Abstract
Introduction
Gln-1062 (Memogain) is a pharmacologically inactive prodrug of galantamine. Owing to its lipophilic nature, it preferentially enters the brain, where it is cleaved into active galantamine. Gln-1062 is expected to have fewer peripheral side effects than other cholinesterase inhibitors, with improved effectiveness.
Methods
This was a double-blind, comparator and placebo-controlled, sequential cohort, single ascending dose study in 58 healthy subjects with Gln-1062 in doses of 5.5, 11, 22, 33, and 44 mg, compared with oral galantamine 16 mg and donepezil 10 mg. Safety, tolerability, pharmacokinetics, and pharmacodynamics were assessed.
Results
Gln-1062 doses up to 33 mg were well tolerated and induced a dose-dependent increase in the plasma concentrations of Gln-1062 and galantamine. Gln-1062 had a dose-dependent positive effect on verbal memory and attention, mainly in the first hours after drug administration.
Discussion
Gln-1062 was better tolerated than galantamine in doses with the same molarity and led to improved effects in cognitive tests. This is most likely caused by the more favorable distribution ratio between peripheral and central cholinesterase inhibition. These results give reason for further exploration of this compound.
Keywords: Pharmacology, Galantamine, Donepezil, Side effects, Alzheimer's disease
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia. Its pathogenesis involves the progressive development of amyloid plaques and tangles, loss of cholinergic neurons, and cholinergic deficiency. Recent trials with disease-modifying compounds, such as gamma secretase inhibitors and monoclonal antibodies against amyloid β, have had negative results [1], [2], [3]. Post hoc analysis of trial data of studies with solanezumab in patients with mild AD and the first results of trials with aducanumab in patients with mild or prodromal AD seem to underline the idea that disease modification might only be useful in earlier stages of the disease [4], [5]. All trials in patients with moderate or severe AD with disease-modifying compounds have been negative so far. The first registered treatment in line for the symptoms of mild-to-moderate AD are cholinesterase inhibitors (ChEIs). Although not curative, ChEIs can reduce symptoms for 6–36 months [6]. However, this positive effect is only seen in 14%–36% of patients [7], [8], [9], [10], [11]. Administration of higher doses, for example, 24 mg of galantamine or 23 mg of donepezil, leads to an increase in peripheral side effects, such as nausea, vomiting, and diarrhea, which overshadows a possible positive effect on cognition and functioning in daily life [12], [13]. As disease modification has not yet been demonstrated for any drug in patients with AD, it is worthwhile to optimize the available symptomatic drugs. Therefore, Gln-1062 (Memogain) was developed as a modification of galantamine having much higher lipophilicity and hence higher preference for the brain than the parent drug. Gln-1062 was designed as an inactive prodrug (in casu a benzoic ester) of galantamine that, after entering the brain, is cleaved into active galantamine by a carboxy-esterase. Gln-1062 is administered intranasally to prevent cleavage to galantamine in the acidic environment of the stomach, and in the presence of carboxy-esterases known to be expressed in the intestines and the liver. In female Wistar rats, intravenous administration of 5.0-mg/kg Gln-1062 led to a maximum concentration (Cmax) of 650 ng/mL in blood with an AUClast of 528 ng h/mL and a Cmax of 13,627 ng/mg in the brain with an AUClast of 9717 ng h/g. The brain-to-blood AUC ratio of Gln-1062 was, therefore, 18.40. After intranasal administration of 5.0 mg/kg, this ratio was 8.1 and intranasal administration of 20.0 mg/kg resulted in a ratio of 10.2 (Supplementary Material).
Owing to its more favorable brain-to-blood ratio, Gln-1062 is expected to have fewer peripheral side effects than galantamine and other ChEIs and a comparable, or possibly an improved, effectiveness in cognition enhancement. In this study, safety, pharmacokinetic, and pharmacodynamic effects of Gln-1062 were assessed and compared with orally administered galantamine and donepezil in healthy young and elderly male subjects.
2. Methods
2.1. Trial design and subjects
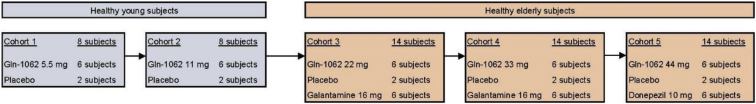
This was a double-blind, double dummy, double comparator, and placebo controlled, sequential cohort single ascending dose study (i.e., each subject received Gln-1062 nasal spray or placebo and capsules of either dummy or active substance for both comparator drugs). Five dose levels of intranasal Gln-1062, one dose level of oral galantamine, and one dose level of oral donepezil were tested in healthy, nonsmoking, male subjects. Main exclusion criteria were a mini-mental state examination of 27 or lower, impaired renal or liver function, use of interfering concomitant medication, and intranasal abnormalities. The first two cohorts each consisted of eight healthy young male subjects. In each cohort, six subjects received a single dose of intranasal Gln-1062 5.5 mg (cohort 1) or 11 mg (cohort 2) and two subjects received placebo. The last three cohorts each consisted of 14 healthy elderly male subjects. In each cohort, six subjects received a single dose of Gln-1062 22 mg (cohort 3), 33 mg (cohort 4), or 44 mg (cohort 5). Oral galantamine 16 mg was administered to 12 subjects in total (spread over cohorts 3 and 4) and oral donepezil 10 mg was administered to six subjects (cohort 5). In each cohort, two subjects received double placebo (six subjects in total; Fig. 1). In cohorts 3 and 4, all drugs were administered at the same time. In cohort 5, donepezil or placebo was administered 3 hours before administration of Gln-1062 or placebo to have the expected time of maximum concentration (Tmax) at approximately 3–4 hours after dosing at the same time point as the Tmax of Gln-1062, which was expected to be approximately 0.5–1 hour after dosing. All subjects gave written informed consent for participation in the study. The study was approved by the ethics committee of the Leiden University Hospital, the Netherlands. The study was conducted according to the Dutch Act on Medical Research Involving Human Subjects (WMO) and in compliance with Good Clinical Practice (ICH-GCP) and the Declaration of Helsinki. The trial was registered in the European Union Clinical Trials Register (2013-004354-25).
