Abstract
Introduction
The preclinical phase of Alzheimer's disease (AD) is optimal for identifying early pathophysiological events and developing prevention programs, which are shared aims of the ALFA project, including the ALFA registry and parent cohort and the nested ALFA+ cohort study.
Methods
The ALFA parent cohort baseline visit included full cognitive evaluation, lifestyle habits questionnaires, DNA extraction, and MRI. The nested ALFA+ study adds wet and imaging biomarkers for deeper phenotyping.
Results
A total of 2743 participants aged 45 to 74 years were included in the ALFA parent cohort. We show that this cohort, mostly composed of cognitively normal offspring of AD patients, is enriched for AD genetic risk factors.
Discussion
The ALFA project represents a valuable infrastructure that will leverage with different studies and trials to prevent AD. The longitudinal ALFA+ cohort will serve to untangle the natural history of the disease and to model the preclinical stages to develop successful trials.
Keywords: Alzheimer's disease, Dementia, Cohort studies, Cognition, Prevention, Biomarkers, Risk factors
1. Introduction
The increase in average life expectancy that has occurred in developed countries during the last 50 years has been accompanied by an increment in the prevalence of age-associated disorders. Alzheimer's disease (AD), more specifically, late-onset AD (LOAD) is the first cause of neurological disability in the elderly causing enormous social and economic burden in modern societies [1]. Currently, >45 million people suffer from dementia worldwide and with the progressive aging of the population, this figure is expected to increase to up to 130 million in 2050 [2]. Despite having described the AD clinicopathological hallmarks over a century ago, its precise etiology remains unknown. It is noteworthy that, to date, all clinical trials evaluating disease-modifying drugs performed have failed [3].
In the last decade, several in vivo AD biomarkers, such as β-amyloid (Aβ) and tau concentration in cerebrospinal fluid (CSF [4]), hippocampal atrophy [5], [6], temporoparietal hypometabolism [7], [8], and cerebral amyloid deposition measured by positron emission tomography (PET [9], [10]), have been extensively characterized. The results of these studies show that AD pathology develops for several years or even decades before clinical symptoms appear; this silent asymptomatic period of the disease is referred to as the preclinical stage of AD [11]. The detection of this preclinical stage opens up novel opportunities for the development of new therapeutic strategies. If new treatments capable of delaying the evolution of the disease and the appearance of dementia emerge in the next years, they will be especially useful during the preclinical phase of the disease [12]. In fact, as AD burden increases with the aging of the population, a treatment capable of delaying the onset of dementia by only a few years would have a tremendous impact on the social cost of the disease. Indeed, it has been calculated that a delay in dementia's onset of only 5 years could reduce a 33% of the economic cost of AD [13]. In this scenario, it has been hypothesized that, to increase the possibilities of success, drugs that have failed in trials performed on AD patients should be essayed in cognitively healthy subjects that are at elevated risk of developing AD. Even more, the preclinical stage could be the optimal timeframe to evaluate new therapeutic strategies directed against targets not only related to the amyloid cascade but also focused on delaying neuronal loss [14]. Moreover, interventional preventive strategies may help to better understand the relationship between the different medical, environmental, and genetic factors and the onset of symptoms.
The setup of preventive studies requires the identification of individuals with an increased risk of developing AD in the near future that are suitable to be recruited as asymptomatic subjects in clinical trials. With this in mind, a number of research projects have been designed and are currently ongoing such as the Wisconsin Registry for Alzheimer's Prevention program [15], the Adult Children Study [16], and the more recently developed PREVENT programme [17] and the longitudinal cohort study of the European Prevention of Alzheimer's Dementia (EPAD) project [18], among others.
Following the same rationale and aiming at increasing our knowledge of the pathophysiology and pathogenic factors emerging at early preclinical AD stages, the Barcelonaβeta Brain Research Center (BBRC) started the ALFA (for Alzheimer and Families) project for the prospective follow-up of a cohort of cognitively normal subjects, most of which are the offspring of AD patients. The ALFA project consists of the ALFA registry, the ALFA parent cohort, and the nested ALFA+ cohort study. The ALFA registry contains basic demographic data of persons willing to participate in future BBRC projects. The ALFA parent cohort is composed of cognitively normal participants aged between 45 and 74 years, who were administered a series of cognitive tests and from which we collected their clinical history and information related to lifestyle and a blood sample for further genetic analysis. The ALFA parent cohort will serve as the basis for the establishment of research protocols and studies, both observational and interventional, of preclinical participants at risk of cognitive impairment due to AD. Furthermore, it will be replenished over time through new recruitment and from the ALFA registry. Participants of the ALFA parent cohort will be offered the option of entering currently active projects at the BBRC such as EPAD [18] or future clinical trials and other research studies.
A subset of the ALFA parent cohort participants will be invited to take part in a nested longitudinal long-term study, named the ALFA+ study, in which a more detailed phenotyping will be performed. On top of a similar characterization as in the ALFA parent cohort, it will entail the acquisition of both wet (CSF, blood, and urine sample collection) and imaging (magnetic resonance imaging [MRI] and PET) biomarkers.
In this article, the ALFA parent cohort and the longitudinal ALFA+ study are introduced. Furthermore, a basic sociodemographic profile and a description of AD risk-associated variables of the ALFA parent cohort participants at baseline are also presented.
2. Methods
2.1. The ALFA parent cohort
The ALFA parent cohort represents a research platform that will supply related studies such as the longitudinal ALFA+.
2.1.1. Inclusion and exclusion criteria
Inclusion criteria were being cognitively normal Spanish and/or Catalan-speaking persons aged between 45 and 74 years that agreed with the study procedures and tests: clinical interview and questionnaires associated to risk factors, cognitive tests, a blood sample extraction for DNA analysis, and MRI. Furthermore, a close relative was involved in the volunteer's functional evaluation and both of them had to grant their consent. A high percentage of the individuals recruited were cognitively normal offspring of AD patients.
