013;13:495–503. doi: 10.1586/ern.13.39. [DOI] [PubMed] [Google Scholar]
- 51.Cohen P., Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 52.Noble W., Planel E., Zehr C., Olm V., Meyerson J., Suleman F. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caccamo A., Oddo S., Tran L.X., LaFerla F.M. Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol. 2007;170:1669–1675. doi: 10.2353/ajpath.2007.061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudduth T.L., Wilson J.G., Everhart A., Colton C.A., Wilcock D.M. Lithium treatment of APPSwDI/NOS2-/- mice leads to reduced hyperphosphorylated tau, increased amyloid deposition and altered inflammatory phenotype. PLoS One. 2012;7:e31993. doi: 10.1371/journal.pone.0031993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 56.Kozlovsky N., Amar S., Belmaker R.H., Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3 beta protein levels in rodent frontal cortex. Int J Neuropsychopharmacol. 2006;9:337–342. doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.F., Huang A.S., Snowman A.M., Teuscher C., Snyder S.H. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardie D.G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 59.Tek C., Kucukgoncu S., Guloksuz S., Woods S.W., Srihari V.H., Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. 2016;10:193–202. doi: 10.1111/eip.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller D.J., Kennedy J.L. Genetics of antipsychotic treatment emergent weight gain in schizophrenia. Pharmacogenomics. 2006;7:863–887. doi: 10.2217/14622416.7.6.863. [DOI] [PubMed] [Google Scholar]
- 61.Souza R.P., Tiwari A.K., Chowdhury N.I., Ceddia R.B., Lieberman J.A., Meltzer H.Y. Association study between variants of AMP-activated protein kinase catalytic and regulatory subunit genes with antipsychotic-induced weight gain. J Psychiatr Res. 2012;46:462–468. doi: 10.1016/j.jpsychires.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Choi Y., Jeong H.J., Liu Q.F., Oh S.T., Koo B.S., Kim Y. Clozapine Improves Memory Impairment and Reduces Abeta Level in the Tg-APPswe/PS1dE9 Mouse Model of Alzheimer's Disease. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9636-x. [DOI] [PubMed] [Google Scholar]
- 63.Vingtdeux V., Davies P., Dickson D.W., Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol. 2011;121:337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takashima A. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: role of GSK-3beta in adult brain. J Pharmacol Sci. 2009;109:174–178. doi: 10.1254/jphs.08r29fm. [DOI] [PubMed] [Google Scholar]
- 65.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iqbal K., Zaidi T., Bancher C., Grundke-Iqbal I. Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett. 1994;349:104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- 67.Schneider A., Biernat J., von Bergen M., Mandelkow E., Mandelkow E.M. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 68.Barini E., Antico O., Zhao Y., Asta F., Tucci V., Catelani T. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;11:16. doi: 10.1186/s13024-016-0082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murray P.S., Kumar S., Demichele-Sweet M.A., Sweet R.A. Psychosis in Alzheimer's disease. Biol Psychiatry. 2014;75:542–552. doi: 10.1016/j.biopsych.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koppel J., Greenwald B.S. Optimal treatment of Alzheimer's disease psychosis: challenges and solutions. Neuropsychiatr Dis Treat. 2014;10:2253–2262. doi: 10.2147/NDT.S60837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinberg G.R., Kemp B.E. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura Y., Okuno S., Kitani T., Otake K., Sato F., Fujisawa H. Immunohistochemical localization of Ca(2+)/calmodulin-dependent protein kinase kinase beta in the rat central nervous system. Neurosci Res. 2001;39:175–188. doi: 10.1016/s0168-0102(00)00209-1. [DOI] [PubMed] [Google Scholar]
- 73.Salminen A., Kaarniranta K., Haapasalo A., Soininen H., Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer's disease. J Neurochem. 2011;118:460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]