Fig. 1.
Schematic overview of cohorts.
2.2. Dosing rationale
2.2.1. Gln-1062
In a 28-day intranasal toxicity study in Wistar rats, a NOAEL for intranasal Gln-1062 was observed at a dose level of 5 mg/kg. The human equivalent dose was estimated to be 48 mg. With a 10-fold safety margin, a starting dose of 5.5 mg was chosen.
2.2.2. Galantamine
The recommended starting regimen for galantamine (slow release formulation) in patients with AD is a titration period of 4 weeks on 8 mg daily after which the dose can be increased to 16 mg daily, and, if necessary, to 24 mg daily. In previous clinical trials, immediate release formulations without preceding dose titration have been given to healthy subjects as a single dose up to 15 mg [14], [15]. Three of eight subjects not pretreated with a peripheral anticholinergic drug as antidote experienced nausea at a dose of 15 mg, and one of eight patients vomited. Because the main advantage of Gln-1062 would be a reduction of side effects, we chose to give a single oral dose of galantamine 16 mg.
2.2.3. Donepezil
The recommended starting dose for donepezil (tablet formulation) is 5 mg/d and is administered as a single daily dose, usually in the morning. The dose can be increased to 10 mg/d as needed. Donepezil 10 mg was chosen because it was the highest dose that was previously given as a single oral dose to healthy subjects without titration [16].
2.3. Pharmacokinetic assessment
Venous blood samples were obtained via an indwelling catheter before administration of Gln-1062 or galantamine or placebo and at 15 minutes, 30 minutes, 1 hour, 1 hour 30 minutes, 2 hours, 2 hours 30 minutes, 3 hours, 3 hours 30 minutes, 4 hours, 5 hours, 7 hours, 10 hours, and 23 hours after administration. In cohorts 4 and 5, the sample at 7 hours after drug administration was replaced by samples at 6 hours and 8 hours and an extra sample at 30 hours after drug administration was added. Plasma concentrations of Gln-1062 and galantamine were determined at WIL Research Europe (Den Bosch, the Netherlands) by a validated method using high performance liquid chromatography coupled to tandem-mass spectrometry.
2.4. Pharmacodynamic assessments
The “NeuroCart” is a battery of sensitive tests for a wide range of central nervous system (CNS) domains that was developed to examine different kinds of CNS-active drugs. The N-back test was used to evaluate working memory [17], [18], [19], the Stroop test evaluated inhibition, interference, and controlled versus automatic processing [20], adaptive tracking measured attention and eye-hand coordination [21], [22], [23], [24], [25], [26], the visual analogue scale according to Bond and Lader was used to assess subjective states [27], [28], pharmaco-electroencephalography, eye movements, and pupil size were used to monitor any drug effects, which can be interpreted as evidence of penetration and activity in the brain [24], [26], [29], [30], [31], body movements were measured with the body sway meter [32], the face encoding and recognition task evaluated visual memory [33], and the visual verbal learning test (VVLT) measured the whole scope of learning behavior (i.e., acquisition, consolidation, storage and retrieval) [34].
All tests with this device were performed twice at baseline and repeated at 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 8 hours, and 10 hours after administration of Gln-1062 or galantamine or placebo. In cohort 5, an additional measurement was performed 2 hours after administration of donepezil or placebo (i.e., 1 hour before administration of Gln-1062 or placebo). The only exceptions were VVLT, which was only performed 1 hour 30 minutes after dosing of Gln-1062 or placebo, and face recognition, which was performed before the dose and 1 hour 45 minutes after administration of Gln-1062 or placebo. Measurements were performed in a quiet room with ambient illumination with only one subject per session in the same room.
2.5. Safety assessments
All subjects underwent medical screening, including medical history, physical examination, nasal examination, vital signs measurement in supine and standing position, 12-lead electrocardiogram (ECG), urinalysis, drug screen, and safety chemistry and hematology blood sampling. During study periods, safety was assessed using monitoring of adverse events (AEs), nasal examination, vital signs, ECG, and safety chemistry and hematology blood sampling.
2.6. Statistics
All pharmacodynamic end points are summarized (mean and standard deviation of the mean, median, and minimum and maximum values) by treatment and time. To establish whether significant treatment effects could be detected, repeatedly measured variables were analyzed with a mixed model analysis of variance with treatment, time and treatment by time as fixed factors, and subject as random factor and the (average) baseline measurement as covariate [35]. Single measured variables were analyzed by a mixed model analysis of variance with fixed factor treatment. The young subjects receiving active treatment were compared with the young subjects on placebo and the elderly subjects on active treatment were compared with the elderly on placebo.
3. Results
3.1. Subjects
The study was conducted between November 2013 and April 2014. A total of 16 healthy young and 42 healthy elderly male subjects participated in this study. The healthy young males had a mean age of 42.9 years (range 19–62), a mean body weight of 77.6 kg (range 58.4–92.9), and a mean body mass index (BMI) of 24.2 kg/m2 (range 18.7–28.6). The healthy elderly males had a mean age of 71.2 years (range 66–89), a mean body weight of 81.8 kg (range 62.6–121.5), and a mean BMI of 25.9 kg/m2 (range 20.4–32.4). There were no dropouts after drug administration.
