Sir,
Scrub typhus occurs in almost all parts of India and is endemic1. Many researchers have reported the profile of scrub typhus from various parts of India with diagnosis based on IgM ELISA2,3,4. Eschar is pathognomonic for rickettsiosis including scrub typhus, but its presence has not been reported as a marker of disease severity. Kim et al5 found absence of eschar to have prognostic significance in their study. We report here results of a retrospective analysis of IgM positive adult patients with scrub typhus showing eschar as a marker of severe rickettsiosis.
This retrospective, hospital record-based observational study was conducted in Dr Rajendra Prasad Government Medical College, Kangra, Tanda, Himachal Pradesh, India. The files of all fever patients from the record section who were admitted between January and December 2015, were reviewed. Those patients who had a diagnosis of scrub typhus based on confirmed IgM-positive ELISA test were included. The IgM kits used for testing were obtained from InBios International, Seattle, WA, USA. Those with co-infections such as malaria, leptospirosis, typhoid, tuberculosis and hepatitis, patients diagnosed as scrub typhus but IgM −ve and those diagnosed as scrub typhus on clinical suspicion where IgM ELISA was not done, were excluded. The data were extracted from the files and analyzed using SPSS software (version 23, SPSS, Inc., Chicago, IL, USA).
The patients were labelled as having sepsis, severe sepsis, septic shock and multi-organ dysfunction syndrome according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee6. Patients were divided into two groups: eschar +ve and eschar −ve for analysis. Distribution of continuous variables in two groups, eschar +ve and eschar −ve, was compared using unpaired t test and Fisher's exact for categorical variables. Multivariate logistic regression was used to model the strength of association of other biologically plausible variables such as age and gender in addition to eschar on each of the outcome variables that were found to have significantly high distribution in patients with eschar.
A total of 74 records of fever with a diagnosis of scrub typhus were obtained, but only 61 patients had a documented IgM ELISA-positivity for scrub typhus. Since, no test was done to rule out leptospirosis in the first week of illness, all patients were given doxycycline (100 mg b.i.d) for 5-7 days and ceftriaxone injection (1g b.i.d) for 7-10 days to cover for both scrub typhus and leptospirosis. Scrub typhus was confirmed in these patients using IgM ELISA. No patient was found with co-infection of scrub typhus and leptospirosis. Malaria was ruled out in all patients using malaria rapid antigen card test.
Totally, 61 IgM+ scrub typhus patients were included, of whom 42 (69%) were females. Age range was 18-69 yr, with a mean of 41.19±13 yr. Mean hospital stay was 5.1±0.57 days. Mean fever duration before admission was 6.1 days, and mean temperature was 39.31°C. The distribution of clinical and biochemical variables in scrub typhus patients is shown in Table I. Among the symptoms, myalgia 49 (80%), pain abdomen 30 (49%), shortness of breath 28 (46%) and headache 27 (44%) were common. Jaundice 17 (28%), meningoencephalitis 6 (10%), rash 2 (3%), pleural effusion 1 (1.5%) and ascites 1 (1.5%) were other less common complaints.
Table I.
Distribution of variables (clinical and biochemical) in scrub typhus patients (n=61)
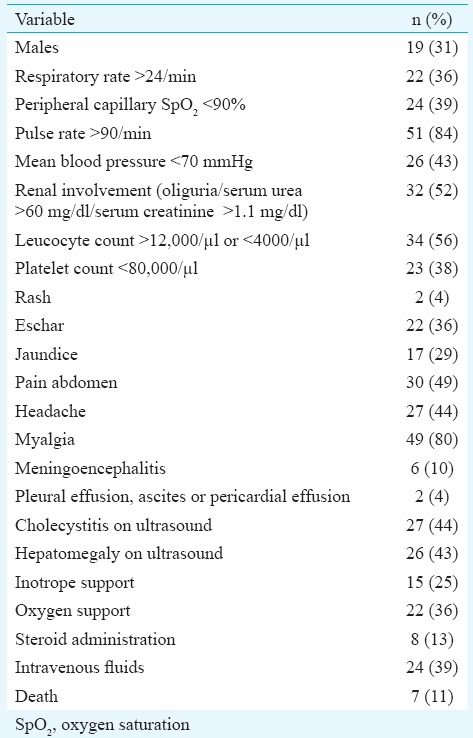
Sepsis was established in 56 (92%), of whom 52 (85%) had severe sepsis. Mean blood pressure <70 mm Hg was present in 26 (42%) patients, and septic shock needing vasopressors was present in 15 (25%). Steroids were given to eight (13%), and supplemental oxygen was given to 22 (36%). Multi-organ dysfunction syndrome was diagnosed in 28 (46%). Ultrasound was suggestive of cholecystitis in 27 (44.26%) and that of hepatomegaly in 26 (43%).
