Abstract
A series of in vivo and in vitro assays were conducted to characterize the pharmacological effects of the first generation abused synthetic cannabinoid CP47,497, a racemic bicyclic cannabinoid that is similar in structure to the potent, high-efficacy synthetic cannabinoid CP55,940. CP47,497 was less efficacious than CP55,940 in activating G-proteins and dose-dependently produced common CB1 receptor-dependent pharmacological effects (i.e. catalepsy, hypothermia, antinociception, and hypolocomotion). CP47,497 also substituted for Δ9-tetrahydrocannabinol (THC) in the mouse drug discrimination, indicating that both drugs elicited a similar interceptive stimulus. The pharmacological effects of CP47,497 underwent tolerance following repeated administration and showed cross-tolerance following repeated THC administration, further suggesting a common cannabimimetic mechanism of action. Finally, the CB1 receptor antagonist rimonabant precipitated similar magnitudes of somatic withdrawal responses in mice treated repeatedly with THC or CP47,497. Taken together, these data verify the acute cannabimimetic effects of CP47,497, and indicate tolerance and dependence following repeated administration. The assays used here provide a straightforward approach to characterize the emerging next generation of abused synthetic cannabinoids.
Keywords: CB1 receptor, dependence, designer drugs, synthetic cannabinoid, tolerance
Introduction
Cannabis has been utilized for medicinal and recreational purposes for millennia and remains the most commonly used illicit drug in the world [1]. Much of our understanding of the mechanisms by which marijuana and specifically its primary psychoactive constituent, Δ9- tetrahydrocannabinol (THC), produce its pharmacological effects can be attributed to the development of synthetic cannabinoids and their in vitro and in vivo characterization. For example, the potent, bicyclic cannabinoid receptor agonist CP55,940 was used to identify the CB1 receptor [2, 3], which is responsible for the majority of the psychoactive effects of marijuana. Many of these ligands, originally developed as pharmacological tools and potential therapeutic agents, have emerged as a prevalent new class of abused drugs that are generally added to herbal incense products and smoked [4–7]. The nonclassical cannabinoid, CP47,497, represents one of the first synthetic cannabinoids used illicitly in the US, which was identified as a powdered material confiscated by the Wisconsin State Crime Laboratory [4–8]. CP47,497 binds CB1 receptors with considerably higher CB1 receptor affinity (Ki = 2.2±0.47 nM) than the phytocannabinoid THC (Ki = 40.2±0.47 nM) [9–13]. CP47,497 has been identified in previously abused herbal preparations [14–16], highlighting a need to characterize its pharmacological effects following acute and repeated administration [17].
Initial studies demonstrated equipotent analgesic activity of CP47,497 when compared to morphine across several acute pain models in mice and rats [11–13]. The initial structure-activity relationship studies conducted with this compound and related compounds established the benzopyran ring of THC as nonessential for analgesic activity. Previous studies show that cannabinoids reliably produce dose-dependent cataleptic, antinociceptive, hypothermic, and hypolocomotive effects in rodents [18–22]. Additionally, many studies have demonstrated that in vitro binding affinity is generally a good predictor of cannabimimetic in vivo effects [23–26]. Repeated treatment with THC or synthetic cannabinoids leads to desensitization and downregulation of CB1 receptors that occurs concomitantly with tolerance to in vivo responses [27]. Moreover, mice given repeated injections of cannabinoids and challenged with the CB1 receptor antagonist rimonabant display precipitated somatic withdrawal signs that include paw flutters and headshakes [28].
Given the high affinity of CP47,497 to the CB1 receptor [10, 12], it poses a significant risk for abuse, tolerance, and dependence. Moreover, the abuse of potent synthetic cannabinoids such as CP47,497 as an alternative to marijuana represents a public health hazard [29], and further scientific evaluation is required to understand the consequences of use and abuse. Therefore, the purpose of the present study was to examine in vitro and in vivo pharmacological effects of CP47,497 relative to THC. CP47,497 was tested in assays predictive of cannabimimetic activity, including agonist-stimulated G-protein activity [10, 25]; the tetrad assay, consisting of hypomotility, antinociception, catalepsy, and hypothermia [9, 24, 30]; and substitution for THC in the drug administration paradigm [31–33]. To determine the pharmacology of CP47,497, complementary pharmacological and genetic approaches were employed, utilizing the selective CB1 receptor antagonist rimonabant [34] in Institute for Cancer Research/CD-1 (ICR) mice and CB1 (+/+) and (−/−) mice [35]. Tolerance to cannabimimetic behavioral effects in the tetrad assay was evaluated after repeated administration of CP47,497. Lastly, dependence liability was assessed by giving mice daily injections of CP47,497, challenging them with rimonabant, and scoring somatic withdrawal signs.