3.2. Pharmacokinetics
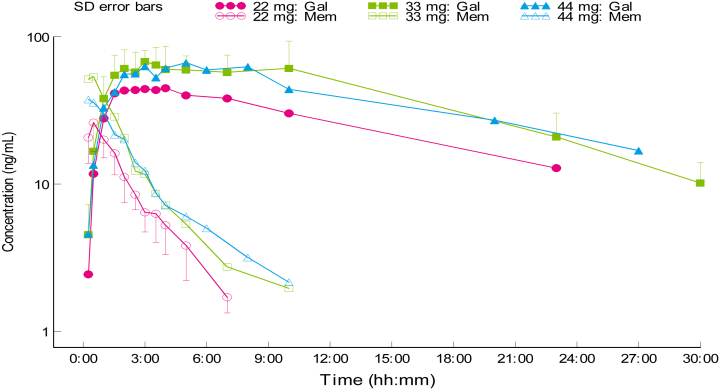
After administration of Gln-1062, concentrations of Gln-1062 and galantamine were measured. Based on the noncompartmental pharmacokinetic analysis of the plasma Gln-1062 concentrations, a dose-dependent increase in exposure was observed up to a dose of 33 mg (Table 1, Fig. 2). The 44-mg Gln-1062 dose led to a mean exposure that was comparable with the 33-mg dose; however, in view of the considerable interindividual variability in exposure, the number of subjects per dose level (n = 6) and the limited increase in dose from 33 mg to 44 mg (+33%), it cannot be concluded from these data that a dose-dependent increase in exposure is not present beyond a dose of 33 mg. In general, subjects with a relatively high Cmax for Gln-1062 also had a relatively high Cmax for Gln-1062–derived galantamine. The Tmax Gln-1062 was 15–45 minutes, whereas the Tmax for galantamine after administration of Gln-1062 was 2–4.5 hours. The half-life of Gln-1062 increased with the administered dose form 1.07 to 2.08 hours. For galantamine derived from Gln-1062, the half-life was approximately 10 hours for all dose levels.
Table 1.
Pharmacokinetic parameters
| Parameter | Cohort 1: Gln-1062 5.5 mg |
Cohort 2: Gln-1062 11 mg |
Cohort 3: Gln-1062 22 mg |
Cohort 4: Gln-1062 33 mg |
Cohort 5: Gln-1062 44 mg |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mem | Gal | Mem | Gal | Mem | Gal | Mem | Gal | Mem | Gal | |
| Cmax (ng/mL) | 15.2 (6.51–29.9) | 17.6 (13.0–21.6) | 19.4 (7.40–5.42) | 27.7 (19.0–41.2) | 26.5 (12.3–39.2) | 46.5 (29.2–67.2) | 58.5 (16.4–103) | 76.1 (49.2–121) | 43.2 (16.9–97.3) | 74.7 (42.7–114) |
| Tmax (h) | 0.29 (0.25–0.50) | 2.27 (1.00–3.53) | 0.48 (0.25–1.00) | 4.35 (1.53–10.0) | 0.60 (0.50–1.00) | 3.28 (1.57–4.02) | 0.59 (0.25–1.53) | 4.58 (2.00–9.89) | 0.71 (0.25–1.67) | 4.66 (2.82–8.00) |
| AUC 0-inf (ng*h/mL) | 20.0 (11.6–35.8) | 268 (166–445) | 32.9 (16.2–71.8) | 367 (273–489) | 69.1 (40.8–95.4) | 799 (629–954) | 125 (49.3–221) | 1190 (926–1800) | 112 (69.6–177) | 1530 (826–3270) |
| T1/2 (h) | 1.07 (0.68–1.51) | 9.85 (6.28–19.9) | 1.37 (0.83–1.93) | 7.94 (4.92–10.4) | 2.64 (1.37–4.64) | 8.71 (6.85–12.1) | 2.34 (1.39–2.87) | 9.24 (6.67–11.2) | 2.80 (1.79–3.97) | 11.1 (4.27–18.1) |
Abbreviations: AUC, area under the curve; T1/2, half-life; Cmax, maximum concentration; Tmax, time of maximum concentration.
NOTE. Mean, range in parentheses.
Fig. 2.
Plasma concentrations of Gln-1062 and galantamine in cohort 3–5. Abbreviations: Mem, Gln-1062; Gal, galantamine; SD, standard deviation.
3.3. Safety
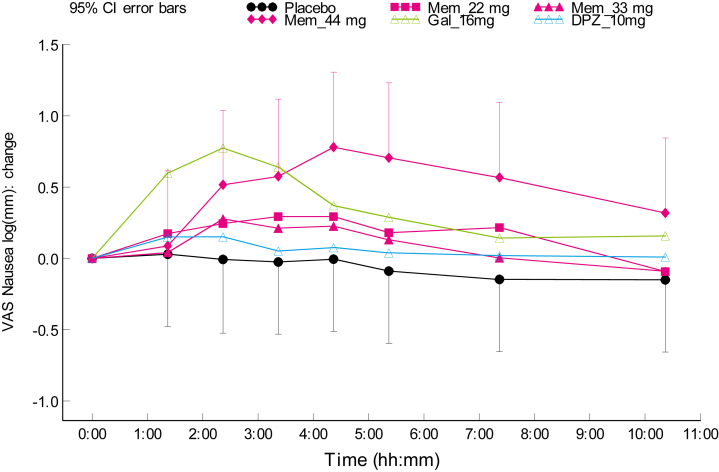
On each treatment, at least 50% of the subjects experienced one or more treatment emergent AE (Table 2). Nasal symptoms, such as nasal discomfort, rhinorrhea, and sneezing, were reported most frequently and exclusively in the Gln-1062 dosing groups, except for one case of nasal discomfort in the donepezil group. Nasal symptoms subsided in most cases within half an hour (data not presented). No clear dose relationship was observed. Cholinergic symptoms (e.g., nausea, vomiting, diarrhea and hyperhidrosis) were reported on all treatments, except for Gln-1062 5.5 mg and placebo. After administration of Gln-1062 11 and 22 mg, one subject in each cohort (16.7%) experienced nausea. Gln-1062 at the highest dose levels led to nausea in 50% of subjects (n = 3), which was higher than the incidence of nausea in the galantamine 16 mg group (33.3%, n = 4) and in the donepezil 10 mg group (16.7%, n = 1). Although 33 mg of Gln-1062 led to a higher incidence of nausea compared with galantamine, the severity, measured with the VAS nausea, was on average lower for Gln-1062 33 mg (Fig. 3). The results on VAS nausea also indicated a difference in time profile. The peak of nausea occurred 2 hours after administration of galantamine, versus 4 hours after administration of Gln-1062.
