Abstract
Propofol, an intravenous anesthetic, has been shown to offer superior analgesic effect clinically. Whether propofol has preventive analgesic property remains unexplored. The present study investigated the antinociceptive effect of propofol and underlying molecular and cellular mechanisms via pre-emptive administration in a formalin-induced inflammatory pain model in rats. Male adult Sprague–Dawley rats were randomly allocated into four groups: naïve (Group Naïve), formalin injection only (Group Formalin), and formalin injection at 30 min (Group P-30 min) or 2 h (Group P-2 h) after intravenous infusion of propofol (0.6 mg kg−1 min−1) for 1 h. Nociceptive responses and protein expression of phosphorylated- or pan-GluN2B, ERK1/2, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in the spinal dorsal horn were evaluated. Alteration of intracellular Ca2+ concentration induced by N-methyl-D-aspartate (NMDA) receptor agonists with or without pre-treatment of propofol was measured using fluorometry in SH-SY5Y cells while neuronal activation in the spinal dorsal horn by immunofluorescence. Pre-emptive propofol reduced pain with a delayed response to formalin and a reduction in hypersensitivity that lasted at least for 2 h. The formalin-induced activation of spinal GluN2B and ERK1/2 but not p38 or c-Jun N-terminal kinase was also diminished by propofol treatment. Preconditioning treatment with 3 µM and 10 µM of propofol inhibited Ca2+ influx mediated through NMDA receptors in SH-SY5Y cells. Propofol also reduced the neuronal expression of c-Fos and p-ERK induced by formalin. This study shows that pre-emptive administration of propofol produces preventive analgesic effects on inflammatory pain through regulating neuronal GluN2B-containing NMDA receptor and ERK1/2 pathway in the spinal dorsal horn.
Keywords: Analgesia, inflammatory pain, N-methyl-D-aspartate receptors, propofol, pre-emptive
Introduction
The role of anesthetics in nociceptive processing has long been of interest in anesthesiology. Propofol, a commonly used general anesthetic for induction and maintenance of general anesthesia (i.e. total intravenous anesthesia, TIVA) during surgical procedures,1 has been reported to have analgesic property in management of acute postoperative pain as compared with other anesthetics.2,3 However, the findings from clinical or pre-clinical studies are widely varied from analgesia,4,5 no analgesia,6,7 or even hyperalgesia.8 The natures of noxious stimulation and administration timing of propofol, e.g., pre- versus post-noxious stimulation play roles in these discrepant results.6,8,9
Spinal N-methyl-D-aspartate (NMDA) receptors were reported to be involved in the antinociceptive effect of propofol.10 Distinct from its GABAergic mechanism underlying its general anesthetic properties, the inhibitory effects of propofol on NMDA receptor11 and calcium ion currents in primary afferent neurons12 may play an essential role in the analgesic property of propofol. NMDA receptors, widely existing in the central nervous system, play critical roles in generation and maintenance of central sensitization associated with hyperalgesia and allodynia in the spinal cord.13 GluN2 receptor, a subunit of NMDA receptors, includes four types of GluN2A, 2B, 2 C, and 2D. Among them, GluN2B subtype dominates at synapses of lamina I in adult spinal dorsal horn and is involved in diversified pain processing via mediating nociceptive signal transmission in the spinal cord.14–17 For instance, in the animal model of formalin-induced inflammatory pain, GluN2B exhibited highest expression in the spinal cord compared to other NMDA subunit receptors.18 Moreover, propofol was found to produce antinociception in hot-plate test and acetic acid-induced writhing test in mice through interaction with spinal NMDA receptor.19 Therefore, GluN2B-containing NMDA subunits may contribute to the analgesic action of propofol.
Mitogen-activated protein kinases (MAPKs) family, including extracellular signal-regulated kinase1/2 (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase (JNK), transduces a wide range of extracellular stimuli into various intracellular responses by transcriptional or non-transcriptional regulation.20 Accumulating evidence shows that the activation of MAPKs in both neurons and glia cells involves in the induction and maintenance of pain hypersensitivity under different types of pain conditions.21 MAPKs activation can be blocked by NMDA receptor antagonists in vivo and in vitro and that blocks the noxious-signal transmission induced by ERK activation consequently.22–26
Pre-emptive analgesia that modulates sensory input before surgery has been widely used as an analgesic strategy to manage post-surgical pain by preventing or suppressing spinal mechanisms of neuronal sensitization.27 Pre-emptive single dose of intravenous propofol was found to reduce post-surgical analgesia requirements in comparison with ketamine or remifentanil clinically.9 Inflammatory pain is the most often post-surgical sequela partially due to tissue injury and release of inflammatory mediators.28 In this study, using the well-established inflammatory pain model induced by formalin injection and adopting the same modality used in clinical administration by intravenous infusion of propofol, we attempted first to investigate whether propofol infused before inflammation challenge possesses preventive analgesic effects on inflammatory pain; second to evaluate whether GluN2B subunit-containing NMDA receptor and downstream of MARK cascades play a role in the analgesic property of propofol at the spinal cord level following inflammatory pain; and finally, to identify the cellular mechanisms underlying its preventive analgesic effect at the spinal dorsal horn level.
Materials and methods
Animals
Male adult Sprague–Dawley rats weighing approximately 250–300 g were used throughout the study. Rats were housed in cages with ad libitum food and water on a standard 12:12 h light/dark cycle. Animal experiments were conducted by animal license holders authorised by Department of Health, The Government of Hong Kong Special Administrative Region and approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR, reference number #3383–14) at the University of Hong Kong. Rats were euthanatized by overdose of sodium pentobarbital (Virbac, Milperra, Australia) by intraperitoneal injection followed by cervical decapitation after all the experiments.
Experimental design
Rats were randomly divided into four groups using an online software (www.randomization.com): Naive group without any treatment (Group Naïve), formalin treatment group with formalin injection only (Group Formalin, MilliporeSigma, St. Louis, MO, USA), and formalin injection with pretreatment of propofol followed by a recovery time of 30 min (Group P-30 min) or 2 h (Group P-2 h). In the groups with pretreatment of propofol, rats received 0.6 mg kg−1 min−1 of propofol (B. Braun, Melsungen, Germany) via tail vein for 1 h. Prior to formalin injection, either 30 min or 2 h recovery time was allowed for animals to recover from the anesthetic effect of propofol for Group P-30 min and Group P-2 h, respectively. The animals that did not conform to the recovery criteria, based on the modified system for post-anesthesia recovery scoring, were eliminated from this study.29
Formalin-induced inflammatory pain
At 30 min or 2 h after propofol infusion, inflammatory pain was induced by injection of 50 µl of 2.5% formalin solution (MilliporeSigma) into the plantar of right hind paw using a 30-gauge, ultra-fine needle (Becton, Dickinson and Company, NJ, USA). Pain severity was evaluated using the composite pain score- weighted scores technique 0,1,2 × time (Composite pain score [CPS]-WST0,1,2) after the injection of formalin.30 Rats were housed individually in plexiglass chambers on a metal mesh for acclimation to the chamber, and observation of the animal’s behavior was made in consecutive 5-min periods for 60 min after formalin administration. In each 5-min period, the total time the animal spent in three different behavioral categories was recorded: (1) the injected paw had little or no weight placed on it; (2) the injected paw was raised; and (3) the injected paw was licked, shaken, or bitten. The CPS was calculated according to the following formula
To avoid experimental bias, the tester was blind to the study groups.