Exclusion criteria were (1) Cognitive performance falling outside the established cutoffs: Mini-Mental State Examination [19], [20] (MMSE) <26, or Memory Impairment Screen [21], [22] (MIS) < 6, or Time-Orientation subtest of the Barcelona Test II [23] (TO-BTII) <68, or semantic fluency [24], [25] (animals; SF) < 12. (2) Clinical Dementia Rating scale [26]; CDR > 0. (3) Major psychiatric disorders (according to DSM-IV-TR) or diseases that could affect cognitive abilities (current major depression or general anxiety disorder, bipolar disorder, schizophrenia, and dementia). The Goldberg Anxiety and Depression Scale [27], [28] (GADS) was used to screen for mood disorders. Whenever the scores were dubious, the rater assessed whether the subject met the DSM-IV-TR criteria for general anxiety disorder or major depressive episode and was excluded if this was the case. (4) Severe auditory and/or visual disorder, neurodevelopmental and/or psychomotor disorder. (5) Significant diseases that could currently interfere with cognition (renal failure on hemodialysis, liver cirrhosis, chronic lung disease with oxygen therapy, solid organ transplantation, fibromyalgia, active cancer in treatment, or any other disease the investigator considered could affect the participant's cognition). (6) Neurological disorders, such as Parkinson's disease, stroke, epilepsy under treatment with frequent seizures (>1/month) in the past year, multiple sclerosis, or other serious neurological diseases. (7) Brain injury that could interfere with cognition: history of head trauma with parenchymal lesion or extraaxial macroscopic large vessel ischemic stroke or hemorrhagic stroke, brain surgery, brain tumors, or other causes that could generate acquired brain damage such as cerebral chemotherapy or radiotherapy, and, finally, (8) suspected pattern of family history of autosomal dominant AD: three affected individuals in two different generations with an onset before the age of 60 years. The ALFA study inclusion and exclusion criteria are listed in Table 1.
Table 1.
The ALFA parent cohort inclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Abbreviations: CDR, Clinical Dementia Rating scale; MMSE, Mini-Mental State Examination; MIS, Memory Impairment Screen; TO-BTII, Time-Orientation subtest of the Barcelona Test II; SF, Semantic Fluency.
2.1.2. Baseline assessment
Recruited subjects were received at the BBRC, Barcelona, Spain, where they were evaluated by a trained neuropsychologist. Once the volunteer had given informed consent and the study inclusion criteria had been met, the subject's medical history and a number of sociodemographic characteristics were recorded, and the cognitive assessment was performed. A study nurse was in charge of taking the subject's blood pressure and height, weight, and waist diameter measurements as well as of obtaining a blood sample for further genetic characterization.
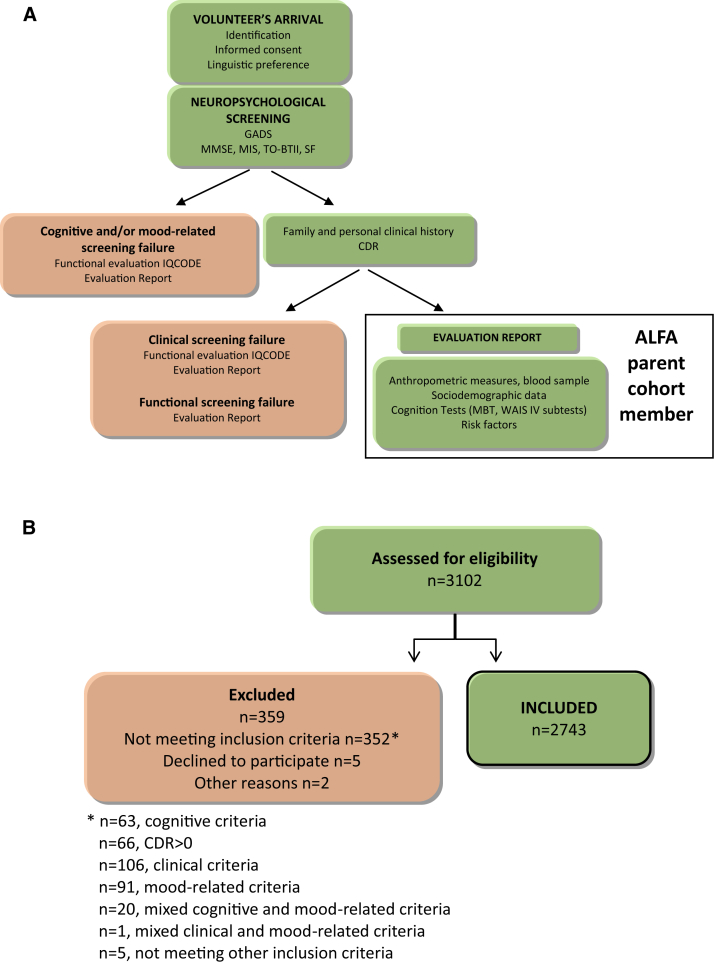
The baseline visit was structured as follows (Fig. 1A):
Fig. 1.
Schematic representation of the ALFA parent cohort baseline visit and screening process. (A) Regardless of their eligibility to enter or not the ALFA parent cohort, all subjects assessed received a specific report on the screening results. (B) Cognitive eligibility was assessed by the administration of the cognitive screening tests (MMSE, MIS, TO-BTII, and SF). The Goldberg Anxiety and Depression Scale was used to screen for mood disorders.
2.1.2.1. Revision of the study's inclusion criteria
To ensure compliance with the study inclusion criteria, an initial evaluation of the volunteers' neuropsychological status was performed through the screening tools mentioned above. Given the bilingual reality of the social context where the visit took place, participants were previously asked for their preferred language (Catalan or Spanish). A specific report on the screening results was given independently of whether the subject was eligible as an ALFA population member. The CDR was the last assessment tool at screening. In the case that the subject was previously excluded for any other reason, the functional assessment was performed through the IQCODE [29], [30] (provided it is shorter and there will not be a follow-up of the volunteer), and the result was included in the delivered report (Fig. 1A). Subjects excluded due to cognitive or functional performance falling outside the established cutoffs were advised to visit their primary care physician if they had previously noticed a significant change in their everyday mental performance. Those excluded due to mood-related criteria were also recommended to ask for medical advice.
After initial cognitive, functional, and mood status evaluation, subjects were asked about their familiar and personal medical history with special emphasis on psychiatric and neurological disorders, and they were excluded if a condition that was incompatible with their inclusion in the study was detected.
2.1.2.2. Neuropsychological evaluation
After initial evaluation, eligible subjects were administered an experimental cognitive test battery for the potential detection of early impairment in longitudinal follow-ups. This battery assessed episodic verbal memory [the Memory Binding Test [31], [32] (MBT)], psychomotor speed, visual processing, executive function, and non-verbal and verbal reasoning (Coding, Visual Puzzles, Digit Span, Matrix Reasoning, and Similarities of the WAIS IV [33]).
