Abstract
Background & objectives:
Gestational diabetes mellitus (GDM) can cause adverse perinatal outcome if not treated. Although insulin therapy has been the main treatment modality over decades but considering its cost and parenteral mode of administration, it does not seem to be appropriate, especially in low-resource settings. The objective of this study was to evaluate the role of metformin in GDM and know its efficacy as well as adverse effect on foetus and mother.
Methods:
All pregnant women with GDM who were not controlled on medical nutrition therapy and required metformin therapy were included in the study. Careful monitoring of blood sugar was done. Development of any maternal or foetal complications and adverse effect were recorded.
Results:
A total of 2797 pregnant women were screened, of whom 233 (8.3%) were found to have GDM. Of the 64 women with GDM (28.7%) who required metformin therapy, majority (93.8%) achieved blood sugar control, whereas three (4.7%) women failed. Caesarean section rate was 54 per cent, and 15.6 per cent neonates were large for gestational age. Only two (3.1%) women had gastrointestinal side effects which were minor and got resolved with time. No case of hypoglycaemia or perinatal mortality was reported.
Interpretation & conclusions:
Our findings indicate that metformin may be used as a safe and effective oral hypoglycaemic agent in GDM, especially in low-resource settings where cost, storage and compliance are logistic issues. However, long-term follow up studies are needed to solve issues related to its safety in pregnancy.
Key words: Gestational diabetes mellitus, insulin, low resource metformin, oral hypoglycaemic agent
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance which is detected for the first time during pregnancy1. The prevalence of GDM is increasing globally, and it varies according to the diagnostic criteria used and the population screened. Uncontrolled GDM may lead to a number of maternal, foetal and neonatal complications such as increased risk of infection, pregnancy-induced hypertension (PIH), preterm labour, polyhydramnios, macrosomia, higher chances of operative delivery, development of type 2 diabetes in later life, respiratory distress syndrome (RDS), large for gestational age (LGA) and other metabolic complications such as hypoglycaemia, polycythaemia and hypocalcaemia2,3,4.
It has been now proved that adequate blood sugar control can significantly improve perinatal outcome5,6. Although lifestyle modification including diet control and exercise is considered the first line of treatment, insulin needs to be added if the blood sugar is not optimally controlled with the above measures. Insulin does not cross the placenta; therefore, historically, it has been the only choice to establish euglycaemia in pregnant women when the diet therapy fails to achieve blood sugar control. However, considering the cost and parenteral mode of administration, it doesnot seem to be appropriate, especially in low-resource settings with poor literacy and insulin storage facilities. Moreover, multiple daily injections and risk of hypoglycaemia and weight gain make it undesirable for many patients.
Metformin is a second-generation biguanide which acts as an insulin sensitizer that reduces insulin resistance and basal plasma insulin levels. It also suppresses hepatic glucose output, increases insulin-mediated glucose uptake and utilization and improves lipid profile by decreasing triglyceridemia, fatty acid and low-density lipoprotein cholesterol levels whereas slightly increasing high-density lipoprotein cholesterol and decreasing intestinal absorption of glucose7. As metformin crosses placenta, there are concerns regarding its safety in pregnant women, but it has not been reported to cause any harm in animal or human studies done so far8. Unlike sulphonylureas, metformin does not cause hypoglycaemia and foetal hyperinsulinemia9. Metformin has the advantage of its oral route of administration, low cost and better patient acceptability. Therefore, it may be a preferred alternative to insulin therapy. With this background, this prospective non-randomized interventional study was conducted to evaluate the efficacy and safety of metformin among pregnant women with GDM.
Material & Methods
This prospective non-randomized interventional study was conducted among the pregnant women attending the antenatal clinic at department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, from October 1, 2011 to August 30, 2014. All women were screened by 75 g oral glucose tolerance test first in early pregnancy and then at 24 wk of gestation if found negative earlier. Cut-off values were taken according to American Diabetes Association (ADA) criteria 2011 (fasting plasma glucose ≥92, one hour ≥180 and two hour ≥153 mg/dl)10. Women were labelled as having GDM if one or more values were found abnormal. All women with GDM were started with medical nutrition therapy (MNT) and exercise. Four-point blood sugar profile (fasting and post-meals) was done after two weeks of starting MNT. Those who were not controlled on diet (≥2 abnormal plasma glucose values: fasting >95 mg/dl and two-hour post-meal value >120 mg/dl) were started on metformin therapy. Informed written consent was obtained from all the women who were started on metformin after explaining its possible side effects. The exclusion criteria included those with grossly deranged liver or kidney function tests, history of any prior adverse reaction (lactic acidosis) or allergy to metformin or any history of major congenital anomaly in previous pregnancies. Ethical clearance for this study was obtained from Ethics Committee of the Institute (CTRI/2011/08/001956).