Materials and methods
Subjects
Naïve, male, ICR (CD-1) mice (Harlan Laboratories, Dublin, VA, USA) weighing 22–32 g were used in cumulative dose-response, time-course, antagonism, tolerance, and withdrawal experiments, described below. Male and female CB1 (+/+) and CB1 (−/−) mice weighing 19–27 g backcrossed more than 15 generations onto a C57BL/6J background were obtained from the Transgenic Colony at Virginia Commonwealth University and used to infer CB1 receptor mechanism of action. Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) weighing 20–25 g were utilized in drug discrimination experiments.
Mice were housed in clear plastic cages in a temperature-controlled (22±2 °C) animal care facility on a 12-h light/dark cycle with ad libitum water and standard rodent chow (7012 Teklad Mouse/Rat Diet, Harlan Laboratories), unless otherwise described. Mice were individually housed the day prior to behavioral testing for acute dosing and drug discrimination experiments and housed four to six mice per cage for repeated administration (tolerance and dependence) experiments.
Brain tissue used for agonist-stimulated [35S]-GTPγS experiments was harvested from mice sacrificed by rapid decapitation and stored at −80 °C until use. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals 8th edition as promulgated by the U.S. National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. All work complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving animals.
Drugs and chemicals
Racemic CP47,497 was purchased from Cayman Chemical (Ann Arbor, MI, USA), CP55,940 was purchased from Tocris Bioscience (Minneapolis, MN), and THC and rimonabant were obtained from the National Institute of Drug Abuse Drug Supply Program (Rockville, MD, USA). All drugs were dissolved in ethanol (5% of total volume), emulphor was added (5% of total volume), and finally 0.9% NaCl isotonic saline (90% of total volume) was added. Vehicle was composed of this same 1:1:18 ratio of ethanol:emulphor:saline, and all treatments were administered with an injection volume of 10 μL/g.
[35S]-guanosine-5′-O-(3-[35 S]thiotriphosphate (GTPγS) (1250 Ci/mmol) was purchased from PerkinElmer (Waltham, MA, USA), and scintillation fluid (ScinitSafe Econo 1) and Whatman GF/B glass fiber filters were obtained from Fisher Scientific (Pittsburgh, PA, USA). Guanosine diphosphate (GDP), GTPγS, adenosine deaminase, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Agonist-stimulated [35S]-GTPγS binding in brain
The effects of CP47,497 and CP55,940 on receptor-mediated G-protein activity were investigated using agonist-stimulated [S35]-GTPγS binding [36]. Whole brains were collected and stored at −80 °C. Upon analysis, brains were thawed and homogenized in membrane buffer (50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4) and then centrifuged at 50,000 × g for 10 min at 4 °C. The supernatant was discarded, and pellets were suspended with 5 mL of assay Buffer A (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4). Protein concentration was determined according to Bradford [37]. Samples were incubated for 15 min at 30 °C with adenosine deaminase (3 mU/mL), to reduce basal activity by adenosine receptors. Concentration-effect curves were generated by incubating 5 μg of membrane protein with CP55,940 (0.001–30 μM) or CP47,497 (0.0001–30 μM) and 30 μM GDP, 0.1 nM [S35]-GTPγS in assay Buffer B (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4, and 1.25 g/L BSA) in a total volume of 0.5 mL. Nonspecific binding was assessed in the presence of 20 μM unlabeled [35S]-GTPγS, and basal binding was determined in the absence of agonist. Samples were incubated for 2 h at 30 °C, and the reaction was terminated by rapid vacuum filtration through Whatman G/FB glass fiber filters, followed by three rinses in cold Tris buffer (50 mM Tris-HCl, pH 7.4). Bound radioactivity was determined by liquid scintillation spectrophotometry at 95% efficiency after over-night extraction in Econo-Safe scintillation fluid. Data are reported as mean±SEM of three experiments performed in triplicate. Nonspecific binding was subtracted from each sample. Net stimulated [35S] GTPγS binding was defined as agonist-stimulated minus basal [35S] GTPγS binding, and percent stimulation was defined as (net stimulated/basal [35S]GTPγS binding) × 100%.