2.1.2.3. Sociodemographic data and medical history
Basic sociodemographic data were registered. All participants were asked about their familiar and personal medical history, and chronic medication use was recorded.
Participants' cognitive reserve was assessed with the administration of the Cognitive Reserve Questionnaire [34] consisting of 8 items, namely formal education, parental formal education, attendance to courses, occupation, musical education, languages spoken and frequency of reading, and cognitively stimulating activities.
2.1.2.4. Anthropometric measurements and blood sample extraction
Participants' weight, height, blood pressure, and waist and hip circumference were measured. Their weight and height measurements were used to calculate their body mass index (BMI) that allows their categorization in BMI ≤30 or BMI >30, cutoffs considered in the CAIDE (cardiovascular risk factors, aging, and incidence of dementia) dementia risk score [35]. Similarly, participants' systolic blood pressure measurements allow their classification in two groups (≤140 and >140) that imply distinct degrees of risk.
A 10 mL of blood were obtained in an EDTA tube and centrifuged to separate the plasma from the cellular fraction. Both fractions were immediately stored at −20°C at the Hospital del Mar facilities and further transferred to the Biobank of Institut Hospital del Mar d'Investigacions Mèdiques (Barcelona, Spain) and kept at −80°C until further use.
2.1.3. Apolipoprotein E genotyping
Total DNA was obtained from the blood cellular fraction by proteinase K digestion followed by alcohol precipitation. Using the following primers (APOE-F 5′-TTGAAGGCCTACAAATCGGAACTG-3′ and APOE-R 5′-CCGGCTGCCCATCTCCTCCATCCG-3′) samples were genotyped for two SNPs, rs429358 and rs7412, determining the possible APOE alleles: ε1, rs429358 (C) + rs7412 (T); ε2, rs429358 (T) + rs7412 (T); ε3, rs429358 (T) + rs7412 (C); and ε4, rs429358 (C) + rs7412 (C).
2.1.4. Other epidemiologic data collected
Additional information was obtained by means of self-reporting questionnaires. The EQ-5D-3L scale [36], [37] was used to obtain the participants' perceived quality of life; physical activity was measured with the Spanish short version of the Minnesota Leisure Time Physical Activity Questionnaire [38]; dietary habits were gathered by a 166-item food frequency questionnaire; alcohol, tobacco, and drug consumption were collected by a simplified version of the 2009 Spanish Ministry of Health National Plan on Drugs Questionnaire; sleeping habits questionnaire [39]; a revised version of the Eysenck personality questionnaire [40] (EPQ-R) was used to assess personality traits; and, finally, information related to social activity and religious practices was also gathered.
2.1.5. Data management and quality control
Data were systematically collected using two databases: one for the screening procedure that allowed the automatic creation of a report with the results (NeuroCog; www.neuro-cog.com), and the rest of the information was directly introduced in a data management system (OpenClinica; www.openclinica.com) that allowed the generation of reports for further analyses. Source documents (2095 of 3102, i.e., 67.5%) for the cognitive assessment were reviewed by a group of raters specially trained on monitoring and quality control procedures and all detected mistakes or inconsistencies were reported, registered, and accordingly amended.
Data quality control was performed for all the visited cases using complete reports of incidences within both e-databases and compared to our personal registries. Queries were corrected using source data verification. Moreover, the whole data were thoroughly reviewed by the data management team in search of inconsistencies or missing information and corrective measures were undertaken whenever possible and/or necessary. Additionally, and throughout the whole study, research personnel received newsletters on a regular basis where, among other information, the most frequent errors and inconsistencies were highlighted and measures to avoid them were established.
2.1.6. Statistical analyses
The chi square goodness of fit test was used to assess for statically significant differences between the frequencies of the APOE genotypes between the parent ALFA cohort and those reported in control subjects in the AlzGene database (http://www.alzgene.org/meta.asp?geneID=83). The same test (chi square) was used to assess for significant differences in the prevalence of clinical features and midlife CAIDE dementia risk scores between ALFA parent cohort members and the general population [35], [41]. SPSS 15.0 for Windows was used for all the analyses. Differences were considered to be significant at P < .05.
2.2. The ALFA+ study
A subset of participants of the ALFA parent cohort will be invited to join the ALFA+ study: a nested long-term longitudinal study aiming to understand the early pathophysiological changes of AD during its preclinical stage. The ALFA+ longitudinal cohort will include 440 individuals, which will be invited to participate based on their specific AD risk profile. This profile will be determined by an algorithm in which participants' AD parental history and APOE status, verbal episodic memory score and CAIDE score will be taken into consideration. In addition, participants will be stratified by age and gender.
The ALFA+ study will start during 2016 and complete follow-up visits will be performed every 3 years. Inclusion and exclusion criteria will be similar to the ALFA parent cohort ones (Table 1), with the addition of the acceptance to all the procedures described below.
2.2.1. Magnetic resonance imaging
Magnetic resonance images will be acquired in a 3.0-T scanner, and the protocol will include a 3D T1-weighted sequence with sub-millimetric isotropic voxel size and clinical T2-weighted sequences oriented along the hippocampal main axis to enable accurate segmentation of the hippocampal subfields. In addition, a high-angular-resolution diffusion imaging sequence and a 9-minute task-free functional MRI sequence will be acquired with isotropic spatial resolution. The ALFA platform will enable the setting up of additional imaging sub-studies including the implementation and validation of MRI research sequences.
2.2.2. Lumbar puncture
CSF will be collected by lumbar puncture between 9 and 12 a.m. in polypropylene tubes. Samples will be processed within 1 hour and will be centrifuged at 4°C for 10 minutes at 2000 g, stored in polypropylene tubes and frozen at −80°C. Core AD biomarkers (namely Aβ42, Aβ40, total Tau, and p tau) and other molecules of interest (such as YKL-40, neurogranin and Aβ oligomers) will be analyzed. Determinations will be carried out in Prof. Kaj Blennow's laboratory (Institute of Neuroscience and Physiology, Department of Psychiatry and Neurochemistry, The Sahlgrenska Academy at the University of Gothenburg, Sweden).
2.3. Amyloid positron emission tomography
A single intravenous dose of 185 MBq of 18F-Flutemetamol will be administered in a maximum volume of 10 mL with a maximum product mass of 6 ng/mL. The PET procedure, lasting 30 minutes (6 frames of 5 minutes), will start 90 minutes after radiotracer injection.
