Abstract
Aims:
The aim of this study is to evaluate the clinical efficacy of computed tomography (CT)-guided radiofrequency (RF) ablation as a minimally invasive therapy for osteoid osteoma.
Materials and Methods:
This is a retrospective analysis of prospectively maintained data of 43 symptomatic osteoid osteoma patients who were treated by radiofrequency ablation (RFA). Forty out of 43 patients were naive cases and underwent primary treatment for osteoid osteoma with RFA, whereas 3 patients included in the study underwent RFA for local recurrence after having undergone surgical treatment. Diagnosis was based on clinical and characteristic imaging findings, and biopsy was done for cases with atypical presentation. Pre and post procedure Visual Analog Score (VAS) was documented in all cases. Monopolar RFA system was used in all patients, and the electrode was placed within the lesion nidus under CT guidance coaxially through 11G introducer needle. Ablation was performed at 90° C for 5 min.
Results:
Technical success rate of intranidal placement of electrode was 100%. The primary clinical success in our study was 97.7% (42 of 43), and the secondary clinical success was 100%. Pre and postprocedure VAS score in our study group was 7.8 and 0.4, respectively. Mean follow-up period in our study was 48 months (Range: 4–129 months). One patient had recurrence of pain 4 years after treatment and was treated successfully by a second session. Minor complications were seen in 3 patients with two cases of RF pad burns and one case of skin burn at the treatment site, and these were managed conservatively. No patients developed temporary/permanent neurological deficits, and no procedure-related mortality was seen in our study.
Conclusion:
CT-guided percutaneous RFA is a simple, safe, minimally invasive, and highly effective treatment option for osteoid osteoma with good long-term pain control and potentially low disease recurrence.
Keywords: Bone ablation, bone RFA, osteoid osteoma, PET-CT, radio frequency ablation
Introduction
Osteoid osteoma is a painful benign bony condition that typically occurs in children (particularly adolescents) and young adults. It characteristically causes night-pain which is relieved on using non-steroidal anti-inflammatory drugs. They have typical radiological finding of lucent nidus <1.5 cm and surrounding solid periosteal reaction. It is not uncommon for it to present with growth disturbances, bone deformity, painful scoliosis, joint effusion, restricted joint motion, or contracture depending on the site of occurrence. Surgical removal or ablation of the nidus is key to the successful treatment outcome in this condition, and open surgery has been considered traditionally to be a gold standard in the treatment of osteoid osteoma. However, surgery is not devoid of technical difficulties and morbidities and suffers from local recurrence rates ranging 9–28%, as reported in the literature. To circumvent these issues various minimally invasive image-guided percutaneous procedures have been tried to treat this painful condition, of which radiofrequency ablation (RFA) is proving to be an attractive alternative to surgery. The objective of this retrospective analysis was to evaluate the long-term clinical effectiveness of computed tomography (CT)-guided RFA as a minimally invasive therapy for osteoid osteoma.
Materials and Methods
A retrospective analysis of prospectively maintained data of 43 symptomatic osteoid osteoma patients who were treated by RFA using CT guidance in our institute between March 2006 and September 2016 was performed. Institutional Review Board (IRB) clearance was obtained for this retrospective cohort analysis. Diagnosis was based on clinical and characteristic imaging findings, and biopsy was done for cases with atypical presentation. Decision to treat by RFA was taken in consultation with orthopedic oncologist in a joint clinic. Clinical criteria included local pain, pain worsening at night, and pain relief after intake of nonsteroidal anti-inflammatory agents. Radiological criteria included typical findings on radiographs and CT and/or magnetic resonance (MR) images, with clear depiction of a nidus smaller than 1.5 cm in maximum diameter with surrounding bone sclerosis. Pre and postprocedure regional positron emission tomography (PET)-CT was done to assess completeness of ablation. Contrast-enhanced dynamic MRI was also done pre and postprocedurally to assess treatment response on follow-up.