Western blot
In another cohort of animals with identical treatment, at 25 min after the injection of formalin when the pain sensitivity reached peak, both ipsilateral and contralateral dorsal horns of lumbar spinal cord (from segments L3 to L5) were dissected after a laminectomy under deep anesthesia of isoflurane (Abbott Laboratories, Berkshire, UK). The dorsal horn sample was homogenized in ice-cold Laemmli buffer containing 50 mM Tris-HCl, pH7.5, 0.5% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, and 1% protease inhibitor cocktail (MilliporeSigma). The proteins were separated on SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. Membranes were then incubated with different primary antibodies including phosphorylated or pan-GluN2B (Merck Millipore, Darmstadt, Germany), ERK1/2, p38 MAPK, JNK (Cell Signaling Technology, Danvers, MA, USA), and the loading control glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Merck Millipore, Darmstadt, Germany) separately overnight at 4℃. Membranes were then incubated with goat anti-rabbit IgG (Cell Signaling Technology, Danvers, MA, USA) or goat anti-mouse IgG for 1 h at room temperature. Proteins were detected by enhanced chemiluminescence (Bio-Rad Laboratories, Hercules, CA, USA) and visualized on X-ray films. Densitometry was analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). The density of specific bands of Western blot was then normalized to corresponding loading control bands.
Calcium fluorometry in vitro
The change in intracellular calcium concentration induced by the activation of NMDA receptors is important for neuron hyperexcitation. The concentration of intracellular calcium was thus evaluated using the visible light-excitable calcium indicator kits on neuroblastoma cell line SH-SY5Y cells, which are undifferentiated human-derived neuroblastoma cell line. SH-SY5Y cells of passage 10–12 were seeded onto polystyrene plates pre-coated with 0.01% collagen (MilliporeSigma), with a plating density of 6 × 104 cells/well. Cells were maintained in 100 µl Dulbecco’s modified eagle medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin-fungizone (P/S, Thermo Fisher Scientific). SH-SY5Y cells were kept in an incubator with humidified atmosphere and 5% CO2 at 37℃. After 24 h, Fluo-4 NW calcium dye (Thermo Fisher Scientific) was used to monitor the alteration of intracellular calcium concentration according to the manufacturer’s instruction. Cells were divided into seven groups including (1) three stimulation groups in which cells were stimulated by NMDAR agonists NMDA and glycine (NG group) or with 1 h pre-incubation of 3 µM of propofol (MilliporeSigma; P3 + NG group) or 10 µM of propofol (P10 + NG group), (2) two propofol groups without NMDAR stimulation in which cells were incubated with 3 µM of propofol (P3 group) or 10 µM of propofol (P10 group), and (3) two control groups in which cells had no treatment as a negative control group or were stimulated with 5 µM of Ionomycin (Thermo Fisher Scientific), which induced a maximal calcium influx as a positive control group. The two concentrations of propofol 3 µM and 10 µM were approximately equivalent to propofol blood concentration that have been reported at 2 h and 30 min after infusion in rats.31 The intracellular calcium concentration was detected by micro-plate reader (Thermo Fisher Scientific) with 494 nm excitation wavelength, 12 nm excitation bandwidth, and 519 nm emission wavelength. All data were recorded after the administration of 30 µM of NMDA receptor agonist NMDA (MilliporeSigma) and 15 µM of co-agonist glycine (MilliporeSigma) immediately by SkanIt software (Thermo Fisher Scientific). The final data were normalized relative to Ionomycin.
Immunofluorescence staining
Animals were deeply anesthetized with sodium pentobarbital and perfused transcardially with ice-cold 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Lumbar spinal segments L3–L5 were collected and post-fixed in 4% paraformaldehyde and then dehydrated overnight in 30% sucrose at 4℃. The tissues were frozen in tissue freezing medium and sliced transversely at 15 µm using a cryostat (Leica Microsystem, Wetzlar, Germany). The sections were then blocked with 10% normal goat serum in phosphate-buffered saline (PBS) at room temperature for 1 h and followed by incubation with primary antibody(s), including mouse anti-c-Fos (1:100, Abcam, Cambridge, UK) mixed with rabbit anti-NeuN antibody (1:500, Abcam), rabbit anti p-ERK antibody (1:100, Cell Signaling Technology) mixed with mouse anti-NeuN antibody (1:500, Abcam) at 4℃ for overnight. After washing with PBS, the sections were incubated with fluorescent-conjugated secondary antibodies (1:1000, goat anti-mouse IgG conjugated with Alexa Fluor 488 or 568 and goat anti-rabbit IgG conjugated with Alexa Fluor 568 or 488, Thermo Fisher Scientific Corporation). The sections were mounted with mounting medium with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Vector Laboratories, Burlingame, CA, USA). The immunoreactive cells were identified under a confocal microscope (LSM 780, Carl Zeiss, Oberkochen, Germany) and positive labeled cells were counted and expressed as a percent of total cells under the same fields.
Statistical analysis
All data in this study are expressed as mean ± standard error of the mean. Calculations were performed by GraphPad Prism software (GraphPad software Inc, La Jolla, CA, USA). Data of time course of recovery times were analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, while other data were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test. P values less than 0.05 were considered as statistically significant.
Results
Propofol administrated before formalin challenge reduces nociceptive responses
Changes in nociceptive response following formalin injection
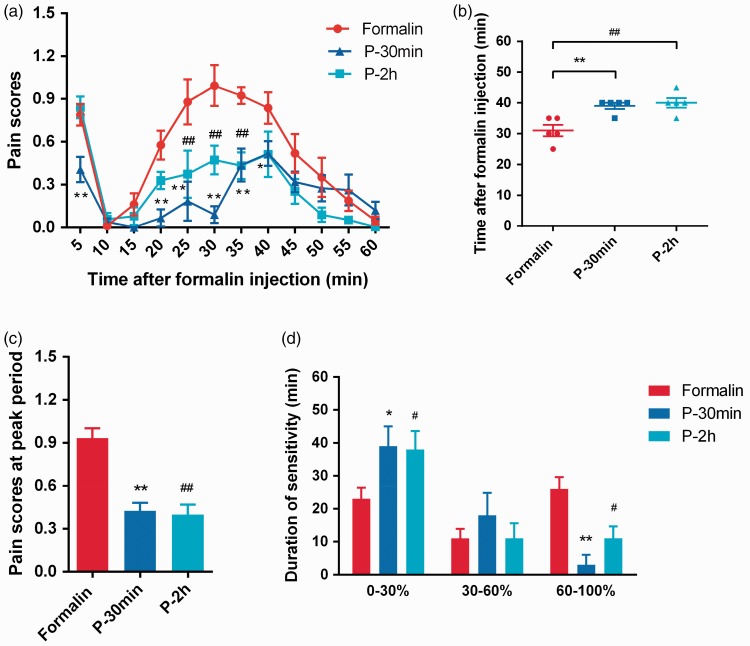
Consistent to previous reports,32,33 bi-phasic nociceptive responses including an early phase (0–10 min and a late phase) (15–60 min) was induced after subcutaneous injection of 2.5% formalin into the hind paw (Figure 1(a)). In the Formalin group, the rats displayed pain-related behaviors including hind paw lifting, flinching, and licking. In the early phase, pain scores increased in 5 min and quickly returned to baseline in 10-min post-injection. Although in the late phase, pain scores gradually increased and peaked at 25–35 min before returning to baseline in 60 min post-injection (Figure 1(a)).