2.3.1. 18F- Fludeoxyglucose (FDG) PET
Individuals will receive a single intravenous standard dose of 370 MBq in a volume of 1 to 10 mL. The PET procedure, lasting 30 minutes (6 frames of 5 minutes), will start 45 minutes after radiotracer injection.
2.3.2. Ethical considerations
The ALFA study protocol was approved by the Independent Ethics Committee Parc de Salut Mar Barcelona and registered at Clinicaltrials.gov (Identifier: NCT01835717). It was conducted in accordance with the directives of the Spanish Law 14/2007, of 3rd of July, on Biomedical Research (Ley 14/2007 de Investigación Biomédica). As mentioned before, all participants in the ALFA study accepted the study procedures by signing an informed consent form and had a close relative volunteering to participate in the functional assessment procedure of the participant, who also granted his or her consent. A prerequisite for entering the ALFA+ study (Clinicaltrials.gov Identifier NCT02485730) will be accepting the study procedures and understanding that the CSF results will not be disclosed.
3. Results
To date, we have conducted the baseline visit of the ALFA study that includes, mainly, a sociodemographic, cognitive, and APOE haplotype characterization of the participants.
The 17th September 2012, a press conference was held where the main aims of the ALFA project and inclusion criteria of participants were explained. This resulted in around 5000 persons showing their interest in becoming part of the ALFA parent cohort and, from these, 3102 were visited and assessed for eligibility from April 2013 to November 2014 at the BBRC. Nearly 2000 individuals were not visited due to a variety of reasons, such as not complying with inclusion criteria (e.g. age range), were not reachable, had difficulties to attend the visit, and so forth.
As previously mentioned, and to ensure fulfillment of the study inclusion criteria, the 3102 assessed participants underwent a cognitive screening. Those subjects that fell outside the defined cutoffs (see Methods) were discontinued from the study. Furthermore, their family and personal medical history were also gathered to ensure compliance with the clinical inclusion criteria. In this regard, 359 volunteers were discontinued due to screening failures or voluntary dropouts, which resulted in the final inclusion of 2743 individuals aged between 45 and 74 years, mostly offspring of AD patients that constitute the ALFA parent cohort (a diagram of the participants' flow is presented in Fig. 1B). Of all the subjects that were excluded due to cognitive performance falling below the established cutoffs (n = 63), 21 obtained a MMSE <26, 22 a MIS <6, 14 a SF < 12, 3 a MMSE <26 and a MIS <6, one a MIS <6 and a SF < 12, and, finally, 2 did not reach the cutoffs for the MMSE, the MIS, and the SF tests. Regarding the cognitive performance of subjects excluded by both cognitive and mood-related criteria (n = 20), 10 obtained a MMSE <26, 7 a MIS <6, one a SF < 12, one a MIS <6, and a SF <12 and, finally, one did not reach the cutoffs for the MMSE, the MIS, and the TO-BTII tests.
The ALFA parent cohort members' mean (SD) age was of 55.8 (6.7) years, 36.8% were men and 63.2% were women with an average years of formal education of 13.3 (3.5) years. Regarding inclusion criteria, the mean MMSE score was of 29.0 (1.1), MIS score of 7.8 (0.5), TO-BTII score of 70.0 (0.1) and SF score of 22.5 (5.1). A baseline characterization of the ALFA cohort members stratified by age groups is summarized in Table 2. Concerning cognitive reserve, participants were grouped into four categories (C1–C4) being C1 the one corresponding to the lowest score and C4 to the highest one [34]. Most of the participants (69%) lay within the highest category (C4), 27% in C3 and only 4% and 1% were categorized as C2 and C1, respectively.
Table 2.
Baseline characterization of the ALFA parent cohort participants
| Descriptor | Total (n = 2743) | Group 1 (n = 500) | Group 2 (n = 666) | Group 3 (n = 631) | Group 4 (n = 617) | Group 5 (n = 265) | Group 6 (n = 64) |
|---|---|---|---|---|---|---|---|
| Age (y) | 55.8 (6.7) | 46.7 (1.4) | 51.4 (1.5) | 56.3 (1.5) | 61.5 (1.6) | 66.1 (1.5) | 71.2 (1.4) |
| Men/women (%) | 36.8/63.2 | 33.6/66.4 | 36.9/63.1 | 34.4/65.6 | 42.9/57.1 | 34.3/65.7 | 35.9/64.1 |
| Education (y) | 13.3 (3.5) | 14.0 (3.3) | 13.7 (3.4) | 13.1 (3.4) | 13.0 (3.7) | 12.7 (3.6) | 12.6 (3.5) |
| GADS-A (0-9)∗ | 0.6 (1.2) | 0.7 (1.3) | 0.7 (1.4) | 0.6 (1.1) | 0.4 (0.9) | 0.5 (1.0) | 0.4 (0.9) |
| GADS-D (0-9)∗ | 0.2 (0.7) | 0.2 (0.6) | 0.2 (0.7) | 0.2 (0.6) | 0.1 (0.6) | 0.2 (0.8) | 0.3 (0.9) |
| MMSE (0–30)∗ | 29.0 (1.1) | 29.2 (1.0) | 29.1 (1.0) | 29.0 (1.1) | 29.0 (1.1) | 28.9 (1.2) | 28.6 (1.1) |
| MIS (0–8)∗ | 7.8 (0.5) | 7.8 (0.4) | 7.8 (0.5) | 7.8 (0.5) | 7.7 (0.6) | 7.6 (0.6) | 7.7 (0.5) |
| TO-BTII (0–70)∗ | 70.0 (0.1) | 70 (0.0) | 70.0 (0.0) | 70.0 (0.1) | 70.0 (0.1) | 70.0 (0.0) | 70.0 (0.0) |
| SF (0–…)∗ | 22.5 (5.1) | 24.0 (5.0) | 23.3 (5.1) | 22.5 (5.0) | 21.2 (4.9) | 20.9 (4.8) | 19.9 (4.2) |
| MBT-TPR (0–32)† | 23.7 (5.3) | 24.8 (5.0) | 24.2 (5.4) | 23.6 (5.0) | 22.9 (5.7) | 22.4 (5.0) | 22.8 (5.1) |
| Coding-TS (0–135)† | 65.8 (14.8) | 73.9 (12.4) | 70.7 (13.2) | 65.8 (13.6) | 60.1 (14.0) | 54.9 (12.2) | 51.0 (13.5) |
| VP (0–26)† | 13.3 (4.3) | 15.0 (4.4) | 14.2 (4.2) | 13.3 (4.0) | 12.0 (3.9) | 11.6 (3.9) | 11.0 (3.2) |
| DS-TS (0–48)† | 24.8 (5.2) | 25.6 (5.3) | 25.2 (5.1) | 24.8 (5.4) | 24.2 (5.0) | 23.8 (5.1) | 22.1 (3.6) |
| Matrix R (0–26)† | 16.6 (4.4) | 18.2 (4.0) | 17.8 (4.0) | 16.4 (4.2) | 15.4 (4.3) | 14.5 (4.6) | 13.0 (4.1) |
| Similarities (0–36)† | 22.1 (4.7) | 22.8 (4.6) | 22.7 (4.6) | 22.2 (4.7) | 21.5 (4.7) | 20.9 (4.9) | 20.4 (5.1) |
Abbreviations: GADS-A, Goldberg anxiety scale; GADS-D, Goldberg depression scale; MMSE, Mini-Mental State Examination; MIS, Memory Impairment Screen; TO-BTII, Time-Orientation subtest of the Barcelona Test II; SF, Semantic Fluency (animals); MBT-TPR, Memory Binding Test Total Paired Recall; Coding-TS, Coding Total Score; VP, Visual Puzzles; DS-T, Digit Span Total; Matrix R, Matrix Reasoning.