Table 2.
Most frequent occurring treatment emerging adverse events
| Event | Gln-1062 5.5 mg | Gln-1062 11 mg | Gln-1062 22 mg | Gln-1062 33 mg | Gln-1062 44 mg | Galantamine 16 mg | Donepezil 10 mg | Placebo |
|---|---|---|---|---|---|---|---|---|
| Any event | 6 (100) | 6 (100) | 5 (83.3) | 5 (83.3) | 5 (83.3) | 10 (83.3) | 3 (50) | 5 (50) |
| Nasal discomfort | 6 (100) | 6 (100) | 4 (66.7) | 4 (66.7) | 3 (50) | — | 1 (16.7) | — |
| Rhinorrhea | 2 (33.3) | 4 (66.7) | 2 (33.3) | 1 (16.7) | 3 (50) | — | — | — |
| Sneezing | 1 (16.7) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 3 (50) | — | — | — |
| Nausea | — | 1 (16.7) | 1 (16.7) | 3 (50) | 3 (50) | 4 (33.3) | 1 (16.7) | — |
| Vomiting | — | 1 (16.7) | — | 2 (33.3) | 2 (33.3) | 5 (41.7) | 1 (16.7) | 1 (10) |
| Diarrhea | — | — | 1 (16.7) | 1 (16.7) | 1 (16.7) | 1 (8.3) | 2 (33.3) | — |
| Cold sweat or hyperhidrosis | — | 1 (16.7) | — | — | 3 (50) | 4 (33.3) | — | — |
| Headache | 2 (33.3) | 2 (33.3) | 3 (50) | 2 (33.3) | — | 1 (8.3) | 2 (33.3) | 3 (30) |
NOTE. Number of subjects, percentage in parentheses.
Fig. 3.
Scores on VAS nausea in cohort 3–5. Abbreviations: VAS, visual analogue scale; Mem, Gln-1062; Gal, galantamine; DPZ, donepezil; CI, confidence interval.
Vomiting did not occur after administration of Gln-1062 5.5 mg in healthy young subjects or after administration of 22 mg in healthy elderly subjects. Gln-1062 11 mg led to vomiting in one healthy young subject (16.7%). Gln-1062 33 and 44 mg led to vomiting in two subjects in each cohort (33.3%), which was lower than the incidence of vomiting after administration of galantamine 16 mg, which led to vomiting in five of 12 subjects (42%). After administration of donepezil 10 mg, one subject (16.7%) vomited. One subject (10%) who was administered placebo vomited. Diarrhea did not occur after administration of Gln-1062 5.5 or 11 mg in healthy young subjects, and administration of Gln-1062 22, 33 mg, and 44 mg in healthy elderly subjects led to diarrhea in one subject (16.7%) in each cohort. This incidence was higher than after administration of galantamine 16 mg (8.3%, n = 1) but lower than after administration of donepezil 10 mg (33.3%, n = 2).
Cold sweat or hyperhidrosis was seen in one subject (16.7%) on Gln-1062 11 mg, three subjects (50%) on Gln-1062 44 mg, and four subjects (33.3%) on galantamine. Headache was frequently reported in all dose groups. All AEs were self-limiting, and most AEs were mild in intensity, except for moderate nausea in one subject on 44 mg of Gln-1062, one subject on galantamine and one subject on donepezil, one subject with moderate vomiting on placebo, and two subjects with moderate postural dizziness on galantamine. No severe AEs occurred.
On nasal examination, three subjects in the Gln-1062 44-mg group had dry white plaques in the nostrils, which were not seen at the follow-up visit approximately 1 week after dosing. Of these subjects, one had red and irritated nasal mucosa at follow-up. One subject in the donepezil group had red and irritated nasal mucosa at follow-up, whereas no nasal abnormalities were seen during the day of drug administration. There were no clinically relevant abnormalities in vital signs, ECG, or chemistry and hematology values in any of the subjects.
3.4. Pharmacodynamics
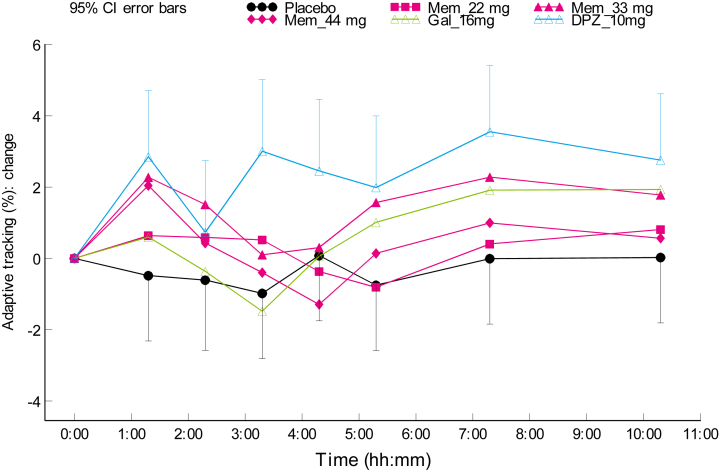
Pharmacodynamic effects of Gln-1062 compared with placebo are summarized in Table 3. An improvement on the adaptive tracking performance was seen in the healthy young subjects receiving Gln-1062 11 mg and healthy elderly subjects receiving Gln-1062 33 or 44 mg (Fig. 4).
Table 3.