Figure 1.
Propofol reduced formalin-induced nocifensive reflexes. Time courses of pain score in Formalin group and two propofol groups P-30 min and P-2 h after formalin injection (a), time of sensitization at peak (b), pain score at peak (c), and duration of low, medium, and high sensitivity (i.e., the time when animals showed pain scores below 30%, 30%–60%, and above 60% of the peak score induced by formalin) (d) were illustrated. *P < 0.05, **P < 0.01: P-30 min group versus Formalin group; #P < 0.05, ##P < 0.01: P-2 h group versus Formalin group; n = 5. (a) Two-way ANOVA followed by Tukey’s multiple comparisons test. (b) to (d) One-way ANOVA followed by Dunnett’s multiple comparisons test.
Preventive analgesic effects of propofol on inflammatory pain induced by formalin
As shown in Figure 1(a), the increased pain scores induced by formalin were significantly reduced by pre-emptive infusion of propofol in both Group P-30 min and Group P-2 h.
Firstly, in the early phase, the pain score in the P-30 min propofol treatment group was significantly lower than that in the Formalin group (0.41 ± 0.09 vs. 0.79 ± 0.07, P = 0.0109, n = 5). Secondly, in the late phase, the rats displayed a slow response to formalin challenge with a 10-min delay (Figure 1(b)). The time to reach peak sensitization after injection in P-30 min and Formalin groups were 30 ± 2 and 39 ± 1 min (P = 0.007, n = 5), respectively. Thirdly, P-30 min group showed a significant reduction in peak pain scores (Figure 1(c)). The peak pain scores summarized in the period of 35–45 min (P-30 min group) and 25–35 min (Formalin group) post-formalin injection were 0.43 ± 0.05 and 0.93 ± 0.07 (P < 0.0001, n = 5), respectively. Last but not least, pre-emptive treatment of propofol significantly prolonged the duration of low sensitivity (i.e. the time when animals showed pain scores below 30% of the peak score induced by formalin) and reduced the duration of high sensitivity (i.e. the time when animals showed pain scores above 60% of the peak score induced by formalin, Figure 1(d)). Durations below 30% of maximum in P-30 min and Formalin groups were 39 ± 6 min versus 23 ± 3 min (P = 0.0352, n = 5) and that above 60% of maximum were 3 ± 7 versus 26 ± 4 min (P = 0.0023, n = 5). However, there was no significant difference between P-30 min and Formalin groups during 30–60% of maximum (18 ± 7 and 11 ± 3 min, P = 0.4619, n = 5, Figure 1(d)).
In contrast to P-30 min group, P-2 h treatment in the P-2 h propofol treatment group did not show significant effect on pain scores induced by formalin in the early phase (P-2 h versus Formalin: 0.84 ± 0.08 versus 0.79 ± 0.07, P = 0.9140, n = 5, Figure 1(a)). Nevertheless, in the late phase, the pain scores in P-2 h group were lower than that in Formalin group at 25, 30, and 35 min after formalin injection (P < 0.001, n = 5). Rats in P-2 h group also displayed a slow response to formalin challenge (Figure 1(b)). The time to reach the peak sensitization after formalin injection in P-2 h and Formalin groups were 40 ± 2 and 30 ± 2 min (P = 0.0035 n = 5), respectively. Moreover, rats in P-2 h group also showed a significant reduction on pain scores at the peak of sensitization (Figure 1(c)). The peak pain scores summarized during 25–35 min after formalin injection in P-2 h and Formalin group were 0.40 ± 0.07 and 0.93 ± 0.07 (P < 0.0001, n = 5), respectively. Similar to P-30 min group, pre-emptive treatment of propofol in P-2 h group also significantly increased the duration of low sensitivity and decreased the duration of high sensitivity (Figure 1(d)). Durations below 30% of maximum in P-2 h and Formalin groups were 38 ± 6 and 23 ± 4 min (P = 0.05, n = 5) and the periods above 60% of maximum were 11 ± 4 and 26 ± 4 min (P = 0.05, n = 5), respectively.
Taken together, these results demonstrated that intravenous propofol administrated before formalin challenge (pre-emptively) reduced formalin-induced nocifensive behaviors in both duration and intensity. It is worth noticing that this analgesic effect of propofol lasted for at least 2 h after propofol administration.
Propofol inhibits the expression of spinal phosphorylated GluN2B induced by formalin injection
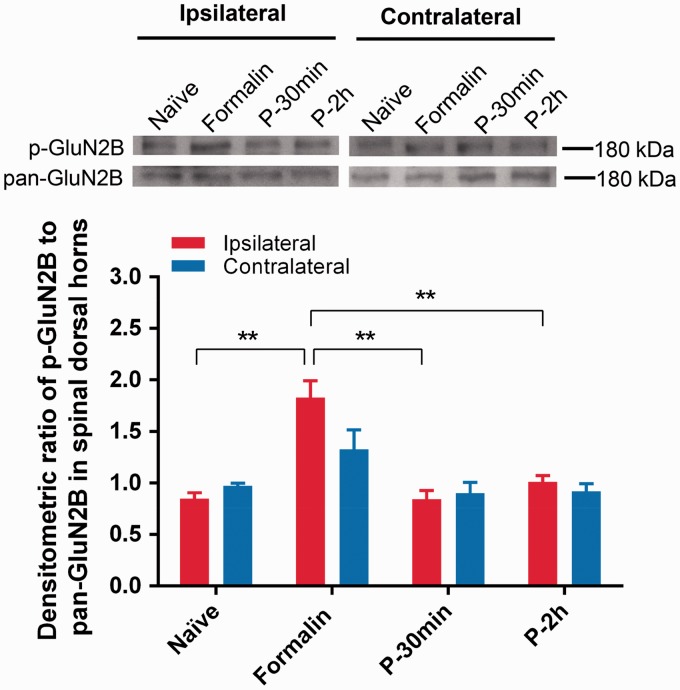
Spinal GluN2B subunit-containing NMDA receptors play an important role in central sensitization in nociceptive processing,14 which might be a potential target of propofol under its antinociceptive effect in this inflammatory pain model. We thus detected the expression of phosphorylated and pan-GluN2B in the spinal dorsal horn following formalin injection with pre-treatment of propofol. As shown in Figure 2, the expression of phosphorylated GluN2B was significantly increased in the ipsilateral spinal dorsal horns after formalin injection compared to that in Naive group (1.83 ± 0.16 versus 0.85 ± 0.06, P = 0.001, n = 5). Interestingly, pre-emptive treatment of propofol prevented the formalin-induced activation of spinal GluN2B in both P-30 min and P-2 h groups (P-30 min, P-2 h versus Formalin: 0.73 ± 0.13, 0.92 ± 0.10 versus 1.83 ± 0.16, P = 0.0003, n = 5, Figure 2). In contrast, there was no detectable difference in the contralateral dorsal horns among all the groups (Figure 2). These results indicate that propofol reduces nocifensive behaviors caused by formalin via preventing the activation of GluN2B subunit-containing NMDA receptors in the spinal cord dorsal horn in the inflammatory pain animal model.