Metformin was started with 500 mg tablet once 15 min before breakfast, and blood sugar profile was done after one week. If two or more values were deranged, the dose was increased to twice daily dose and then increased gradually upto a maximum of 2500 mg according to the requirement. Liver and kidney function tests were repeated at monthly interval for monitoring of side effects. Routine antenatal care was provided. Home blood sugar monitoring was done every two weekly with seven-point blood sugar profile. Dose adjustments were made based on the glycaemic status. Antenatal surveillance was started with biophysical profile from 28 wk and non-stress test from 32 wk period of gestation (POG). Obstetric ultrasound was performed once at 32 wk POG for foetal growth parameters, liquor and placenta and was repeated if required. Development of any antenatal complications such as PIH, preterm labour, chorioamnionitis, polyhydramnios, cholestasis of pregnancy, macrosomia or intrauterine growth restriction was also recorded.
Mode of delivery was individualized according to the obstetric indication. Those who failed to go into spontaneous labour were induced at 38 wk POG. All the neonates were assessed at birth by neonatologist and monitored for the development of hypoglycaemia and other metabolic complications including hyperbilirubinemia, polycythaemia, hypokalaemia and development of RDS. Neonate was considered LGA if birth weight was >90th percentile based on growth standard11. Hypoglycaemia was defined as blood sugar value <40 mg/dl. Hyperbilirubinemia was defined as serum bilirubin of at least 12 mg/dl within first seven days of birth. Polycythaemia was defined as haematocrit >60 per cent measured in cord blood of all infants. RDS was defined as the need for respiratory support with oxygen, continuous positive pressure or intermittent positive pressure ventilation during first 24 h after delivery. In addition, neonatal intensive care unit admission rates, congenital anomalies, birth injuries, exchange transfusions and the need for phototherapy were also documented.
Statistical analysis
An earlier study conducted in India showed that GDM prevalence based on single abnormal value was 10.87 per cent12. Considering this value as a hypothetical prevalence by allowing 2 per cent error margin, the required sample size for 80 per cent power at 5 per cent level of significance was calculated to be 1919, and by allowing a 15 per cent dropout rate a minimum sample size was fixed as 2210. Data were analyzed using SPSS, IBM Version 19.0 (IBM Corp., Armonk, NY, USA). For continuous variables, means and standard deviation were calculated. Frequency data were summarized as percent values. Results obtained were analyzed for outcomes and presented as percentage.
Results
A total of 2797 pregnant women were screened during the study period of whom 233 were found abnormal (using ADA criteria 2011) making the occurrence of GDM to be 8.3 per cent. Of these 233, 10 (4.3%) women with GDM were excluded on the basis of exclusion criteria. Of the remaining 223 women, 28.7 per cent (n=64) required metformin therapy and the rest were controlled on MNT alone Figure.
Figure.