Pre and post procedure Visual Analog Score (VAS) was documented in all cases. Monopolar RFA system was used in all patients. RFA electrode was placed within the lesion nidus under CT guidance coaxially using a 11-G introducer needle, and ablation was performed.
The following variables were assessed:
Technical success defined as placement of the active tip within the nidus, with the nidus border not exceeding the active tip by more than 5 mm
Clinical outcome – primary clinical success, defined as pain relief after one session of RFA. Secondary clinical success, defined as pain relief after more than one session of RFA.[1]
Technical considerations
Procedure was done with CT guidance (GE healthcare and Miyabi Angio-CT, Siemens Healthneers, Global) under general anesthesia. Monopolar RFA system (RITA System - Model: RF generator 1500X, Angiodynamics, USA) was used in all the cases, and consequently, two self-adhesive grounding pads were attached to the patient prior to treatment initiation. Based on the CT scan images, limb or body was positioned in such a manner to have safe and easy access to the nidus. During ablation of the vertebral (pedicular) osteoid osteoma, epidural sheath was inserted and air insufflation was done to prevent damage to the cord and adjacent nerve roots. A small skin incision was made at the entry site. A 11 -G bone biopsy needle (Murphy - M2, Osteosite cook medical, USA) was introduced till the periosteum, and an attempt was made to enter the nidus by manual rotatory movements of the needle. In cases that had significant cortical thickening, the nidus was accessed by using a hand drill, introduced coaxially via the bone biopsy needle. When nidus was reached, the drill was removed and the RF electrode [Starburst-XL or side deploying (SDE) electrode] was introduced. The choice of the electrode was based on the location of the nidus within the bone.
Care was taken to ensure that there was at least 1 cm distance between the tip of the cannula and the active portion of electrode. If this distance was not adequate, the murphy needle was exchanged with RITA starburst access system (hard introducer) over “K”wire. This system has insulation which would prevent inadvertent ablation of tract along the needle even if electrode tip is in contact with the cannula. Once the RF electrode tip was optimally placed intranidally, ablation was initiated at 90° C for a period of 5 min.
Postprocedural care
Patients were given adequate analgesia overnight. PET-CT and dynamic MRI was performed next day for assessing the completeness of ablation along with assessment of pain score. Patients were discharged after confirming absence of enhancement on dynamic MRI and non-avidity on PET-CT of the treated lesion. Oral antibiotics were administered for 5 days post procedure.
Follow-up
Patients were asked about the pain score telephonically after 1 week, and were followed up with dynamic MRI after 6 weeks. Patients were reviewed and assessed clinically 6 monthly for 1 year and annually thereafter for 2 years. Subsequently, patients were telephonically reviewed annually and reviewed clinically SOS. No radiological evaluation was done during these follow-up visits if patients were asymptomatic, whereas patients with lesions close to joints were assessed for any complications with follow-up MRI.
Results
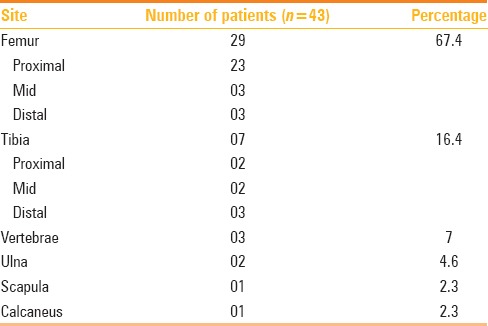
Our study group showed male preponderance [Males (35), Females (08)] with mean age of study population being 18.1 years (age range: 3–46 years). Anatomical lesion distribution is summarized in Table 1.
Table 1.
Anatomical distribution of lesion in our study
All 43 patients had significant improvement in pain score. Technical success rate of intranidal placement of electrode was 100%. The primary clinical success in our study was 97.7% (42 of 43) and secondary clinical success was 100%. Pre and postprocedure VAS score in our study group was 7.8 and 0.4, respectively. Patients were pain free within a week after ablation and off analgesics with return to full range of routine activity. Mean follow-up period in our study was 48 months (Range: 4–129 months). One patient had recurrence of pain 4 years after treatment and was treated successfully by second session. Minor complications were seen in 3 patients with two cases of RF pad burns and one case of skin burn at the treated site, which were managed conservatively. No patients developed temporary/permanent neurological deficits, and no procedure-related mortality were seen in our study.