Figure 2.
Protein expression of phosphorylated GluN2B (p-GluN2B) and pan-GluN2B in spinal dorsal horns of L3–L5. Relative intensity of p-GluN2B to pan-GluN2B in ipsilateral and contralateral dorsal horns was illustrated. *P < 0.05, **P < 0.01 versus Formalin group, n = 5, one-way ANOVA followed by Dunnett’s multiple comparisons test.
Propofol inhibits the calcium influx induced by NMDA receptor agonists in SH-SY5Y cells
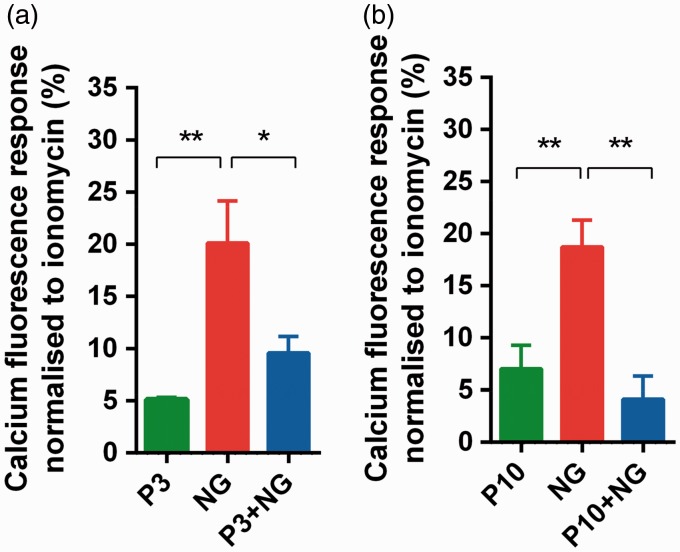
We further identified whether calcium influx through NMDA receptors could be altered by pre-treatment with propofol in vitro. It was found that pre-emptive treatment with 3 µM of propofol for 1 h significantly reduced the calcium fluorescence intensity evoked by NMDA receptor agonists NMDA and glycine (P3 + NG versus NG: 9.58 ± 4.70 versus 20.11 ± 4.04, P = 0.017 n = 9, Figure 3(a)). Similarly, 10 µM of pre-emptive propofol also diminished the elevation of intracellular calcium caused by NMDA receptor agonists (P10 + NG versus NG: 4.15 ± 2.19 versus 18.73 ± 2.57, P = 0.0012, n = 13, Figure 3(b)). Thus, these results in vitro confirmed that pre-emptive propofol inhibits NMDA receptor activation and subsequently reduces the calcium influx.
Figure 3.
Propofol blocked NMDA receptor-mediated calcium influx in vitro. In each group, fluorescence responses were normalized to maximal calcium fluorescence induced by Ionomycin. The effects of pre-emptive 3 µM (a) and 10 µM (b) propofol were illustrated. *P < 0.05, **P < 0.01 versus NG group, n = 13, one-way ANOVA followed by Dunnett’s multiple comparisons test. P3: 3 µM of propofol; NG: NMDA and glycine; P3 + NG: pre-treatment with 3 µM of propofol for 1 h followed by NMDA and glycine; P10: 10 µM of propofol; P10 + NG: pre-treatment with 10 µM of propofol for 1 h followed by NMDA and glycine.
Propofol inhibits the activation of spinal MAPKs ipsilateral to formalin challenge
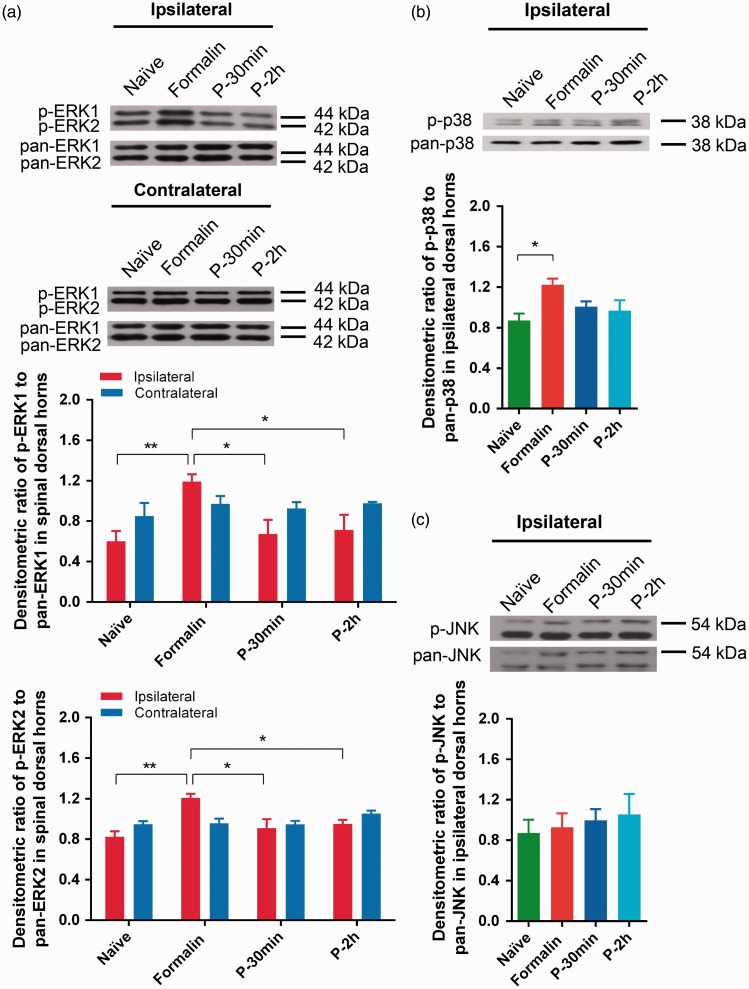
Since accumulating evidence showed that MAPK signaling pathways contribute to pain sensitization after nerve injury and inflammation,21,34,35 the role of MAPK cascades in the pre-emptive antinociceptive effects of propofol was further investigated. As shown in Figure 4(a), following formalin injection, phosphorylated ERK1/2 (p-ERK1/2) protein level was elevated in the ipsilateral dorsal horn compared with that in Naive group (Formalin versus Naïve—for ERK1: 1.19 ± 0.07 versus 0.60 ± 0.10, P = 0.0059, n = 5; for ERK2: 1.21 ± 0.04 versus 0.82 ± 0.05, P = 0.0017, n = 5, Figure 4(a)), which was blocked by pre-emptive treatment of propofol in both P-30 min and P-2 h groups (P-30 min, P-2 h, versus Formalin—for ERK1: 0.67 ± 0.14, 0.71 ± 0.15, versus 1.19 ± 0.07, P = 0.0145, n = 5; for ERK2: 0.91 ± 0.09, 0.95 ± 0.04, versus 1.19 ± 0.07, P = 0.029, n = 5, Figure 4(a)). There was no statistical difference in ERK activation on the contralateral sides among all the treatment groups (Figure 4(a)).