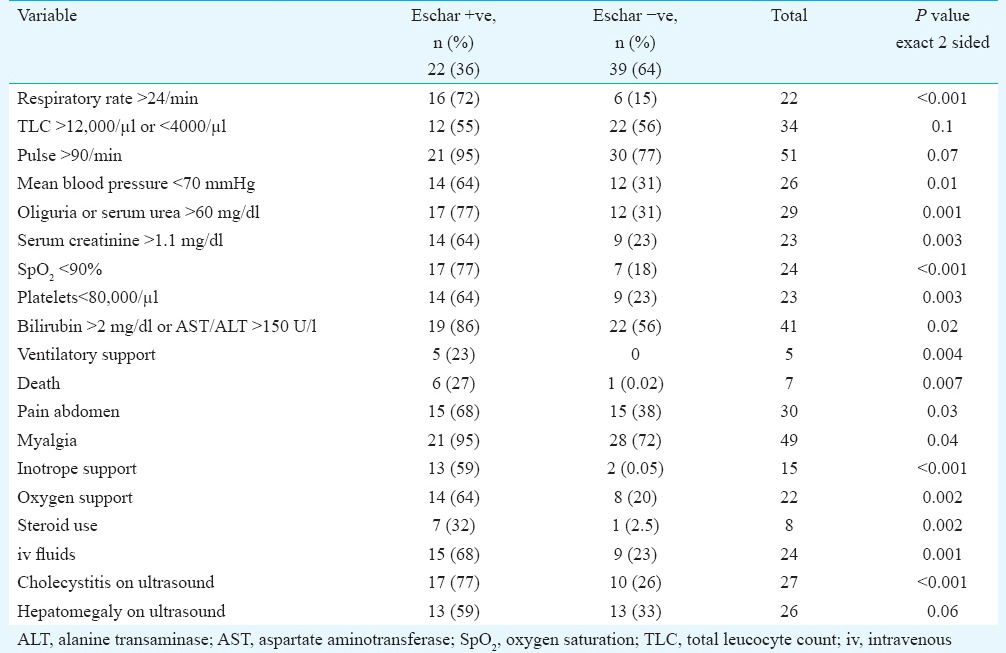
Eschar +ve 22 (36%) patients were compared with eschar −ve 39 (64%) patients to see if the distribution of clinical, biochemical and outcome variables was different in these two groups (Table II). The baseline characteristics such as age and gender were comparable. Mean age in eschar +ve group was 42.5±14 yr, while it was 40.4±12 yr in the eschar −ve group. Multivariate logistic regression was used to model the strength of association of age, gender and eschar on each of the parameters that showed significantly high distribution in eschar +ve patients. Keeping age and gender constant, eschar was found to have significant independent association with respiratory rate >24/min, mean blood pressure <70 mmHg, oliguria or serum urea >60 mg/dl, serum creatinine >1.1 mg/dl, oxygen saturation <90 per cent, platelet count <80,000/μl, bilirubin >2 mg/dl (or AST/ALT) >150 U/l, need for inotrope support, need for oxygen support, need for intravenous fluids, steroid use, pain abdomen, myalgia, findings of cholecystitis on ultrasound and duration of hospital stay (Table II).
Table II.