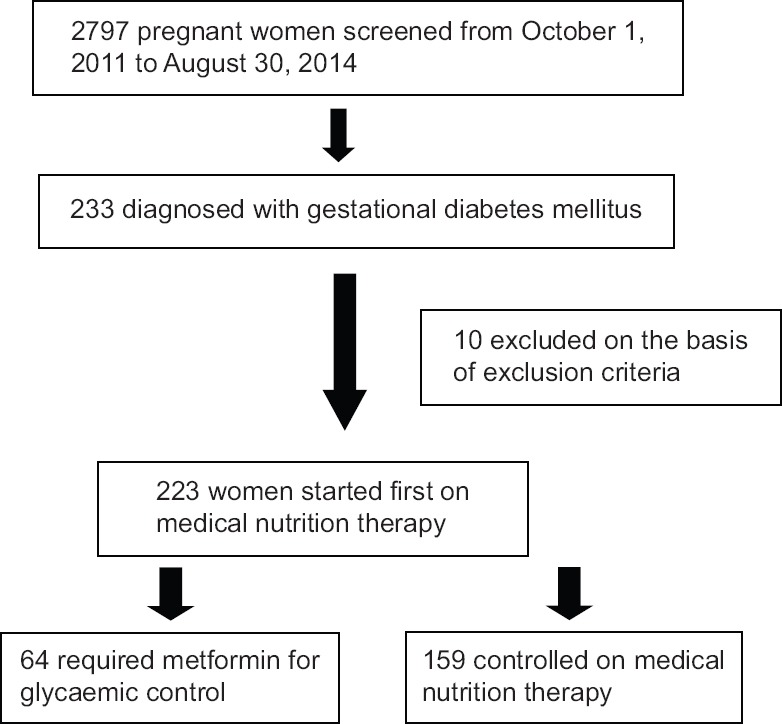
Flow chart showing enrolment of participants.
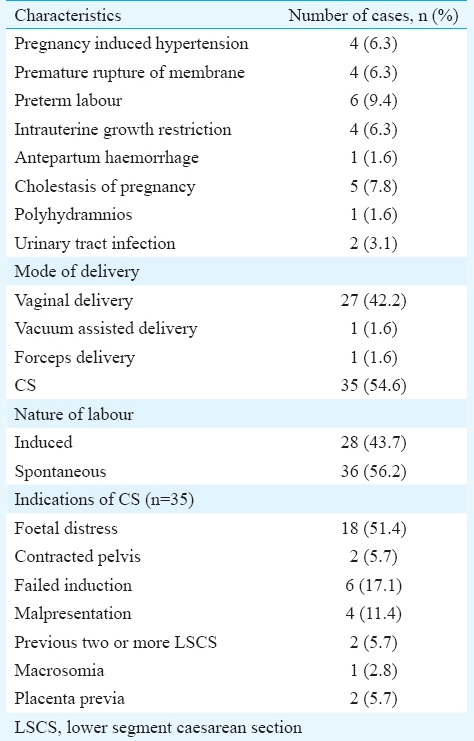
The demographic and clinical profile of the enrolled patients is summarized in Table I. The mean dose of metformin required was 1226.5±397.1 mg (500-2500 mg). Table II summarizes the details of patients according to the gestational age of starting metformin and its impact on treatment. Majority (n=60, 93.8%) of the pregnant women achieved blood sugar control on metformin therapy, whereas three (4.7%) women failed. Two (3.1%) women had gastrointestinal side effects which were minor and got resolved with time. Table III shows the details of maternal complications and mode of delivery among these women. No episode of hypoglycaemia was reported among the pregnant women who received metformin. There was no case of perinatal mortality.
Table I.
Demographic and clinical profile of patients (n=64)
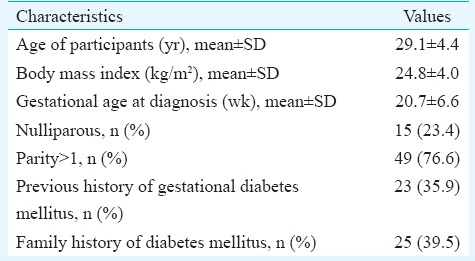
Table II.
Gestational age of start of metformin therapy and its effect on treatment (n=64)
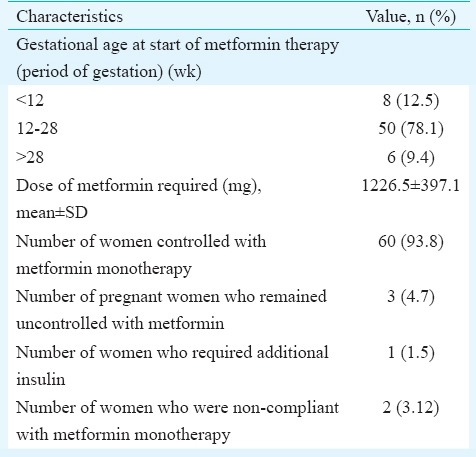
Table III.