Figure 4.
Protein expression of phosphorylated or pan-ERK1/2, p38 MAPK, and JNK in dorsal horn of L3–L5. Relative intensity of phosphorylated MAPKs to pan MAPKs is shown in the graphs. Ipsilateral and contralateral ERK1/2 (a), ipsilateral p38 MAPK (b), and JNK (c) were illustrate. *P < 0.05, **P < 0.01 versus Formalin group, n = 5, one-way ANOVA followed by Dunnett’s multiple comparisons test.
An increase in the activation of p38 MAPK was found following formalin injection in the ipsilateral spinal dorsal horn. This elevated phosphorylated p38 MAPK (p-p38) was not altered by pre-emptive treatment of propofol (Formalin versus Naïve: 1.22 ± 0.06 versus 0.81 ± 0.07, P = 0.0393, n = 5, Figure 4(b)). Unlike ERK1/2 and p38 MAPK, phosphorylated JNK (p-JNK) was neither altered by formalin nor propofol pre-treatment (Figure 4(c)). These results showed that only ERK activation in the MARK cascades was involved at the spinal level in the pre-emptive antinociceptive effect of propofol in this inflammatory pain model.
Propofol suppresses the neuronal expression of c-Fos and p-ERK induced by formalin challenge in spinal dorsal horn
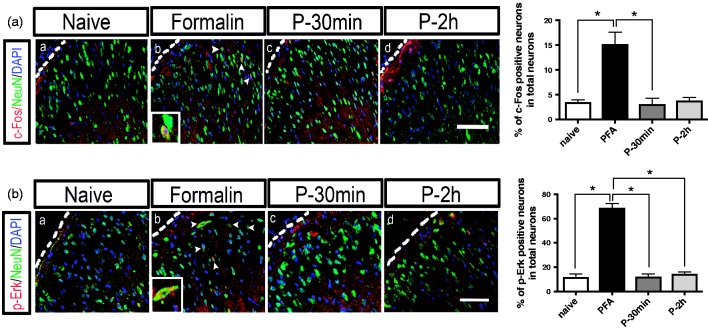
To further examine whether propofol produces its preventive analgesic effect through directly modulating the neuronal activity in this inflammatory pain model, double immunofluorescence labeling was used to identify the neuronal activity in the spinal dorsal horn following formalin injection. As shown in Figure 5(a), formalin injection induced a significant increase in c-Fos-labeled cells (15.19% ± 2.47, n = 3, P < 0.05) compared to that in the Naive group (3.44% ± 0.56, n = 3). Interestingly, these c-Fos-positive cells were co-expressed with the neuronal marker NeuN (Figure 5(a) to (b)), suggesting that these c-Fos-labeled cells are neuronal soma. These double-labeled cells were predominantly distributed in the superficial laminae of lumber spinal dorsal horn (Figure 5(a) to (b)). However, the formalin-induced increase in c-Fos/NeuN double-labeled cells were reduced by pre-emptive treatment of propofol in both P-30 min (3.11% ± 1.21, P < 0.05, n = 3, Figure 4(a) to (c)) and P-2 h groups (3.82% ± 0.65, P < 0.05, n = 3, Figure 5(a) to (d)).
Figure 5.
Distribution and co-localization of c-Fos-positive and p-ERK-positive-labeled neurons in the spinal cord dorsal horn following different treatment groups, including Naive group without any treatment (Naïve), formalin treatment group with formalin injection only (Formalin), and formalin injection with pretreatment of propofol followed by a recovery time of 30 min (P-30 min) or 2 h (P-2 h). (a) Confocal fluorescence images illustrate the co-localization of c-Fos-positive cells (red), NeuN (green), and DAPI (blue) in the different treatment groups. (b) Confocal fluorescence images illustrate the co-localization of p-ERK-positive cells (red), NeuN (green), and DAPI (blue) in the different treatment groups. Dotted lines show the estimated edges of laminae I. Scale bar: 50µm. The column figures on the right panel show the summary data following different treatments. *P < 0.05, n = 3, one-way ANOVA on ranks followed by Dunn’s post hoc test.
Furthermore, it was also found that the number of p-ERK immunoreactive cells increased after formalin injection, and these p-ERK-positive cells were also double labeled with the NeuN-positive cells, indicating that ERK is primarily activated in dorsal horn neurons located primarily in the superficial laminae of lumber spinal dorsal horn (Figure 5(b)). As shown in Figure 5(b), formalin induced a robust increase in the double-labeled p-ERK/NeuN-labeled cells (68.76% ± 3.77, n = 3, P < 0.05, Figure 5(b)) compared with the Naive group (11.74% ± 2.59, n = 3), whereas pre-emptive treatment with propofol significantly reversed this increase in both P-30 min (11.87% ± 2.67, n = 3, P < 0.05, Figure 5(b) to (c)) and P-2 h groups (14.28% ± 1.83, n = 3, P < 0.05, Figure 5(b) to (d)). Given that the activation of ERK in superficial spinal dorsal horn after noxious stimulation or inflammation is specifically essential for the induction of central sensitization,36,37 activation (phosphorylation) of ERK in dorsal horn neurons has been served as a marker for central sensitization. Thus, these results show that pre-emptive propofol infusion produces its preventive analgesic effect on formalin-induced inflammatory pain via suppressing of neuronal p-ERK activation and central sensitization in the lumbar spinal dorsal horn.
Discussion
This study demonstrates that intravenous infusion of propofol before inflammatory (formalin) challenge produces preventive analgesic effects characterized by a delayed response to formalin and a reduction in hypersensitivity. This preventive analgesic property of propofol appears to be attributed to its inhibitory effects on calcium influx through NMDA receptor and the downstream molecules of ERK1/2 MAPKs in the spinal dorsal horn neurons.
While clinical studies have reported that surgical patients receiving propofol-based anesthesia intraoperatively experienced less postsurgical pain,38–40 the findings from clinical and pre-clinical studies vary widely from analgesic effect,5,9 no analgesia,7 or hyperalgesia.8 Animal studies reported that topical or intrathecal propofol produced antinociceptive effects on heat-evoked responses41 or antihyperalgesic effect on formalin-induced pain,42 whereas intravenous infusion of propofol showed no analgesia on the second phase of formalin-induced nocifensive responses.43 Even in the same rat model of formalin-induced inflammatory pain, Merrill et al.6 reported that propofol lacks analgesic property because it does not prevent the formalin-induced Fos-like expression, a neuronal marker for noxious stimulation. However, Gilron et al.44 found that when given pre-emptively before formalin injection, propofol did suppress the formalin-induced spinal Fos-like immunoreactivity, suggesting its important analgesic property of propofol. Apparently, not only does the stimulus nature but more notably the administration timing of propofol, e.g., pre- versus post-formalin injection, plays a role in the discrepant results.