Discussion
Osteoid osteoma is a common benign osteogenic tumor predominantly affecting children and young adults and accounts for 13.5% of all benign tumors.[2] It often presents clinically as localized severe bone pain typically in the night, which is characteristically relieved by nonsteroidal anti-inflammatory drugs.[3] Alternatively it may present with growth disturbances, bone deformity, painful scoliosis, and if located within a joint capsule, there might be joint effusion, synovitis, restricted joint motion, or contracture.[4] Osteoid osteoma may be self-limiting, and spontaneous regression of some lesions has been reported though generally a delayed event.[5] The mechanism of involution is not clearly known though it has been attributed to tumor nidus infarction.
Characteristic radiographic findings [Figure 1A] include a nidus of vascular osteoid tissue in the bone cortex surrounded by reactive sclerotic bone. CT is the modality of choice for detecting this tumor and provides the best characterization of the nidus and the surrounding sclerotic bone [Figure 1B].[6] MRI, though not as specific as CT,[6,7,8,9,10] very well depicts the adjacent marrow edema and synovial changes in the lesion close to the joint capsule. Further, on dynamic contrast enhanced MRI, nidus often demonstrate strong enhancement [Figure 1C].[11,12]
Figure 1 (A-D).
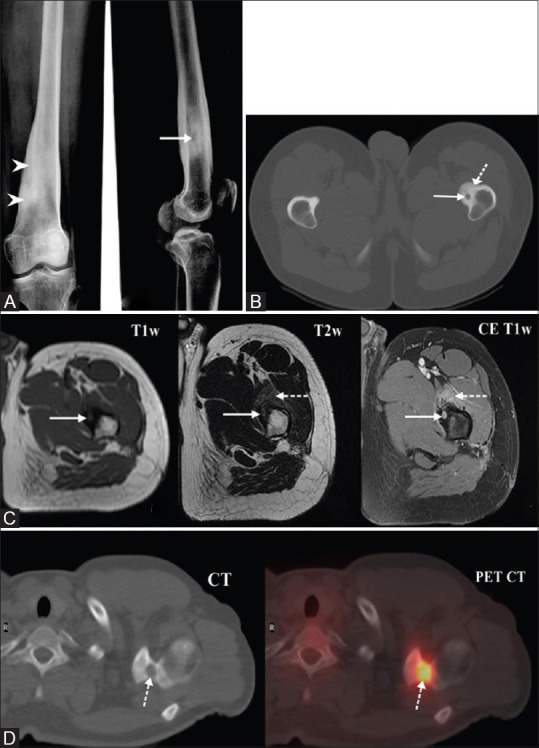
(A) Plain radiograph revealed radiolucent nidus (white arrow) with surrounding sclerosis (arrowheads). (B) CT image of osteoid osteoma in the proximal femur with lucent nidus (white arrow) and surrounding thickened sclerotic cortex (dotted arrow). (C) MRI images reveal osteoid osteoma in the proximal femur with nidus (solid arrow) which enhances avidly in post contrast scan. Adjacent muscle shows hyperintensity and enhancement secondary to inflammation (dotted arrow). (D) PET-CT shows a metabolically avid lesion (dotted arrow) in the base of the acromion process of left scapula
PET/CT using F-18 and fluorodeoxy glucose may have role in the diagnosis and posttreatment response evaluation. The nidus exhibits avid glucose metabolism [Figure 1D], whereas surrounding sclerosis does not.[13,14]
In our study cohort, osteoid osteoma was mainly seen in the Femur and Tibia (84%) with spine, upper limb, and foot less common site of occurrence ([Figure 2 and 3] summarize osteoid osteoma cases in femur and spine).