Pharmacodynamic effects compared with placebo
| Test parameter | Cohort 1: Gln-1062 5.5 mg | Cohort 2: Gln-1062 11 mg | Cohort 3: Gln-1062 22 mg | Cohort 4: Gln-1062 33 mg | Cohort 5: Gln-1062 44 mg |
|---|---|---|---|---|---|
| Adaptive tracking (%) | 1.96 (−0.88 to 4.80) P = .1581 | 3.47 (0.52 to 6.42) P = .0247 | 0.64 (−1.06 to 2.35) P = .4474 | 1.79 (0.07 to 3.52) P = .0424 | 0.74 (−0.99 to 2.48) P = .3887 |
| VVLT: immediate word recall trial 1 | 3.42 (−0.73 to 7.56) P = .0984 | 3.92 (−0.23 to 8.06) P = .0621 | 2.67 (0.17 to 5.16) P = .0367 | 1.97 (−0.65 to 4.58) P = .1355 | 2.57 (−0.05 to 5.18) P = .0541 |
| VVLT: immediate word recall trial 2 | 3.17 (−1.93 to 8.26) P = .2025 | 1.83 (−3.26 to 6.93) P = .4510 | 3.50 (0.33 to 6.67) P = .0315 | 1.77 (−1.56 to 5.09) P = .2880 | 1.77 (−1.56 to 5.09) P = .2880 |
| VVLT: immediate word recall trial 3 | 5.00 (−0.58 to 10.58) P = .0749 | 3.00 (−2.58 to 8.58) P = .2662 | 0.67 (−3.23 to 4.56) P = .7302 | 0.60 (−3.49 to 4.69) P = .7672 | 1.00 (−3.09 to 5.09) P = .6222 |
| VVLT: delayed word recall | 3.42 (−2.62 to 9.45) P = .2431 | 3.25 (−2.79 to 9.29) P = .2657 | −0.33 (−3.58 to 2.91) P = .8354 | −0.00 (−3.63 to 3.63) P = 1.0000 | −1.00 (−4.63 to 2.63) P = .5780 |
| VVLT: word recognition | −0.15 (−8.05 to 7.75) P = .9674 | 1.45 (−6.45 to 9.35) P = .6939 | 0.87 (−4.09 to 5.82) P = .7231 | −0.67 (−6.45 to 5.12) P = .8153 | 2.17 (−3.11 to 7.45) P = .4083 |
| N-back, 0-back (correct-incorrect/total) | 0.04 (−0.02 to 0.10) P = .1696 | 0.03 (−0.03 to 0.09) P = .3145 | 0.02 (−0.02 to 0.05) P = .3729 | 0.03 (−0.01 to 0.06) P = .1690 | 0.03 (−0.00 to 0.07) P = .0654 |
| N-back, 1-back (correct-incorrect/total) | −0.00 (−0.17 to 0.16) P = .9835 | −0.05 (−0.21 to 0.12) P = .5597 | 0.02 (−0.02 to 0.06) P = .3402 | −0.03 (−0.07 to 0.02) P = .2212 | −0.01 (−0.06 to 0.03) P = .5016 |
| N-back, 2-back (correct-incorrect/total) | 0.09 (−0.06 to 0.25) P = .2199 | 0.13 (−0.02 to 0.28) P = .0867 | −0.02 (−0.09 to 0.06) P = .6835 | −0.02 (−0.10 to 0.06) P = .5957 | 0.02 (−0.05 to 0.10) P = .5430 |
| Face recognition number correct | −0.77 (−4.17 to 2.64) P = .6332 | 4.54 (1.22 to 7.87) P = .0116 | −2.58 (−7.22 to 2.06) P = .2642 | −0.54 (.5.15 to 4.08) P = .8135 | −1.63 (−6.30 to 3.05) P = .4822 |
| Stroop (correct congruent – correct incongruent) | −0.39 (−0.94 to 0.15) P = .1411 | −0.60 (−1.21 to 0.01) P = .0535 | 0.10 (−1.05 to 1.25) P = .8606 | 0.58 (−0.52 to 1.68) P = .2909 | 0.11 (−0.98 to 1.20) P = .8406 |
| EEG alpha Fz-Cz (uV) | −13.2% (−30.5% to 8.5%) P = .1914 | −0.8% (−19.5% to 22.1%) P = .9310 | 7.8% (−7.0% to 25.1%) P = .3088 | 21.5% (4.8% to 40.8%) P = .0116 | −10.1% (−24.2% to 6.6%) P = .2121 |
| EEG alpha Pz-Oz (uV) | −8.9% (−23.8% to 8.9%) P = .2782 | −13.1% (−27.2% to 3.7%) P = .1086 | 13.8% (−5.4% to 36.9%) P = .1633 | 19.7% (−0.2% to 43.6%) P = .0530 | 2.1% (−16.3% to 24.5%) P = .8306 |
| Saccadic peak velocity (°/s) | 9.90 (−17.50 to 37.30) P = .4465 | 8.32 (−18.32 to 34.95) P = .5097 | 26.92 (4.11 to 49.73) P = .0223 | 0.92 (−21.84 to 23.68) P = .9349 | 19.19 (−5.54 to 43.92) P = .1242 |
| Saccadic inaccuracy (%) | −1.56 (−2.77 to −0.35) P = .0157 | −1.78 (−3.03 to −0.54) P = .0088 | 0.24 (−1.01 to 1.48) P = .7005 | 0.08 (−1.14 to 1.30) P = .8970 | −0.22 (−1.57 to 1.12) P = .7363 |
Abbreviations: VVLT, visual verbal learning test; EEG, electroencephalogram.
NOTE. Mean, confidence interval in parentheses.
Fig. 4.
Effect on adaptive tracking in cohort 3–5 (healthy elderly males). Abbreviations: Mem, Gln-1062; Gal, galantamine; DPZ, donepezil; CI, confidence interval.
On the VVLT, an improved immediate recall of the words was seen for all doses of Gln-1062 in both young and elderly subjects, when compared with galantamine. In the healthy young subjects, the delayed word recall also improved for both the 5.5- and the 11-mg dose level. Word recognition did not improve on any of the Gln-1062 dose levels.