Pre-emptive analgesia given before surgery has been widely used as an analgesic strategy to reduce postsurgical pain severity and duration and even prevent the development of postsurgical chronic pain.45 Recent study found that pre-emptive single dose of intravenous propofol decreased post-surgical analgesia requirements compared with ketamine or remifentanil.9 Inflammatory pain is the most common sequela after surgery due to tissue injury and tissue injury induced release of inflammatory mediators.28 This study, for the first time using the well-established inflammatory pain model induced by formalin and adopting the same modality used in clinical administration (i.e. TIVA) by intravenous infusion of propofol at a dose of 0.6 mg kg−1 min−1 (the equivalent human dosage 0.1 mg kg−1 min−1 for maintenance of general anesthesia calculated according to a guide for dose conversion between animals and human46), evaluated whether pre-emptive propofol could prevent the development of hyperalgesia following inflammatory pain.
Consistent with previous report,30 the biphasic nocifensive reflexes were found after intra-plantar injection of formalin in this study, including an early phase (0–10 min) and a late phase (15–60 min), which reflect the direct activation of nociceptors and spinal neuronal sensitization with functional alteration, respectively, following formalin injection.44,47 There are several evidence-based findings in this study. First, propofol infusion resulted in preventive analgesic effect on formalin challenge even though it was stopped for 30 min to 2 h when its anesthetic effect disappeared. Second, compared to the P-30 min group in which propofol produced antinociceptive effect on both early and late phases, propofol treatment with 2 h recovery time exhibited pain relieving effect only on the late phase while leaving the early phase unaffected. This finding is supported by Takechi’s report that topical application of propofol suppressed noxious heat-evoked responses maximally at 15 min after application and its antinociceptive effect was faded within 30 min.41 In the same model of formalin-induced inflammatory pain, single bolus of pre-emptive propofol administration (5–20 mg kg−1) before formalin injection showed pain relief in the early phase but not in the late phase.43 The reason is likely that single bolus of propofol (10 mg kg−1) loses its effect quickly (in 4 min)48 so that its action may be transient only in the peripheral nervous system. In the present study, intravenous perfusion of propofol for 1 h led to preventive analgesia in both early and late phases of the formalin pain model, indicating that propofol may need longer time to exert its analgesic effect through modulating the neuronal excitability and responsiveness in the central nervous system such as suppressing the spinal dorsal neuronal c-Fos expression and ERK1/2 activation. Finally, the present study showed that the rats in both P-30 min and P-2 h groups displayed a slow hypersensitization to formalin challenge, less pain severity, and a shorter duration of experiencing hypersensitization. These effects exhibiting in the P-2 h group indicate that the preventive analgesic property of propofol can last for at least 2 h after infusion in this animal model of inflammatory pain.
It is well known that NMDA receptors play critical roles in generating and mediating nociceptive signal transmission and central sensitization at the spinal cord level.49 Intrathecal injection of NMDA receptor agonist is associated with a significant increase in phospho-Tyr1472 GluN2B, whereas no changes were observed in pan-GluN2B expression during inflammatory pain.14,50 Additionally, gene knockdown of GluN2B expression relieved formalin-induced nociception in the late phase in rats.51,52 In concordance with behavioral results, propofol infusion with 30 min or 2 h recovery periods significantly reduced the expression of phosphorylated GluN2B in the ipsilateral spinal dorsal horn, whereas no change was found on the contralateral side. The findings of the antinociceptive effect of pre-emptive propofol (at least 2 h after the infusion) may help to explain the clinical findings that administration of propofol during operations shows superior efficacy in management of postoperative pain as compared with other inhalational anesthetics.38–40 Furthermore, given the fact that the activation of synaptic GluN2B in nociceptive neurons can result in calcium influx and a variety of calcium-mediated cascades that subsequently induce augmentation of neuronal excitability and hypersensitivity to peripheral stimuli,16 our findings that NMDA receptor-mediated calcium influx was significantly inhibited by pre-incubation with either 3 µM or 10 µM of propofol for 1 h in SH-SY5Y cells provide the first evidence in vitro that pre-treatment with propofol inhibits calcium influx through GluN2B-containing NMDA receptor.
ERK1/2 is one of the downstream effectors of GluN2B receptor in the postsynaptic neurons in spinal dorsal horn,53 and ERK1/2 activation induced by noxious stimulus can be reduced by NMDA receptor blockers.36 Activation of ERK1/2 was enhanced after formalin challenge in the spinal dorsal horn neurons, whereas the inhibition of ERK1/2 reduced nociceptive response in the late phase of formalin test.54 Consistent with previous reports,36,55 we found that phosphorylation ERK (p-ERK1/2)-labeled neurons were increased in the superficial dorsal horn after formalin injection, which was suppressed by pre-emptive propofol infusion in both groups with 30 min and 2 h recovery periods. These findings indicate that pre-emptive propofol-induced reversal of the activation of ERK1/2 induced by formalin in ipsilateral dorsal horn contributes to the delayed sensitization and reduced hypersensitivity in inflammatory pain. This feature of propofol is most likely associated with its inhibitory effect on pain hypersensitivity induced by ERK1/2 activation as intrathecal injection of the MEK inhibitor PD 98059, which blocks ERK phosphorylation, dose-dependently reduced the late phase of pain response without changing the early phase following formalin injection.36 Additionally, though formalin induced an increase in p38 MAPK expression compared with that in the Naive group, propofol had no effect on the activation of p38 MAPK in formalin-induced inflammatory pain model (Figure 6). Unlike ERK1/2, p38 MAPK is mainly activated by cytokines after inflammation.36 The current findings indicate that the analgesic property of propofol may not be directly associated with its anti-inflammatory effect.56
Figure 6.
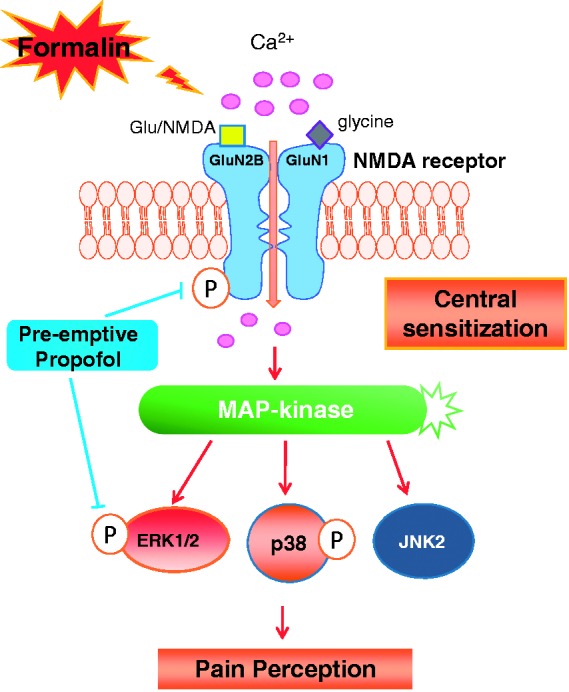
Schematic diagram depicts the GluN2B-contaning NMDA receptor/ERK1/2 signaling pathway underlying the preventive analgesic effect of pre-emptive intravenous propofol on formalin-induced inflammatory pain. Formalin injection results in the upregulation of phosphorylated GluN2B-contaning NMDA receptor, which subsequently induces an increase in calcium influx and activates ERK1/2 and p38 MAPK signal transduction pathways. Pre-emptive intravenous propofol inhibits the formalin-induced activation of spinal GluN2B that, through decreasing calcium influx, blocks ERK1/2 activation, and thereby reduces pain hypersensitivity following nociceptive stimulation. P: phosphorylation; Glu: glutamate.