Figure 2 (A-H).
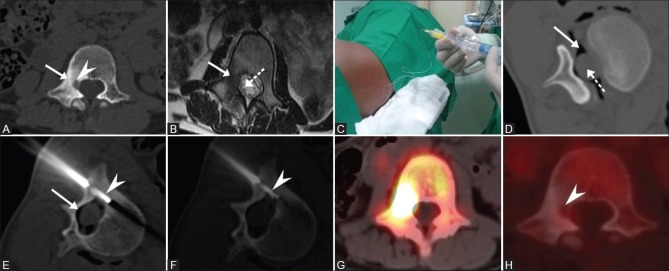
(A) CT image shows nidus (arrowhead) in the right pedicle of L1 vertebra with adjacent sclerosis (solid arrow). (B) T2W axial MR image shows dependent nerve root (dotted arrow) within the thecal sac close to the nidus. (C) Air was insufflated via needle placed within the epidural space. (D) CT axial image shows epidural air (solid arrow) separating the thecal sac (dotted arrow) from the nidus. (E and F) RFA electrode (dotted arrow) placed within the nidus. Pre RFA (F-18) PET CT (G) shows avid uptake within the nidus and complete metabolic regression (arrowhead) in post RFA scan (H)
Figure 3 (A-H).
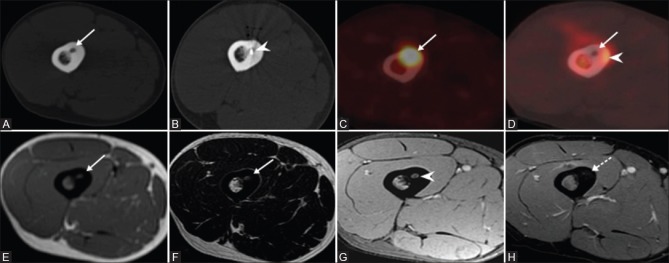
CT image (A) shows osteoid osteoma lesion in the mid shaft of femur with lucent nidus and surrounding thickened bone (solid arrow). CT image (B) showing the electrode tip within the nidus (arrowhead). The lesion is metabolically active (solid arrow) on F-18 PET CT scan (C) and Post ablation PET CT (D) shows no activity within the nidus (solid arrow) and inflammation in the surrounding bone (arrowhead). MRI scan shows nidus (solid arrow) which is isointense on T1W (E), hyperintense on T2W (F) sequence and enhances in post contrast T1w (G) scan. Absent enhancement (arrowhead) in post RFA scan (H)
Open surgical wide resection has been the traditional gold standard treatment option for osteoid osteoma and often requires extensive surgical approach with bone graft and fixation, which ultimately leads to prolonged hospital stay, delayed functional recovery, and its associated morbidity spectrum.[15,16]
Further, some lesions located at anatomically inaccessible areas, such as the femoral head and neck, are difficult to identify and localize precluding safe surgery. Key to a successful treatment of osteoid osteoma is complete removal or destruction of the tumor nidus and not just the removal of the overt or exuberant new bone (osteosclerosis) formed adjacent to the nidus. Consequently, 9–28% of osteoid osteomas tend to recur after surgical resection.[17] In recent years, several CT-guided percutaneous techniques have been employed to achieve removal or destruction of the nidus such as percutaneous trephine resection, drill resection with or without subsequent ethanol injection, thermal destruction by means of laser photocoagulation, and RFA.[18,19,20,21]
The potentially serious complications of surgery have made percutaneous RFA an attractive alternative. RFA of osteoid osteoma is a promising minimally invasive technique in interventional radiology, which was first reported by Rosenthal et al.[8] in 1992. RFA of osteoid osteoma offers several advantages over other treatment modalities. It requires only small osseous access to allow insertion of the electrode. There is minimal loss of bone not causing significant structural weakening. If lesion is located at a vulnerable site, such as proximal femur or surrounding joint space, there are chances of bone resorption during the repair process, which leads to some mechanical weakness requiring restriction of sports activities for a small period.[22] None of our patients showed evidence of this weakness on follow-up.