NOTE. Group 1, 45–49 years; group 2, 50–54 years; group 3, 55–59 years; group 4, 60–64 years; group 5, 65–69 years; group 6, 70–74 years. Possible range scores are indicated in brackets.
screening tests.
Experimental test battery.
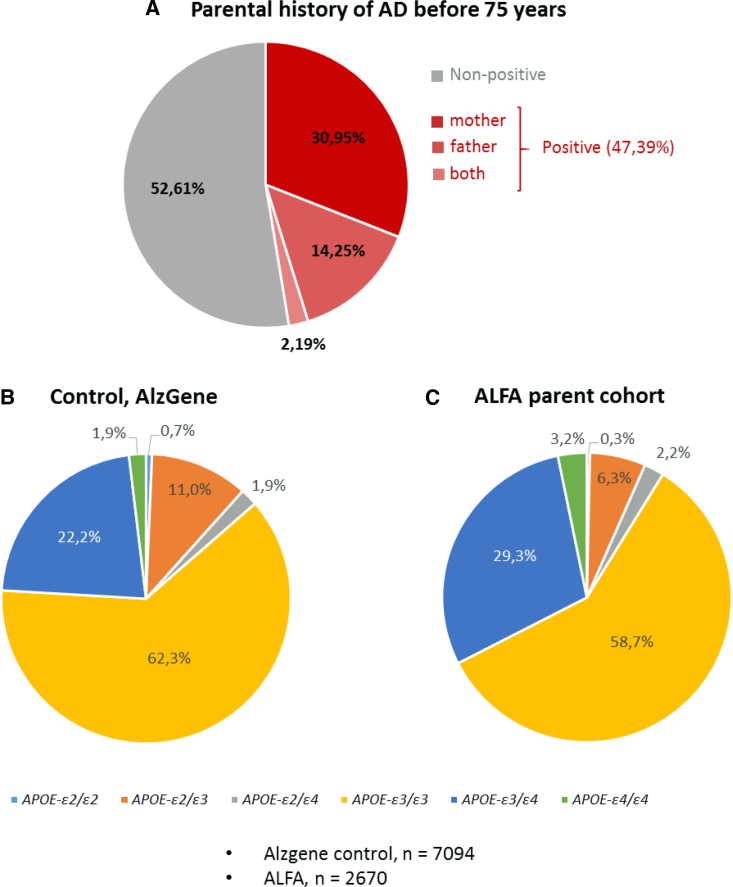
The participants' family history of AD was recorded during baseline visit. In particular, we registered who, their mother and/or father, had been diagnosed with AD. In this regard, 86.3% of the participants had at least one of their parents that had suffered AD. When considering a more strict family history encoding, it is remarkable that 47.4% of the ALFA study participants had at least one of their parents that had been diagnosed with AD before the age of 75 years. Specifically, in 2.2% of the volunteers both parents had been diagnosed with AD (at least one of them before the age of 75 years), in 14.3% their father had been diagnosed with AD, and 31.0% of the ALFA study participants' mother had been diagnosed with AD before the age of 75 years (Fig. 2A).
Fig. 2.
Nonmodifiable risk factors for Alzheimer's disease (AD). (A) Schematic representation of the ALFA study participants' parental history of AD before the age of 75 years. Percentage of APOE genotypes in the ALFA parent cohort population (C) compared to the control (B).
A higher frequency of the APOE-ε4 allele was found in ALFA parent cohort members than in the general population (19% and 14%, respectively; P < .001). Specifically, statistically significant differences were found between the APOE-ε3/ε4 and APOE-ε4/ε4 percentages in the ALFA study group and the control (P < .001). Remarkably, no significant differences were found between the percentage of individuals with the APOEε2/ε4 genotype in the ALFA and control groups (P = .241). Figure 2 shows the percentage of each of the APOE genotypes found in our population (C) compared to cognitively normal individuals' percentages taken from the AlzGene database (B). Of 2670 ALFA members whose genotype could be determined, 9 were APOE-ε2/ε2 homozygotes, 167 were APOE-ε2/ε3 heterozygotes, 59 were APOE-ε2/ε4 heterozygotes, 1567 were APOE-ε3/ε3 homozygotes, 782 were APOE-ε3/ε4 heterozygotes and, finally, 86 were APOE-ε4/ε4 homozygotes.
Other variables of interest obtained during the baseline visit were those lifestyles and cardiovascular risk factors that had been previously suggested as modifiable risk factors that may increase or decrease the risk of cognitive impairment and dementia. These include cardiovascular and endocrino-metabolic comorbidities, the participants' level of physical activity and their smoking habits [35], [42]. Cardiovascular comorbidities were self-reported during baseline visit by 28.4% of the subjects, being current hypertension the most prevalent (64.5%). The 42.4% of the study participants self-reported endocrino-metabolic comorbidities: of these, 69.8% reported current dyslipidemia and 9.8% were currently diagnosed with diabetes. 79.8% of the ALFA cohort members had a BMI ≤30 and 73.6% a measured systolic blood pressure ≤140, both of these ranges are associated with a lower risk of developing cognitive impairment and/or cardiovascular disease. Their physical activity level and smoking habits were gathered by means of self-reported questionnaires that were completed off-site by 84.1% and 84.7% of the ALFA population, respectively. Of our population, 65.4% fell in the “active” category (this is at least 150 minutes per week of moderate exercise or 75 minutes per week of vigorous exercise as recommended by current guidelines), and 34.6% were categorized as inactive; 17.5% of the ALFA population had never smoked, 57.6% had quit smoking for more than a year ago and, finally, 24.9% of them fell in the smokers' category. The main clinical and lifestyle features of the ALFA cohort are compared to those of the Spanish general population [41] in Table 3.