Finally, c-Fos has been used as a neuronal marker to study nociception with its rapid expression characterisitc in neurons in response to various nociceptive stimuli.57 A consistency with previous reports was observed in the current study in c-Fos expression and distribution in the superficial spinal dorsal horn after formalin stimulation.4,6,44 Also, in accordance with previous reports that propofol directly suppressed lumber dorsal horn neuronal responses to noxious stimulation,58 the current findings in propofol suppressing the increased c-Fos-/NeuN-positive neurons is paralleled with the behavioral findings lasted up for 2 h. Thus, propofol at an anesthetic dose inhibits the activity of these projection neurons in the superficial laminae of the spinal dorsal horn and subsequently suppresses the development of central sensitization and blocks nociceptive transmission.
There are clinical implications and limitations for this study. While propofol alone is not encouraged to be used for analgesia in clinical practice because of controversy under different surgical circumstances,59,60 its pre-emptive analgesic property when used as an anesthetic for maintenance of general anesthesia could be an advantage to manage postsurgical pain as compared to other inhalational anesthetics, e.g., isoflurane has recently been reported to impair cognition by increasing the extrasynaptic expression of GluN2B in rat hippocampus.61 Moreover, with the preventive analgesic effects of propofol shown, it can potentially contribute to the multimodal analgesia with other analgesics to better control acute postsurgical pain.
In conclusion, intravenous infusion of propofol administrated pre-emptively attenuated formalin-induced inflammatory pain. This preventive analgesic effect of propofol appears to be mediated by spinal neuronal GluN2B-containing NMDA receptor and ERK1/2 MAPK pathway. The current findings provide evidence-based molecular and cellular support, at the spinal level, for the potential preventive analgesia of propofol in the perioperative management of post-surgical pain.
Acknowledgments
The authors are grateful to Ms. Man Ying Ao in the Laboratory Animal Unit, The University of Hong Kong for her ongoing technical support in our work and the assistance of the Faculty Core Facility in acquiring the confocal images. The authors also would like to thank Mr. Haydn H.C Shiu in the Laboratory and Clinical Research Institute for Pain, Department of Anaesthesiology, the University of Hong Kong for his technical help in popofol infusion during the experiemnts.
Author Contributions
QQ and LS participated in study design, data collection, result interpretation, and drafting the manuscript. PG and XMW contributed to partial experiment design, data collection, and interpretation. XMW and CWC participated in result interpreration, writing, and editing the manuscript. CWC was entirely responsible for the overall study design, overseeing data collection and analysis, as well as manuscript finalization. ACYL, KLW, and SSW contributed to manuscript critical reading and editing process. All authors read and approved the final manuscript. QQ and LS contributed equally to this work.
Declaration of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Small Project Funds (Reference No. 201309176075) from Li Ka Shing Faculty of Medicine, The University of Hong Kong.
References
- 1.White PF. Propofol: its role in changing the practice of anesthesia. Anesthesiology 2008; 109: 1132–1136. [DOI] [PubMed] [Google Scholar]
- 2.Chan AC, Qiu Q, Choi SW, et al. Effects of intra-operative total intravenous anaesthesia with propofol versus inhalational anaesthesia with sevoflurane on post-operative pain in liver surgery: a retrospective case-control study. PloS one 2016; 11: e0149753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu Q, Choi SW, Wong SS, et al. Effects of intra-operative maintenance of general anaesthesia with propofol on postoperative pain outcomes - a systematic review and meta-analysis. Anaesthesia 2016; 71: 1222–1233. [DOI] [PubMed] [Google Scholar]
- 4.Ma D, Sanders RD, Halder S, et al. Xenon exerts age-independent antinociception in Fischer rats. Anesthesiology 2004; 100: 1313–1318. [DOI] [PubMed] [Google Scholar]
- 5.Anker-Moller E, Spangsberg N, Arendt-Nielsen L, et al. Subhypnotic doses of thiopentone and propofol cause analgesia to experimentally induced acute pain. Br J Anaesth 1991; 66: 185–188. [DOI] [PubMed] [Google Scholar]
- 6.Merrill AW, Barter LS, Rudolph U, et al. Propofol’s effects on nociceptive behavior and spinal c-fos expression after intraplantar formalin injection in mice with a mutation in the gamma-aminobutyric acid-type(A) receptor beta3 subunit. Anesth Analg 2006; 103: 478–483. [DOI] [PubMed] [Google Scholar]
- 7.Wilder-Smith OH, Kolletzki M, Wilder-Smith CH. Sedation with intravenous infusions of propofol or thiopentone. Effects on pain perception. Anaesthesia 1995; 50: 218–222. [DOI] [PubMed] [Google Scholar]
- 8.Frolich MA, Price DD, Robinson ME, et al. The effect of propofol on thermal pain perception. Anesth Analg 2005; 100: 481–486. [DOI] [PubMed] [Google Scholar]
- 9.Naghibi K, Kashefi P, Abtahi AM. The comparison of preemptive effects of propofol, remifentanil and ketamine on post-operative pain scores and analgesic requirements in elective lower abdominal surgery under general anesthesia: a randomized, double-blinded study. J Res Med Sci 2013; 18: 567–572. [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg 2008; 106: 264–269. [DOI] [PubMed] [Google Scholar]
- 11.Orser BA, Bertlik M, Wang LY, et al. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol 1995; 116: 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todorovic SM, Lingle CJ. Pharmacological properties of T-type Ca2+current in adult rat sensory neurons: effects of anticonvulsant and anesthetic agents. J Neurophysiol 1998; 79: 240–252. [DOI] [PubMed] [Google Scholar]
- 13.Bardoni R, Magherini PC, MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. J Neurosci 1998; 18: 6558–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W, Zou S, Guan Y, et al. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci 2002; 22: 6208–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan QQ, Li L, Wang WT, et al. Activation of alpha2 adrenoceptors inhibited NMDA receptor-mediated nociceptive transmission in spinal dorsal horn of mice with inflammatory pain. Neuropharmacology 2014; 77: 185–192. [DOI] [PubMed] [Google Scholar]
- 16.Tong CK, MacDermott AB. Synaptic GluN2A and GluN2B containing NMDA receptors within the superficial dorsal horn activated following primary afferent stimulation. J Neurosci 2014; 34: 10808–10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y, Cho HY, Ahn YJ, et al. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain 2012; 153: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 18.Gaunitz C, Schuttler A, Gillen C, et al. Formalin-induced changes of NMDA receptor subunit expression in the spinal cord of the rat. Amino acids 2002; 23: 177–182. [DOI] [PubMed] [Google Scholar]