The primary clinical success in our study was 97.7% and the secondary clinical success was 100% [Figures 2 and 3]. In our series of 43 patients, minor complications were seen in 3 children, with two cases of RF pad burns and one case of skin burn at treatment site, which were managed conservatively (GRADE B – Society of Interventional Radiology Complication Criteria). Pad burns occurred in two children overlying the iliac crest due to suboptimal positioning of the grounding pads. The skin burn occurred at the treated site in a child with tibial osteoid osteoma despite due technical precaution taken (use of ice and hydro dissection) and was likely secondary to the superficial location of the treated lesion. These patients returned to normal activity with no long-term sequelae.
In a series of 38 patients with osteoid osteoma treated with CT-guided RFA using cool tip electrodes, Martel J et al. showed primary success rate of 97% with a 100% secondary success rate.[23] Lindner et al. treated 58 patients of osteoid osteoma with CT-guided RFA, and found a primary cure rate of 95% and a 100% cure rate after second ablation.[24] Motamedi et al. had primary cure rate of 89–95%.[1] In a study by Jhankaria et al. involving 40 patients, primary and secondary clinical success rates of 95% and 100%, respectively, was achieved.[25] Rehnitz et al.[26] in their retrospective analysis of 72 cases reported primary and secondary clinical success rates of 99% and 100%, respectively. Clinical results of our study is comparable to these studies in literature.
Conclusion
CT-guided percutaneous RFA is a simple minimally invasive, safe, and effective technique for the treatment of osteoid osteomas, and should be regarded as the treatment of choice for osteoid osteoma. Our results also suggest that RFA represents a promising method for treating patients with persistent or recurrent pain after unsuccessful surgical treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, et al. Thermal Ablation of Osteoid Osteoma: Overview and Step-by-Step Guide. Radiographics. 2009;29(2127):41. doi: 10.1148/rg.297095081. [DOI] [PubMed] [Google Scholar]
- 2.Unni KK, Dahlin DC. Dahlin's bone tumors. 5th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 1996. Osteoid osteoma; pp. 121–30. [Google Scholar]
- 3.Cantwell CP, O'Byrne J, Eustace S. Radiofrequency ablation of osteoid osteoma with cooled probes and impedance-control energy delivery. AJR Am J Roentgenol. 2006;186:244–8. doi: 10.2214/AJR.04.0938. [DOI] [PubMed] [Google Scholar]
- 4.Kransdorf MJ, Stull MA, Gilkey FW, Moser RP. Osteoid osteoma. Radiographics. 1991;11:671–96. doi: 10.1148/radiographics.11.4.1887121. [DOI] [PubMed] [Google Scholar]
- 5.Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74:179–85. [PubMed] [Google Scholar]
- 6.Liu PT, Kujak JL, Roberts CC, de Chadarevian JP. The vascular groove sign: Anew CT finding associated with osteoid osteomas. AJR Am J Roentgenol. 2011;196:168–73. doi: 10.2214/AJR.10.4534. [DOI] [PubMed] [Google Scholar]
- 7.Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res. 2000:115–24. doi: 10.1097/00003086-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal DI, Alexander A, Rosenberg AE, Springfield DS. Ablation of osteoid osteomas with a percutaneously placed electrode: Anew procedure. Radiology. 1992;183:29–33. doi: 10.1148/radiology.183.1.1549690. [DOI] [PubMed] [Google Scholar]
- 9.Yip PS, Lam YL, Chan MK, Shu JS, Lai KC, So YC. Computed tomography-guided percutaneous radiofrequency ablation of osteoid osteoma: Local experience. Hong Kong Med J. 2006;12:305–9. [PubMed] [Google Scholar]