Pharmaco-EEG, face encoding and recognition test, pupil-to-iris ratio, eye movements, the VAS mood and calmness composite scores, the N-back test, body sway, and Stroop color-word interference test did not show consistent differences compared with placebo for any of the Gln-1062 dose levels.
Administration of galantamine 16 mg did not induce any measurable pharmacodynamic effects compared with placebo. Administration of donepezil 10 mg only led to an improvement in adaptive tracking. The maximum effect on the adaptive tracker test performance of Gln-1062 33 and 44 mg was comparable with the maximum effect of donepezil 10 mg.
4. Discussion
In this study, we examined the pharmacokinetics, side-effect profile, and pharmacodynamic effects of Gln-1062 and compared these to the pharmacodynamics effects and side-effect profile of galantamine and donepezil in healthy male subjects.
Gln-1062 was rapidly absorbed into the systemic circulation with a Cmax in plasma reached after approximately 15–45 minutes and a half-life of 1.1–2.8 hours, depending on the dose administered. The Tmax of galantamine after administration of Gln-1062 was 2.3–4.7 hours in all except the third cohort, which is approximately two half-lives of Gln-1062. This would be consistent with the hypothesis that Gln-1062 rapidly enters the brain, where it may be cleaved into active galantamine. It is established that the approved ChEIs all distribute into the brain according to their lipophilicity. The lipophilic nature of Gln-1062 and the avoidance of the first-pass effect due to the intranasal administration could increase the concentrations of Gln-1062 in the brain. A direct route from the nose to the brain has never been demonstrated in humans [36].
As a prodrug of galantamine, a low exposure of Gln-1062 generally resulted in a low formation of galantamine in most subjects. However, some individuals seemed to reach lower galantamine concentration than expected based on their measured Gln-1062 exposure. This may be indicative of differences between subjects in the rate of conversion of Gln-1062 to galantamine.
All doses of Gln-1062 were safe and reasonably well tolerated. The most frequently reported AEs were related to irritation of nasal mucosa to which Gln-1062 is dispositioned after intranasal administration. The reported irritation was rapidly reversible and will be further studied in the next clinical trial. The subjects generally considered the intranasal administration to be easy and well tolerable and compared it with the use of a nasal spray as is used in case of a common cold.
As Gln-1062 is expected to have fewer peripheral side effects than galantamine and other ChEIs, the comparison of AE's between the different treatments was an important aspect of this study. After administration of galantamine 16 mg, the most frequently reported treatment emergent AEs were nausea, vomiting, and cold sweat or hyperhidrosis, which is consistent with previous studies [37], [38]. Gln-1062 22 mg has the same molarity as the 16 mg dose of galantamine, and based on preclinical studies, Gln-1062 22 mg is expected to lead to at least 10-fold higher galantamine concentrations in the brain compared with orally administered galantamine 16 mg. At this dose of Gln-1062, nausea occurred in 16.7% of subjects, compared with 33.3% in the galantamine subjects, and vomiting did not occur at all, although this was present in 41.7% of subjects on galantamine. The Gln-1062 33 mg dose led to a higher incidence of nausea, compared with galantamine, but a lower severity of nausea, as measured using a VAS for nausea. After administration of Gln-1062 44 mg, both incidence and severity of nausea were higher, compared with galantamine 16 mg. Compared to donepezil, Gln-1062 33 and 44 mg both had a higher incidence of nausea and vomiting but a lower incidence of diarrhea. It can be concluded that single doses of Gln-1062 up to 33 mg seem to be tolerated at least as well as a single dose of galantamine 16 mg but are likely to lead to substantially higher galantamine concentrations in the brain in comparison with an oral dose of 16-mg galantamine. The single dose design of the study is a limitation with respect to the extrapolation of the results to clinical practice because the treatment of symptoms of AD with one of the registered ChEIs will always imply daily dosing with a period of uptitration. The results of this study provide a good base for a multiple dose study to investigate this in more detail. Another way to reduce side effects with classic ChEIs is transdermal administration, which, at this stage, is only possible with rivastigmine. However, this does not alter the ratio of peripheral and central cholinesterase inhibition, although the preclinical data of Gln-1062 and the results of the presented study suggest that this ratio might be more favorable for Gln-1062 compared with the currently registered ChEIs.
The analysis of pharmacodynamic effects in this study was exploratory in nature because the study was not powered to detect differences between treatments and there was no correction for multiple testing. This needs to be taken into account when interpreting the pharmacodynamic results. Previous research has shown that acetylcholine plays an important role in attentional processes and memory and ChEIs also primarily affect these domains in patients with AD [38], [39]. This is in line with the findings in our study, where administration of Gln-1062 led to consistent improvements on adaptive tracking, which is very sensitive to compounds that affect vigilance and arousal, and VVLT, a test of verbal memory. The improvements on the VVLT after administration of Gln-1062 in healthy elderly subjects were observed on the immediate recall trials, suggesting an effect on short-term memory capacity or learning, but not on retrieval of previously stored information, which would be consistent with previous research [39], [40], [41], [42]. The lack of effect of donepezil on VVLT might be explained by the fact that donepezil does not have a direct effect on nicotinic acetylcholine receptors, which galantamine also has [43]. Galantamine allosterically sensitizes neuronal nicotinic acetylcholine receptors, but when orally applied has limited brain penetration, which may explain the lack of acutely measurable pharmacodynamic effects of oral galantamine.