- 19.Xu AJ, Duan SM, Zeng YM. Effects of intrathecal NMDA and AMPA receptors agonists or antagonists on antinociception of propofol. Acta Pharmacol Sin 2004; 25: 9–14. [PubMed] [Google Scholar]
- 20.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912. [DOI] [PubMed] [Google Scholar]
- 21.Ji RR, Gereau RWt, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan J, Gladding CM, Wang L, et al. P38 MAPK is involved in enhanced NMDA receptor-dependent excitotoxicity in YAC transgenic mouse model of Huntington disease. Neurobiol Dis 2012; 45: 999–1009. [DOI] [PubMed] [Google Scholar]
- 23.Katsura H, Obata K, Miyoshi K, et al. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 2008; 56: 723–733. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang ZY, Wen YR, Zhang DR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 2006; 26: 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Mei XP, Wei YY, et al. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain, Behav Immun 2011; 25: 1355–1366. [DOI] [PubMed] [Google Scholar]
- 26.Wei F, Vadakkan KI, Toyoda H, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci 2006; 26: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woolf CJ, Chong MS. Preemptive analgesia-treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77: 362–379. [DOI] [PubMed] [Google Scholar]
- 28.Wang XM, Hamza M, Wu TX, et al. Upregulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: Correlation to clinical pain. Pain 2009; 142: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penderis J, Franklin RJ. Effects of pre- versus post-anaesthetic buprenorphine on propofol-anaesthetized rats. Vet Anaesth Analg 2005; 32: 256–260. [DOI] [PubMed] [Google Scholar]
- 30.Watson GS, Sufka KJ, Coderre TJ. Optimal scoring strategies and weights for the formalin test in rats. Pain 1997; 70: 53–58. [DOI] [PubMed] [Google Scholar]
- 31.Leal N, Calvo R, Agrad FZ, et al. Altered dose-to-effect of propofol due to pharmacokinetics in rats with experimental diabetes mellitus. J Pharm Pharmacol 2005; 57: 317–325. [DOI] [PubMed] [Google Scholar]
- 32.Choi SS, Seo YJ, Shim EJ, et al. Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res 2006; 1108: 28–38. [DOI] [PubMed] [Google Scholar]
- 33.Yoon MH, Bae HB, Choi JI, et al. Evaluation of interaction between intrathecal adenosine and MK801 or NBQX in a rat formalin pain model. Pharmacology 2005; 75: 157–164. [DOI] [PubMed] [Google Scholar]
- 34.Fitzsimmons BL, Zattoni M, Svensson CI, et al. Role of spinal p38alpha and beta MAPK in inflammatory hyperalgesia and spinal COX-2 expression. Neuroreport 2010; 21: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Zhang H, Shao H, et al. ERK MAP kinase activation in spinal cord regulates phosphorylation of Cdk5 at serine 159 and contributes to peripheral inflammation induced pain/hypersensitivity. PloS one 2014; 9: e87788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 2001; 8: 1–10. [DOI] [PubMed] [Google Scholar]
- 37.Karim F, Wang CC, Gereau RWt. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci 2001; 21: 3771–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Mei W, Wang P, et al. Propofol reduces early post-operative pain after gynecological laparoscopy. Acta Anaesthesiol Scand 2012; 56: 368–375. [DOI] [PubMed] [Google Scholar]
- 39.Cheng SS, Yeh J, Flood P. Anesthesia matters: Patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg 2008; 106: 264–269. [DOI] [PubMed] [Google Scholar]
- 40.Tan T, Bhinder R, Carey M, et al. Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesth Analg 2010; 111: 83–85. [DOI] [PubMed] [Google Scholar]
- 41.Takechi K, Carstens MI, Klein AH, et al. The antinociceptive and antihyperalgesic effects of topical propofol on dorsal horn neurons in the rat. Anesth Analg 2013; 116: 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishiyama T, Matsukawa T, Hanaoka K. Intrathecal propofol has analgesic effects on inflammation-induced pain in rats. Can J Anaesth 2004; 51: 899–904. [DOI] [PubMed] [Google Scholar]
- 43.Goto T, Marota JJ, Crosby G. Pentobarbitone, but not propofol, produces pre-emptive analgesia in the rat formalin model. Br J Anaesth 1994; 72: 662–667. [DOI] [PubMed] [Google Scholar]
- 44.Gilron I, Quirion R, Coderre TJ. Pre- versus postinjury effects of intravenous GABAergic anesthetics on formalin-induced Fos immunoreactivity in the rat spinal cord. Anesth Analg 1999; 88: 414–420. [DOI] [PubMed] [Google Scholar]
- 45.Dahl JB, Moiniche S. Pre-emptive analgesia. Br Med Bull 2004; 71: 13–27. [DOI] [PubMed] [Google Scholar]
- 46.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Chen X, Meng J, et al. ED50 and recovery times after propofol in rats with graded cirrhosis. Anesth Analg 2012; 114: 117–121. [DOI] [PubMed] [Google Scholar]
- 49.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991; 44: 293–299. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Cao J, Yang X, et al. NR2B phosphorylation at tyrosine 1472 in spinal dorsal horn contributed to N-methyl-D-aspartate-induced pain hypersensitivity in mice. J Neurosci Res 2011; 89: 1869–1876. [DOI] [PubMed] [Google Scholar]
- 51.Tan PH, Yang LC, Shih HC, et al. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene therapy 2005; 12: 59–66. [DOI] [PubMed] [Google Scholar]
- 52.Zhang RX, Yan XB, Gu YH, et al. Gene silencing of NR2B-containing NMDA receptor by intrathecal injection of short hairpin RNA reduces formalin-induced nociception in C57BL/6 mouse. Int J Neurosci 2013; 123: 650–656. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Li Y, Dun L, et al. Antinociceptive effects of oxymatrine from Sophora flavescens, through regulation of NR2B-containing NMDA receptor-ERK/CREB signaling in a mice model of neuropathic pain. Phytomedicine 2013; 20: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 54.Lee MJ, Jang M, Jung HS, et al. Ethyl pyruvate attenuates formalin-induced inflammatory nociception by inhibiting neuronal ERK phosphorylation. Mol pain 2012; 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji RR, Kohno T, Moore KA, et al. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26: 696–705. [DOI] [PubMed] [Google Scholar]
- 56.Samir A, Gandreti N, Madhere M, et al. Anti-inflammatory effects of propofol during cardiopulmonary bypass: a pilot study. Ann Card Anaesth 2015; 18: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull 1998; 45: 1–8. [DOI] [PubMed] [Google Scholar]
- 58.Antognini JF, Wang XW, Piercy M, et al. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth 2000; 47: 273–279. [DOI] [PubMed] [Google Scholar]
- 59.TerRiet MF, Jacobs JS, Lewis MC, et al. Propofol and analgesia. Anesth Analg 2000; 90: 1455. [DOI] [PubMed] [Google Scholar]
- 60.Jalili M, Bahreini M, Doosti-Irani A, et al. Ketamine-propofol combination (ketofol) vs propofol for procedural sedation and analgesia: systematic review and meta-analysis. Am J Emerg Med 2016; 34: 558–569. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Li Z, Cao Y, et al. Increased extrasynaptic GluN2B expression is involved in cognitive impairment after isoflurane anesthesia. Exp Ther Med 2016; 12: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]