Table 3.
Clinical features of the ALFA parent cohort participants
| Descriptor | General population |
ALFA population |
||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Hypertension∗ | 47.0 | 39.0 | 51.6 | 34.2 |
| Diabetes† | 13.0 | 10.0 | 6.3 | 3.5 |
| Dyslipidemia† | 35 | 32 | 35.8 | 29.4 |
| BMI 25–29.9 | 51.0 | 36.0 | 52.3 | 36.5 |
| BMI ≥30 | 29.0 | 29.0 | 24.3 | 18.0 |
| Smoker | 33.0 | 21.0 | 23.6 | 25.7 |
| Ex-smoker‡ | 36.0 | 14.0 | 61.1 | 55.6 |
| Nonsmoker | 32.0 | 66.0 | 15.3 | 18.7 |
NOTE. Percentages are shown.
Self-reported hypertension + systolic/diastolic ≥140/90 mm Hg.
Self-reported.
For longer than a year. With the exception of BMI 25–29.9, the rest of comparisons were statistically significantly different (P < .05).
Once the main nonmodifiable risk factors for AD (namely parental history and APOE genotype; Figure 2) and cardiovascular risk factors (Table 3) had been defined for the ALFA population, we set out to determine their CAIDE dementia risk score, a well-established approach to assess the probability of dementia in late life according to the risk score categories in middle age [35]. Participants risk scores were calculated using both CAIDE statistical models that differ in whether they do not take (model I) or take (model II) the APOE status into consideration. Table 4 shows the percentage of ALFA cohort participants found in each of the CAIDE score ranges as well as their comparison to the general population percentages obtained from the original publication [35].
Table 4.
CAIDE dementia risk score analyses of the ALFA parent cohort
| CAIDE score model I |
CAIDE score model II |
||||
|---|---|---|---|---|---|
| Score | CAIDE population, n = 1350 | ALFA population, n = 2302 | Score | CAIDE population, n = 1318 | ALFA population, n = 2247 |
| 0–5 | 29.7 | 45.0 | 0–5 | 22.2 | 29.2 |
| 6–7 | 20.0 | 29.1 | 6–8 | 27.5 | 41.1 |
| 8–9 | 23.1 | 18.4 | 9–10 | 20.0 | 17.5 |
| 10–11 | 18.2 | 6.2 | 11–12 | 17.2 | 9.3 |
| 12–15 | 9.0 | 1.3 | 13–18 | 13.1 | 2.9 |
NOTE. *P < .001. Percentages are shown.
The ALFA+ longitudinal cohort study will include a subset of 440 participants. It will be enriched in risk factors for AD such as age, family history of the disease, and APOE-ε4 genotype and matched by gender.
4. Discussion
Accumulating data from biomarker and imaging studies support the existence of a preclinical, asymptomatic phase of AD [11], [43], [44], [45], [46], [47]. In addition, disease-modifying pharmacological interventions on both mild moderate and late-stage AD persons have yet to produce significant clinical benefits [48]. In this scenario, intervention studies and secondary prevention programs on asymptomatic at risk individuals emerge as highly relevant, before substantial irreversible neuronal network dysfunction and loss have occurred. To this end, the identification of AD pathology through biomarkers will contribute to the efficient design and performance of preclinical AD trials and prevention studies.
Taking all the above into consideration and with the long-term aim of identifying risk factors that may predispose to suffering or be indicative of AD in asymptomatic individuals, we set out to establish a study of cognitively normal participants, enriched by family history of AD. The ALFA parent cohort is currently composed of 2743 individuals representing a highly valuable research platform. From this population, specific subgroups will be invited to form part of more technological although smaller sized studies. One of these, the nested ALFA+ cohort study has been designed to include, besides the already described characterization (neuropsychological evaluation, clinical history, APOE genotyping and lifestyle questionnaires), blood and CSF sampling, FDG and amyloid PET and structural and functional MRI. These procedures will be repeated every 3 years.
Regarding the neuropsychological assessment of the ALFA parent cohort participants, the cognitive screening tests show very similar descriptive data among age groups. However, semantic fluency tests constitute an exception to this trend as they show a tendency to decrease with advancing age (Table 2). This is consistent with previous findings that relate cognitive aging with lower performances in fluency tasks [25], [49]. Furthermore, a clear ceiling effect is observable for most of the cognitive tests used for screening purposes: for example, the mean score for the MMSE is of 29 (of 30), 7.8 (of 8.0) for the MIS and 70 (of 70) for the TO-BTII (Table 2). On the other hand, the neuropsychological tests included in the cognitive experimental battery (namely the MBT and the WAIS-IV subtests) are more complex showing no ceiling effects, as shown for the MBT when validated [32], [50]. In fact, the MBT was designed with the intention of overcoming some limitations in the sensitivity of the widely used FCSRT in the detection of subtle episodic memory changes due to AD [31]. On the other hand, the initial purpose of the WAIS subtests is to assess intelligence, which, by definition, has a wide range of performance in the population. Indeed, our results suggest that both the MBT and WAIS subtests may be useful in detecting subtle intra-individual changes in cognitive performance in longitudinal assessments of individuals performing within normal psychometrical ranges and, thus, to serve as cognitive markers of preclinical AD. However, repeated exposure to the same test might lead to practice effects leading to improved performance in subsequent assessments. These improvements may mask subtle preclinical changes, but, on the other hand, reduced practice effects have actually been suggested as a cognitive marker of stage-III of preclinical AD [51].
The thorough cognitive assessment performed in the ALFA parent cohort baseline visit allows us to use participants' scores in the selection algorithm used to assess individuals' AD risk profiles and invite them to form part of currently active projects at the BBRC such as EPAD [18], the ALFA+ longitudinal cohort or future trials and studies. In addition, the comparison of scores between those tests that are common in the ALFA parent cohort baseline visit and the first visit of future studies and/or trials will allow us to obtain longitudinal data with the ultimate aim of assessing cognitive decline.