Strength of association of eschar with various clinical and biochemical parameters
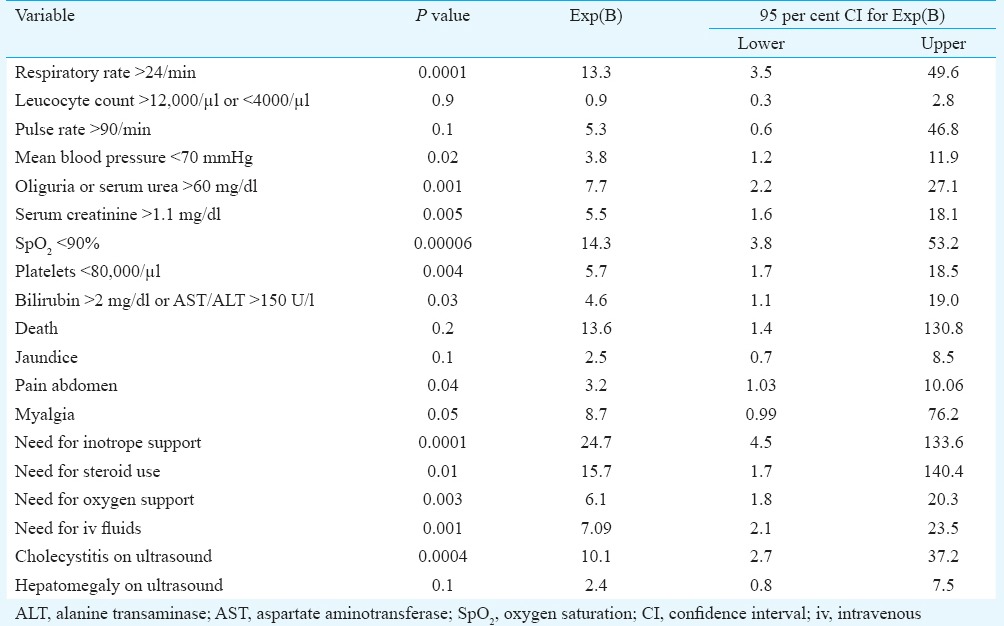
Eschar is a common finding in scrub typhus and spotted fever group rickettsiosis, but its positive association with disease severity has not been established in clinical studies. Sonthayanon et al7 from Thailand found presence of an eschar associated with higher bacterial load in blood, and they speculated that there might be a possible link between the development of an eschar and the size of initial inoculum or the virulence of the infecting strain. This study used molecular techniques for diagnosis and the association of eschar with high viral loads was a significant finding. We could not find any study showing positive association of eschar with the severity in disease in scrub typhus patients. Kim et al5, however, showed the absence of eschar to be associated with severity, which was contrary to our observations. Sriwongpan et al8 found almost equal (56±4%) distribution of eschar in non-severe, severe and deceased scrub typhus patients. On multivariate logistic regression using variables such as age, gender and eschar as predictors, significant independent association of eschar was found with the respiratory rate >24/min, mean blood pressure <70 mmHg, oliguria or serum urea >60 mg/dl, serum creatinine >1.1 mg/dl, oxygen saturation <90 per cent, platelet count <80,000/μl, bilirubin >2 mg/dl (or AST/ALT >150 U/l), need for inotrope support, need for oxygen support, need for intravenous fluids, pain abdomen, findings of cholecystitis on ultrasound and duration of hospital stay (Table III).
Table III.
Multivariate logistic regression showing the strength of association of eschar with the variables
Eschar is produced as a part of immune response to the rickettsial antigens in the body and biopsy of the eschar shows leukocytoclastic vasculitis7. Hyperactive immune response behind eschar formation may also be responsible for renal, respiratory, haematological and circulatory organ involvement and severe illness in eschar +ve scrub typhus patients. Other plausible explanations include high inoculum load in patients with eschar leading to higher DNA loads and thus eschar and severe disease7. It may also be explained on the basis of virulence of the prevalent strains that causes eschar formation, higher DNA loads and severity of disease.
Our study had some limitations. The study was done in a tertiary care setting, so the results cannot be extrapolated to the scrub typhus patients in community. Arterial blood gas analysis (pO2) was not done, so specific diagnosis of acute respiratory distress syndrome could not be made in the patients.
To conclude, the presence of eschar in IgM+ scrub typhus patients was found to be associated with symptom severity including renal, haematological, respiratory and circulatory systems, longer hospital stay and higher mortality in this group compared to eschar −ve IgM+ scrub typhus patients. Further studies need to be done to determine the causes behind this association.
Footnotes
Conflicts of Interest: None.
References
- 1.Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis & management of Rickettsial diseases in India. Indian J Med Res. 2015;141:417–22. doi: 10.4103/0971-5916.159279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koraluru M, Bairy I, Varma M, Vidyasagar S. Diagnostic validation of selected serological tests for detecting scrub typhus. Microbiol Immunol. 2015;59:371–4. doi: 10.1111/1348-0421.12268. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta M, Anandan S, Daniel D, Prakash JAJ. Scrub typhus seroprevalence in healthy Indian population. J Clin Diagn Res. 2015;9:DM01–2. doi: 10.7860/JCDR/2015/14708.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peter JV, Sudarsan TI, Prakash JAJ, Varghese GM. Severe scrub typhus infection: Clinical features, diagnostic challenges and management. World J Crit Care Med. 2015;4:244–50. doi: 10.5492/wjccm.v4.i3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 7.Sonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, Day NP, et al. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol. 2009;47:430–4. doi: 10.1128/JCM.01927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sriwongpan P, Krittigamas P, Kantipong P, Kunyanone N, Patumanond J, Namwongprom S. Clinical indicators for severe prognosis of scrub typhus. Risk Manag Healthc Policy. 2013;6:43–9. doi: 10.2147/RMHP.S52470. [DOI] [PMC free article] [PubMed] [Google Scholar]