The time profiles of the adaptive tracking test in the healthy elderly subjects showed that the administration of Gln-1062 resulted in larger effects compared with oral administration of 16-mg galantamine and placebo mainly due to an improvement that occurred in the first hours after study drug administration. After approximately 4 hours, the adaptive tracker test curves of the Gln-1062 33 and 44 mg cohorts return to the same level as the galantamine 16-mg curve and continue to run in parallel. This is in line with the hypothesis that Gln-1062, as a prodrug of galantamine, enters the CNS to a greater extent than (oral) galantamine in the initial hours after drug administration. The distribution of small molecules such as Gln-1062 and galantamine via the blood-brain barrier is extremely fast. It is the higher level of galantamine that is produced in the brain after enzymatic cleavage of Gln-1062 that causes the higher level of activation of nicotinic receptors and, thus, the higher pharmacodynamics effects compared with oral galantamine. Several hours after the dose (±4 hours), Gln-1062 can be expected to be almost completely converted into galantamine, which is likely to be the reason why the Gln-1062 33 and 44 mg curves are no longer distinguishable from the galantamine 16-mg curve at 4 hours and beyond. Establishment of a pharmacokinetic-pharmacodynamic model may shed more light on the exact relationship between the pharmacodynamics effects observed and the estimated brain concentrations and measured plasma concentrations of Gln-1062 and galantamine. Donepezil showed an improvement in adaptive tracking performance that was similar in magnitude to Gln-1062 33 and 44 mg. However, the donepezil-induced improvement lasted considerably longer. This is consistent with the pharmacokinetic profile of donepezil and its half-life of 70 hours.
In conclusion, this study demonstrates that Gln-1062 is safe and well tolerated at single dose levels up to 33 mg. The pharmacokinetic and pharmacodynamic profile of Gln-1062 as observed in this study are in accordance with the hypothesis that Gln-1062 enters the CNS very rapidly and is then enzymatically cleaved to the active ingredient galantamine, resulting in higher CNS concentrations than can be achieved by oral administration of galantamine. The observation that, in this study, the dose of 22 mg of Gln-1062 induces fewer cholinergic side effects than 16 mg of galantamine, which has the same molarity, supports this hypothesis. Based on these observations, Gln-1062 is expected to be better tolerated and to be more effective than oral galantamine in treating the symptoms of patients with AD and may be a promising compound for an improved symptomatic treatment.
Research in context.
-
1.
Systematic review: The authors searched PubMed for publications regarding the effectiveness and side effects of cholinesterase inhibitors (ChEIs) in patients with Alzheimer's disease (AD).
-
2.
Interpretation: ChEIs can reduce symptoms in patients with mild-to-moderate AD. Even the daily recommended doses induce side effects that lead to discontinuation of medication. The effects of the newly developed prodrug of galantamine have not been investigated before in humans.
-
3.
Future directions: This study provides a good basis for a head-to-head comparison of Memogain and galantamine in patients with AD. In such a study, the long-term safety and efficacy in patients with AD will be determined. Preclinical evidence suggests that in addition to providing a better benefit-risk ratio in symptomatic relief than current drugs, Memogain may lead to disease modification.
Acknowledgments
The authors thank Jasper van der Aart, Anna Uit den Boogaard, Renate van Rijt and Esther Davidse for their contributions to the clinical execution of the study.
The study that led to this article was funded by Neurodyn Inc. Neurodyn commented on the study design and the study report but was not involved in the collection, analysis, or interpretation of the data.
Footnotes
D.G.K. and A.M. are employees of Neurodyn Inc and Galantos Pharma, respectively. All other authors report no conflicts of interest.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2015.12.003.
Supplementary data
References
- 1.Mikulca J.A., Nguyen V., Gajdosik D.A., Teklu S.G., Giunta E.A., Lessa E.A. Potential novel targets for Alzheimer pharmacotherapy: II. Update on secretase inhibitors and related approaches. J Clin Pharm Ther. 2014;39:25–37. doi: 10.1111/jcpt.12112. [DOI] [PubMed] [Google Scholar]
- 2.Prins N.D., Visser P.J., Scheltens P. Can novel therapeutics halt the amyloid cascade? Alzheimers Res Ther. 2010;2:5. doi: 10.1186/alzrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prins N.D., Scheltens P. Treating Alzheimer's disease with monoclonal antibodies: Current status and outlook for the future. Alzheimers Res Ther. 2013;5:56. [Google Scholar]
- 4.Patel K.R. Biogen's aducanumab raises hope that Alzheimer's can be treated at its source. Manag Care. 2015;24:19. [PubMed] [Google Scholar]
- 5.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 6.Wallin A.K., Wattmo C., Minthon L. Galantamine treatment in Alzheimer's disease: Response and long-term outcome in a routine clinical setting. Neuropsychiatr Dis Treat. 2011;7:565–576. doi: 10.2147/NDT.S24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birks J., Grimley E.J., Iakovidou V., Tsolaki M., Holt F.E. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. 2009:CD001191. doi: 10.1002/14651858.CD001191.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Olin J., Schneider L. Galantamine for Alzheimer's disease. Cochrane Database Syst Rev. 2002:CD001747. doi: 10.1002/14651858.CD001747. [DOI] [PubMed] [Google Scholar]