Family studies have shown that having a parental history of AD represents a risk factor for LOAD [52], [53] and the biggest genetic susceptibility factor is the APOE-ε4 allele [54]. As previously mentioned, the ALFA parent cohort is mainly composed of AD patients' offspring (47.4% of them are adult children of AD patients that had shown signs of cognitive impairment before the age of 75) and a higher frequency of APOE-ε4 alleles than in the general population may be expected. In agreement with this, the frequency of APOE-ε3/ε4 and APOE-ε4/ε4 genotypes found among ALFA parent cohort members is significantly higher than that reported for the control population (Fig. 2). Therefore, our results confirm that we have established a research platform that is enriched in genetic risk factors for AD. As a consequence, the proportion of patients presenting altered biomarkers, neuroimaging changes and eventually the development of cognitive decline is also expected to be higher, which will be evaluated in the longitudinal assessments and, specifically, in the ALFA+ study.
In summary, the ALFA parent cohort represents a valuable infrastructure of middle age participants representing the whole spectrum of risk that will leverage with different projects and trials to prevent AD. The longitudinal ALFA+ cohort, through deep phenotyping of middle age subjects, is aimed at studying the earliest stages of preclinical AD, which will be useful to understand early pathophysiological changes together with modeling the preclinical stages to develop successful trials.
4.1. Future clinical applications
Throughout the ALFA project, biological and neuroimaging markers present in the AD preclinical phase will be detected, preceding or informing about the presence of brain Aβ deposition. Furthermore, biomarkers used and validated in these studies may constitute endpoints for the construction of large population interventional randomized controlled trials, which will lead to the development of pharmacological and nonpharmaceutical interventions targeting individuals at risk for AD.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources. Cohort studies aimed at identifying early pathophysiological events of Alzheimer's disease (AD) and developing prevention programs are cited throughout the article.
-
2.
Interpretation: The ALFA project consists of the ALFA registry, the ALFA parent cohort, and the nested ALFA+ study. The parent cohort includes 2743 participants and is enriched for family history of AD and APOE-ε4 genotypes.
-
3.
Future directions: The ALFA parent cohort represents a valuable infrastructure of middle age participants representing the whole spectrum of risk that will leverage with different projects and trials to prevent AD. The longitudinal ALFA+ cohort, through deep phenotyping of middle age subjects, will be useful to understand early pathophysiological changes together with modeling the preclinical stages to develop successful trials.
Acknowledgments
The authors express their most sincere gratitude to the ALFA project volunteers, without whom this research would have not been possible. The research leading to these results has received funding from “la Caixa” Foundation. Additional funding was obtained from: Fondo de Investigación Sanitaria (FIS), Instituto de Salud Carlos III under grant PI12/00326. Biological samples are stored at the MARBiobanc (Barcelona) that receives funding from the Instituto de Salud Carlos III FEDER (RD09/0076/00036). Juan D. Gispert holds a “Ramón y Cajal” fellowship (RYC-2013-13054).
The authors are indebted to the collaborators who have supported our team in the design and implementation of the ALFA project (Carme Junqué, Raquel Sánchez-Valle, Jesús Pujol, Carmen Ayuso, Jordi Sunyer, Jordi Alonso, and Jordi Peña-Casanova), and the scientific international committee (Bruno Dubois, Zaven Khachaturian, Javier Nieto, Ronald Petersen, Hugo Vanderstichele, and Bengt Winblad). We thank Neil Forrest for carefully proof reading this article and the reviewers of this article for their valuable comments.
In memory of Mrs. Maria Thos i Negre, the authors would like to express their gratitude for her donation to the Pasqual Maragall Foundation for research on Alzheimer's prevention.
Contributor Information
José Luis Molinuevo, Email: jlmolinuevo@fpmaragall.org.
Jordi Camí, Email: jordicami@fpmaragall.org.
References
- 1.Takizawa C., Thompson P.L., van Walsem A., Faure C., Maier W.C. Epidemiological and economic burden of Alzheimer's disease: a systematic literature review of data across Europe and the United States of America. J Alzheimers Dis. 2015;43:1271–1284. doi: 10.3233/JAD-141134. [DOI] [PubMed] [Google Scholar]
- 2.World Alzheimer Report 2015 . Alzheimer's Disease International (ADI); London: 2015. The global impact of dementia: an analysis of pervalence, incidence, cost and trends. [Google Scholar]
- 3.Schneider L.S., Mangialasche F., Andreasen N., Feldman H., Giacobini E., Jones R. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molinuevo J.L., Blennow K., Dubois B., Engelborghs S., Lewczuk P., Perret-Liaudet A. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Bernard C., Helmer C., Dilharreguy B., Amieva H., Auriacombe S., Dartigues J.F. Time course of brain volume changes in the preclinical phase of Alzheimer's disease. Alzheimers Dement. 2014;10:143–151.e1. doi: 10.1016/j.jalz.2013.08.279. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M., Tanabe H., Nakagawa Y., Kazui H., Oi H., Yamazaki H. MRI-based quantitative assessment of the hippocampal region in very mild to moderate Alzheimer's disease. Neuroradiology. 1994;36:7–10. doi: 10.1007/BF00599184. [DOI] [PubMed] [Google Scholar]
- 7.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewers M., Insel P.S., Stern Y., Weiner M.W. Cognitive reserve associated with FDG-PET in preclinical Alzheimer disease. Neurology. 2013;80:1194–1201. doi: 10.1212/WNL.0b013e31828970c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson K.A., Sperling R.A., Gidicsin C.M., Carmasin J.S., Maye J.E., Coleman R.E. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. 2013;9(5 Suppl):S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doraiswamy P.M., Sperling R.A., Coleman R.E., Johnson K.A., Reiman E.M., Davis M.D. Amyloid-beta assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79:1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vellas B., Aisen P.S., Sampaio C., Carrillo M., Scheltens P., Scherrer B. Prevention trials in Alzheimer's disease: an EU-US task force report. Prog Neurobiol. 2011;95:594–600. doi: 10.1016/j.pneurobio.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Changing the Trajectory of Alzheimer's Disease: How a Treatment by 2025 Saves Lives and Dollars. Alzheimer's Association; 2010. [Google Scholar]