- 10.Efstathopoulos N, Sapkas G, Xypnitos FN, Lazarettos I, Korres D, Nikolaou VS. Recurrent intra-articular osteoid osteoma of the hip after radiofrequency ablation: Acase report and review of the literature. Cases J. 2009;2:6439. doi: 10.4076/1757-1626-2-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spouge AR, Thain LM. Osteoid osteoma: MR imaging revisited. Clin Imaging. 2000;24:19–27. doi: 10.1016/s0899-7071(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 12.Zampa V, Bargellini I, Ortori S, Faggioni L, Cioni R, Bartolozzi C. Osteoid osteoma in atypical locations: The added value of dynamic gadolinium-enhanced MR imaging. Eur J Radiol. 2009;71:527–35. doi: 10.1016/j.ejrad.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Purandare NC, Rangarajan V, Shah SA, Sharma AR, Kulkarni SS, Kulkarni AV, et al. Therapeutic Response to Radiofrequency Ablation of Neoplastic Lesions: FDG PET/CT Findings. Radio Graphics. 2011;31:201–13. doi: 10.1148/rg.311105033. [DOI] [PubMed] [Google Scholar]
- 14.Imperiale A, Moser T, Ben-Sellem D, Mertz L, Gangi A, Constantinesco A. Osteoblastoma and osteoid osteoma: Morphological characterization by MRI and dynamic F-18 FDG PET/CT before and after radiofrequency ablation. Clin Nucl Med. 2009;34:184–8. doi: 10.1097/RLU.0b013e3181966de6. [DOI] [PubMed] [Google Scholar]
- 15.Libordo G, Ghelman H. Osteoid osteoma. Medscape May. 2015 [Google Scholar]
- 16.Vogl TJ, Helmberger TK, Mack MG. Percutaneous Tumor Ablation in Medical Radiology. Springer Verlag; 2007. ISBN: 3540225188. [Google Scholar]
- 17.Healey JH, Ghelman B. Osteoid osteoma and osteoblastoma.Current concepts and recent advances. Clinic Orthop Relat Res. 1986;204:76–85. [PubMed] [Google Scholar]
- 18.Parlier-Cuau C, Champsaur P, Nizard R, Hamze B, Laredo JD. Percutaneous removal of osteoid osteoma. Radiol Clin North Am. 1998;36:559–66. doi: 10.1016/s0033-8389(05)70044-0. [DOI] [PubMed] [Google Scholar]
- 19.Adam G, Keulers P, Vorwerk D, Heller KD, Füzesi L, Günther RW. The percutaneous CT-guided treatment of osteoid osteomas: Acombined procedure with a biopsy drill and subsequent ethanol injection. Fortschr Rontgenstr. 1995;162:232–5. doi: 10.1055/s-2007-1015871. [DOI] [PubMed] [Google Scholar]
- 20.Gangi A, Dietemann JL, Clavert JM, Dodelin A, Mortazavi R, Durckel J, et al. Treatment of osteoid osteoma using laser photocoagulation. Apropos of 28 cases. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:676–84. [PubMed] [Google Scholar]
- 21.Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop. 2000;373:115–24. doi: 10.1097/00003086-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: Percutaneous radio-frequency ablation. Radiology. 1995;197:451–4. doi: 10.1148/radiology.197.2.7480692. [DOI] [PubMed] [Google Scholar]
- 23.Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol. 2005;56:403–8. doi: 10.1016/j.ejrad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391–6. doi: 10.1302/0301-620x.83b3.11679. [DOI] [PubMed] [Google Scholar]
- 25.Jhankaria B, Burute N. Percutaneous radiofrequency ablation for osteoid osteoma: How we do it. Indian J Radiol Imaging. 2009;19:36–42. doi: 10.4103/0971-3026.44523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehnitz C, Sprengel SD, Lehner B, Ludwig K, Omlor G, Merle C, et al. CT guided Radiofrequency ablation of osteoid osteoma: Correlation of clinical outcome and imaging features. Diagn Interv Radiol. 2013;19:330–9. doi: 10.5152/dir.2013.096. [DOI] [PubMed] [Google Scholar]