Details of maternal outcome (n=64)
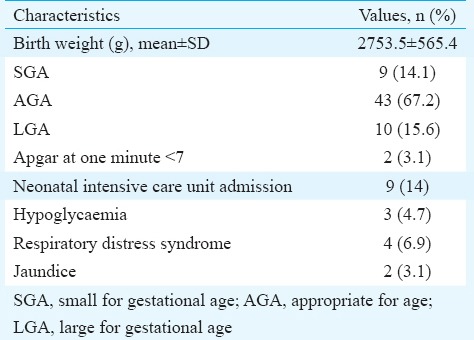
Details of foetal outcomes are presented in Table IV. The mean birth weight was 2753±565.4g. Ten (15.6%) infants were LGA. All of them were monitored for hypoglycaemia for 48 h by checking capillary blood sugar. Five of them developed hypoglycaemia. Of these five, two responded to oral feeding while three required neonatal intensive care unit admission. None of them had shoulder dystocia. There was no perinatal morbidity or mortality.
Table IV.
Details of foetal outcome (n=64)
Discussion
The increase in prevalence of diabetes can be attributed to urbanization, change in lifestyle and dietary habits, and it also varies according to the diagnostic criteria and the population being screened13. The association between timely diagnosis and treatment of GDM cannot be over emphasized. The earliest report about the use of metformin in pregnancy came from South Africa in 1970, where it was used for both type 2 diabetes and GDM12. Some studies concluded that women with GDM treated with metformin had a higher rate of perinatal mortality than the general population, but it was still lower than those who were left untreated14,15,16.
Metformin is an oral biguanide which enhances insulin sensitivity and decreases hepatic glucose output. Unlike insulin, it controls blood sugar level without causing hypoglycaemia or weight gain. Metformin has an ease of administration and storage. As it crosses placenta, there are concerns about its safety, especially in the first trimester. The adverse effects are mainly related to gastrointestinal system. Symptoms are often self-limiting and include nausea, vomiting, abdominal bloating, diarrhoea, anorexia and abdominal pain17. In our study, some of the participants had minor gastrointestinal side effects which resolved gradually. None of them had to discontinue therapy due to adverse effect. In another study, gastrointestinal side effects of metformin were encountered in 1.9 per cent of patients who required discontinuation of therapy18. It is preferred to commence metformin at a low dose (500 mg/day) and always with food to reduce gastrointestinal adverse effect. The women found metformin to be convenient as they did not need to take daily insulin injections. The time and workforce required to train the women for self-administration of insulin could also be avoided.
Vanky et al19 have shown that metformin crosses the placenta and foetus is exposed to levels of metformin comparable to therapeutic level in adults. In a systematic review and meta-analysis it has been concluded that though human experience with metformin during the first trimester of pregnancy is reassuring but there is a need for specific studies designed to evaluate the teratogenicity20. Although 12.5 per cent of the pregnant women were started on metformin in the first trimester, no case of congenital anomaly was observed in the present study.
Sixty women achieved good glycaemic control while four of the remaining failed to achieve euglycemic status with metformin in our study. Three of them were non-compliant to the treatment and one required insulin therapy for glycaemic control. In the metformin versus insulin for the treatment of gestational diabetes (MiG) trial18, 46.3 per cent of the women in metformin arm required supplemental insulin to maintain euglycaemia. In other studies 10, 18 and 14 per cent of the participants have been reported to need insulin, respectively21,22,23. The reason behind this varying number may be difference in age, body mass index and racial composition of the population.
The percentage of women developing hypertensive complications and preterm labour in the present study was 6.3 per cent each whereas it was 12 and 7.2 per cent each in the study by Rowan et al18. Only 15.6 per cent neonates were LGA compared to 19.3 per cent in the study done by Rowan et al18. Although only 4.7 per cent of the women remained with uncontrolled level of GDM with metformin, this higher number of LGA neonates in the present study may be due to other associated factors such as constitutional or genetic. The higher rate of caesarean section (54%) and SGA neonates (14.1%) in the present study may be explained by the fact that the majority of the pregnant women booked in our tertiary referral centre had one or more high-risk factors.