- 14.Vellas B., Carrillo M.C., Sampaio C., Brashear H.R., Siemers E., Hampel H. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Sager M.A., Hermann B., La Rue A. Middle-aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 16.Coats M., Morris J.C. Antecedent biomarkers of Alzheimer's disease: the adult children study. J Geriatr Psychiatry Neurol. 2005;18:242–244. doi: 10.1177/0891988705281881. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie C.W., Wells K., Ritchie K. The PREVENT research programme–a novel research programme to identify and manage midlife risk for dementia: the conceptual framework. Int Rev Psychiatry. 2013;25:748–754. doi: 10.3109/09540261.2013.869195. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie C.W., Molinuevo J.L., Truyen L., Satlin A., Van der Geyten S., Lovestone S. Development of interventions for the secondary prevention of Alzheimer's dementia: the European Prevention of Alzheimer's Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–186. doi: 10.1016/S2215-0366(15)00454-X. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Blesa R., Pujol M., Aguilar M., Santacruz P., Bertran-Serra I., Hernandez G. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia. 2001;39:1150–1157. doi: 10.1016/s0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 21.Buschke H., Kuslansky G., Katz M., Stewart W.F., Sliwinski M.J., Eckholdt H.M. Screening for dementia with the memory impairment screen. Neurology. 1999;52:231–238. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Bohm P., Pena-Casanova J., Gramunt N., Manero R.M., Terron C., Quinones-Ubeda S. Spanish version of the Memory Impairment Screen (MIS): normative data and discriminant validityNeurologia. 2005;20:402–411. [PubMed] [Google Scholar]
- 23.Quinones-Ubeda S. Ramon Llull University; Barcelona: 2009. Psychology. [Google Scholar]
- 24.Ramier A.M., Hécaen H. Role respectif des atteintes frontales et de la lateralisation lesionnelle dans les deficits de la fluence verbale. Rev Neurol (Paris) 1970;123:17–22. [PubMed] [Google Scholar]
- 25.Pena-Casanova J., Quinones-Ubeda S., Gramunt-Fombuena N., Quintana-Aparicio M., Aguilar M., Badenes D. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol. 2009 Jun;24:395–411. doi: 10.1093/arclin/acp042. [DOI] [PubMed] [Google Scholar]
- 26.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg D., Bridges K., Duncan-Jones P., Grayson D. Detecting anxiety and depression in general medical settings. BMJ. 1988;297:897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monton C., Perez Echeverria M.J., Campos R., Garcia Campayo J., Lobo A. Anxiety scales and Goldberg's depression: an efficient interview guide for the detection of psychologic distressAten Primaria. 1993;12:345–349. [PubMed] [Google Scholar]
- 29.Morales Gonzalez J.M., Gonzalez-Montalvo J.I., Del Ser Quijano T., Bermejo Pareja F. Validation of the S-IQCODE: the Spanish version of the informant questionnaire on cognitive decline in the elderlyArch Neurobiol (Madr) 1992;55:262–266. [PubMed] [Google Scholar]
- 30.Jorm A.F., Jacomb P.A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 31.Buschke H. The rationale of the Memory Binding Test. In: Nilsson L.G., Ohta N., editors. Dementia and Memory. Psychology Press; New York: 2014. pp. 55–71. [Google Scholar]
- 32.Gramunt N., Buschke H., Sanchez-Benavides G., Lipton R.B., Pena-Casanova J., Dieguez-Vide F. Reference Data of the Spanish Memory Binding Test in a Midlife Population from the ALFA STUDY (Alzheimer's and Family) J Alzheimers Dis. 2015;48:613–625. doi: 10.3233/JAD-150237. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Pearson; Madrid: 2012. WAIS-IV, Escala de inteligencia de Wechsler para adultos-IV. [Google Scholar]
- 34.Rami L., Valls-Pedret C., Bartrés-Faz D., Caprile C., Solé-Padullés C., Castellvi M. Cognitive reserve questionnaire. Scores obtained in a healthy elderly population and in one with Alzheimer's disease. Rev Neurol. 2011;52:195–201. [PubMed] [Google Scholar]
- 35.Kivipelto M., Ngandu T., Laatikainen T., Winblad B., Soininen H., Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 36.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 37.The EuroQol Group EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 38.Elosua R., Garcia M., Aguilar A., Molina L., Covas M.I., Marrugat J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire In Spanish Women. Investigators of the MARATDON Group. Med Sci Sports Exerc. 2000;32:1431–1437. doi: 10.1097/00005768-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Haro J.M., Palacin C., Vilagut G., Martinez M., Bernal M., Luque I. Prevalence of mental disorders and associated factors: results from the ESEMeD-Spain studyMed Clin (Barc) 2006;126:445–451. doi: 10.1157/13086324. [DOI] [PubMed] [Google Scholar]
- 40.Eysenck H., Eysenck S.B., EPQ-R . 10th ed. TEA ediciones; Madrid: 2001. Cuestionario revisado de personalidad de Eysenck. [Google Scholar]
- 41.Grau M., Elosua R., Cabrera de Leon A., Guembe M.J., Baena-Diez J.M., Vega Alonso T. Cardiovascular risk factors in Spain in the first decade of the 21st Century, a pooled analysis with individual data from 11 population-based studies: the DARIOS studyRev Esp Cardiol. 2011;64:295–304. doi: 10.1016/j.recesp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 43.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knopman D.S., Jack C.R., Jr., Wiste H.J., Weigand S.D., Vemuri P., Lowe V. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mormino E.C., Betensky R.A., Hedden T., Schultz A.P., Ward A., Huijbers W. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–1767. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiman E.M., Quiroz Y.T., Fleisher A.S., Chen K., Velez-Pardo C., Jimenez-Del-Rio M. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 48.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas J.A., Ivnik R.J., Smith G.E., Bohac D.L., Tangalos E.G., Graff-Radford N.R. Mayo's older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 50.Gramunt N., Sanchez-Benavides G., Buschke H., Lipton R.B., Masramon X., Gispert J.D. Psychometric Properties of the Memory Binding Test: Test-Retest Reliability and Convergent Validity. J Alzheimers Dis. 2016;50:999–1010. doi: 10.3233/JAD-150776. [DOI] [PubMed] [Google Scholar]
- 51.Hassenstab J., Ruvolo D., Jasielec M., Xiong C., Grant E., Morris J.C. Absence of practice effects in preclinical Alzheimer's disease. Neuropsychology. 2015;29:940–948. doi: 10.1037/neu0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green R.C., Cupples L.A., Go R., Benke K.S., Edeki T., Griffith P.A. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 53.Cupples L.A., Farrer L.A., Sadovnick A.D., Relkin N., Whitehouse P., Green R.C. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL study. Genet Med. 2004;6:192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 54.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]