- 10.Rosler M., Anand R., Cicin-Sain A., Gauthier S., Agid Y., Dal-Bianco P. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: International randomised controlled trial. BMJ. 1999;318:633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tariot P.N., Solomon P.R., Morris J.C., Kershaw P., Lilienfeld S., Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 12.Knopman D.S. Donepezil 23 mg: An empty suit. Neurol Clin Pract. 2012;2:352–355. doi: 10.1212/CPJ.0b013e318278bebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loy C., Schneider L. Galantamine for Alzheimer's disease and mild cognitive impairment. Cochrane Database Syst Rev. 2006:CD001747. doi: 10.1002/14651858.CD001747.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemann D., Gann H., Dressing H., Muller W.E., Aldenhoff J.B. Influence of the cholinesterase inhibitor galantamine hydrobromide on normal sleep. Psychiatry Res. 1994;51:253–267. doi: 10.1016/0165-1781(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q., Brett M., Van O.N., Huang F., Raoult A., Van P.A. Galantamine pharmacokinetics, safety, and tolerability profiles are similar in healthy Caucasian and Japanese subjects. J Clin Pharmacol. 2002;42:1002–1010. [PubMed] [Google Scholar]
- 16.Mihara M., Ohnishi A., Tomono Y., Hasegawa J., Shimamura Y., Yamazaki K. Pharmacokinetics of E2020, a new compound for Alzheimer's disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol. 1993;31:223–229. [PubMed] [Google Scholar]
- 17.Lim H.K., Juh R., Pae C.U., Lee B.T., Yoo S.S., Ryu S.H. Altered verbal working memory process in patients with Alzheimer's disease: An fMRI investigation. Neuropsychobiology. 2008;57:181–187. doi: 10.1159/000147471. [DOI] [PubMed] [Google Scholar]
- 18.Rombouts S.A., Barkhof F., Van Meel C.S., Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet L.H., Rao S.M., Primeau M., Durgerian S., Cohen R.A. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp. 2006;27:28–36. doi: 10.1002/hbm.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laeng B., Lag T., Brennen T. Reduced Stroop interference for opponent colors may be due to input factors: Evidence from individual differences and a neural network simulation. J Exp Psychol Hum Percept Perform. 2005;31:438–452. doi: 10.1037/0096-1523.31.3.438. [DOI] [PubMed] [Google Scholar]
- 21.Borland R.G., Nicholson A.N. Comparison of the residual effects of two benzodiazepines (nitrazepam and flurazepam hydrochloride) and pentobarbitone sodium on human performance. Br J Clin Pharmacol. 1975;2:9–17. doi: 10.1111/j.1365-2125.1975.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borland R.G., Nicholson A.N. Visual motor co-ordination and dynamic visual acuity. Br J Clin Pharmacol. 1984;18 Suppl 1:69S–72S. doi: 10.1111/j.1365-2125.1984.tb02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gijsman H.J., van Gerven J.M., Tieleman M.C., Schoemaker R.C., Pieters M.S., Ferrari M.D. Pharmacokinetic and pharmacodynamic profile of oral and intravenous meta-chlorophenylpiperazine in healthy volunteers. J Clin Psychopharmacol. 1998;18:289–295. doi: 10.1097/00004714-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 24.van Steveninck A.L., Schoemaker H.C., Pieters M.S., Kroon R., Breimer D.D., Cohen A.F. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50:172–180. doi: 10.1038/clpt.1991.122. [DOI] [PubMed] [Google Scholar]
- 25.van Steveninck A.L., Gieschke R., Schoemaker H.C., Pieters M.S., Kroon J.M., Breimer D.D. Pharmacodynamic interactions of diazepam and intravenous alcohol at pseudo steady state. Psychopharmacology (Berl) 1993;110:471–478. doi: 10.1007/BF02244655. [DOI] [PubMed] [Google Scholar]
- 26.van Steveninck A.L., van Berckel B.N., Schoemaker R.C., Breimer D.D., van Gerven J.M., Cohen A.F. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:10–17. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 27.de Visser S.J., van der Post J.P., de Waal P.P., Cornet F., Cohen A.F., van Gerven J.M. Biomarkers for the effects of benzodiazepines in healthy volunteers. Br J Clin Pharmacol. 2003;55:39–50. doi: 10.1046/j.1365-2125.2002.t01-10-01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- 29.Baloh R.W., Sills A.W., Kumley W.E., Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25:1065–1070. doi: 10.1212/wnl.25.11.1065. [DOI] [PubMed] [Google Scholar]
- 30.Bittencourt P.R., Wade P., Smith A.T., Richens A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 1983;15:259–262. doi: 10.1111/j.1365-2125.1983.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Steveninck A.L. A microcomputer based system for recording and analysis of smooth pursuit and saccadic eye movements. Br J Clin Pharmacol. 1989;27:712P–713P. [Google Scholar]
- 32.Wright B.M. A simple mechanical ataxia-meter. J Physiol. 1971;218 Suppl:27P–28P. [PubMed] [Google Scholar]
- 33.Lezak M.D., Howieson D.B., Loring D.W. 4th ed. Oxford University Press; New York: 2004. Neuropsychological assessment. [Google Scholar]
- 34.de Haas S.L., Franson K.L., Schmitt J.A., Cohen A.F., Fau J.B., Dubruc C. The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA-A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J Psychopharmacol. 2009;23:625–632. doi: 10.1177/0269881108092595. [DOI] [PubMed] [Google Scholar]
- 35.Moser EB. Repeated measures modeling with PROC MIXED. SUGI 29. P188–29. 2004. Ref Type: Conference Proceeding.
- 36.Merkus P., Guchelaar H.J., Bosch D.A., Merkus F.W. Direct access of drugs to the human brain after intranasal drug administration? Neurology. 2003;60:1669–1671. doi: 10.1212/01.wnl.0000067993.60735.77. [DOI] [PubMed] [Google Scholar]
- 37.Farlow M.R. Clinical pharmacokinetics of galantamine. Clin Pharmacokinet. 2003;42:1383–1392. doi: 10.2165/00003088-200342150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Repantis D., Laisney O., Heuser I. Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review. Pharmacol Res. 2010;61:473–481. doi: 10.1016/j.phrs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Pepeu G., Giovannini M.G., Bracco L. Effect of cholinesterase inhibitors on attention. Chem Biol Interact. 2013;203:361–364. doi: 10.1016/j.cbi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Drachman D.A., Leavitt J. Memory impairment in the aged: Storage versus retrieval deficit. J Exp Psychol. 1972;93:302–308. doi: 10.1037/h0032489. [DOI] [PubMed] [Google Scholar]
- 41.Drachman D.A., Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 42.Bracco L., Bessi V., Padiglioni S., Marini S., Pepeu G. Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer's disease patients. J Alzheimers Dis. 2014;40:737–742. doi: 10.3233/JAD-131154. [DOI] [PubMed] [Google Scholar]
- 43.Samochocki M., Hoffle A., Fehrenbacher A., Jostock R., Ludwig J., Christner C. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.