In a meta-analysis, six studies consisting of 1388 participants were analyzed24. The use of oral hypoglycaemic agent (OHA) was not associated with risk of neonatal hypoglycaemia, incidence of caesarean section or incidence of LGA babies. As metformin crosses the placenta, neonatal follow up is required to accept metformin as safe in pregnancy. A trial (Metformin in GDM – The offspring follow up) was conducted among women who had participated in the MiG trial, and body composition was measured in their children at two years age. It was found that children exposed to metformin had more subcutaneous fat, but overall body fat was similar as in children whose mothers were treated with insulin alone but further follow up was recommended25.
In conclusion, metformin may be used as a safe and effective OHA in the management of GDM. It may prove to be a boon in low-resource settings where cost, storage and compliance are logistic issues. However, long-term follow up studies in pregnancy are needed to solve issues related to its safety on foetus and mother.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for financial support (grant number 5/3/8/82/2010-RHN).
Footnotes
Conflicts of Interest: None.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–8. [PubMed] [Google Scholar]
- 2.Korucuoglu U, Biri A, Turkyilmaz E, DogaYildirim F, Ilhan M, Hirfanoglu IM, et al. Glycemic levels with glucose loading test during pregnancy and its association with maternal and perinatal outcomes. Diabetes Res Clin Pract. 2008;80:69–74. doi: 10.1016/j.diabres.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes: A retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878–83. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 5.Contreras M, Sacks D, Bowling F, Cowley D, Liley H, McIntyre H, et al. The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynaecol Obstet. 2011;78:69–77. doi: 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 6.Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 2010;33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209:34.e1–7. doi: 10.1016/j.ajog.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Marques P, Carvalho MR, Pinto L, Guerra S. Metformin safety in the management of gestational diabetes. Endocr Pract. 2014;20:1022–31. doi: 10.4158/EP14018.OR. [DOI] [PubMed] [Google Scholar]
- 9.Langer O. When diet fails: Insulin and oral hypoglycemic agents as alternatives for the management of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2002;11:218–25. doi: 10.1080/jmf.11.4.218.225. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voskamp BJ, Kazemier BM, Schuit E, Mol BW, Buimer M, Pajkrt E, et al. Birth weight ratio as an alternative to birth weight percentile to express infant weight in research and clinical practice: A nationwide cohort study. Obstet Gynecol Int. 2014;2014:749476. doi: 10.1155/2014/749476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajput R, Yadav Y, Nanda S, Rajput M. Prevalence of gestational diabetes mellitus & associated risk factors at a tertiary care hospital in Haryana. Indian J Med Res. 2013;137:728–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna FW, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351–8. doi: 10.1046/j.1464-5491.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 14.Coetzee EJ, Jackson WP. Pregnancy in established non-insulin-dependent diabetics. A five-and-a-half year study at Groote Schuur Hospital. S Afr Med J. 1980;58:795–802. [PubMed] [Google Scholar]
- 15.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002;17:2858–64. doi: 10.1093/humrep/17.11.2858. [DOI] [PubMed] [Google Scholar]
- 16.Coetzee EJ, Jackson WP. Metformin in management of pregnant insulin-independent diabetics. Diabetologia. 1979;16:241–5. doi: 10.1007/BF01221950. [DOI] [PubMed] [Google Scholar]
- 17.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: A randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 18.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 19.Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575–8. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Cassina M, Donà M, Di Gianantonio E, Litta P, Clementi M. First-trimester exposure to metformin and risk of birth defects: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:656–69. doi: 10.1093/humupd/dmu022. [DOI] [PubMed] [Google Scholar]
- 21.Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet Med. 2009;26:798–802. doi: 10.1111/j.1464-5491.2009.02780.x. [DOI] [PubMed] [Google Scholar]
- 22.Tertti K, Ekblad U, Vahlberg T, Rönnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: A retrospective, case-control study. Rev Diabet Stud. 2008;5:95–101. doi: 10.1900/RDS.2008.5.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: Arandomized clinical trial. Diabetes Res Clin Pract. 2012;98:422–9. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycaemic agents vs insulin in management of gestational diabetes: A systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.e1–9. doi: 10.1016/j.ajog.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes mellitus – The offspring follow up (MIFTOFU)-body composition at 2 years of age. Diabetes Care. 2011;34:2279–84. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]