Tetrad behavioral assessment
Mice were acclimated to the test environment for at least 1 h prior to testing for tetrad components: spontaneous activity, catalepsy, antinociception, and hypothermia [30, 24, 38]. In the locomotor studies, subjects were administered vehicle or drug and 5 min later were placed into clear acrylic boxes (approx. 44.5 cm × 22.25 cm × 20.0 cm) contained within sound-attenuating cabinets equipped with an LED light source and fans for general air circulation and creation of white noise. The distance traveled (meters) and time spent immobile (s) for each mouse were collected and recorded for 10 min using Fire- i™ digital cameras purchased from Unibrain (San Ramon, CA, USA) and ANY- maze™ video tracking software purchased from Stoelting Company (Wood Dale, IL, USA).
Mice were assessed for baseline tail withdrawal latencies and body temperature, given an intraperitoneal (i.p.) injection of vehicle or drug (THC or CP47,497), and 30 min later assessed in the following order: catalepsy, tail withdrawal test, and body temperature. Catalepsy was measured using the horizontal bar test in which both fore-limbs of the mouse were placed on a horizontal bar (approximately 1.25 cm in diameter and 4.5 cm parallel to the bench top), with the duration of a fixed and motionless posture (except normal breathing) recorded by stopwatch over a 60 s interval. Antinociception was determined in the warm water (52 °C) tail immersion test whereby the distal end (approximately 1 cm) of the tail was immersed into the water bath and the latency of the mouse to withdrawal its tail recorded (to the nearest 0.1 s). A 10 s cutoff was used to minimize tail damage. Antinociception data were transformed to represent a maximum percent effect (%MPE) by the following formula: %MPE = [(test latency−pretreatment latency)/(10−pretreatment latency)] × 100. Body temperature measurements (recorded to the nearest 0.1 °C) were collected by inserting a rectal probe, lubricated with mineral oil and attached to a telethermometer (Yellow Spring Industries Inc., Yellow Springs, OH, USA), to a 2 cm depth.
Cumulative dose-response studies
A cumulative dosing paradigm was used to construct dose-response curves according to our published methods [39]. ICR mice received increasing doses of drug (CP47,497 or THC) or repeated vehicle every 40 min, with behavioral assessments 30 min after each i.p. injection. Locomotor activity was not assessed due to habituation effects that occur following repeated exposures. The cumulative doses for CP47,497 (0.3, 1, 3, 10, and 30 mg/kg) and THC (3, 10, 30, 100, and 200 mg/kg) were then determined.
For antagonism studies, ICR mice received a subcutaneous (s.c.) injection of rimonabant (3 or 10 mg/kg) 10 min before test drug and were assessed in the tetrad, as described above. CB1 (+/+) and CB1 (−/−) mice received an i.p. injection of vehicle or CP47,497 (30 mg/kg) and were tested for tetrad behaviors 30 min later.
Drug discrimination assay
C57BL/6J mice were maintained at 85%–90% of free feeding body weight by restricting the daily ration of standard rodent chow, with ad libitum water available except during training and testing sessions. Training and test sessions were conducted at similar times during the light phase of a 12 h light/dark cycle. Sound- and light-attenuated mouse operant conditioning chambers (MED Associates, St. Albans, VT, USA) were used for behavioral training and testing; each chamber housed an acrylic operant box (18 × 18 × 18 cm) equipped with a house light and two nose poke apertures (left and right) with a recessed well between the two apertures. Houselights were illuminated during all operant sessions, and fan motors provided ventilation and masked outside noise. A computer with Logic “1” interface and MED-PC software (MED Associates) controlled contingency schedules and data recording. Mice were trained to respond in one aperture following administration of 5.6 mg/kg THC and to respond in the opposite aperture following vehicle administration. A sweetened pellet served as reinforcement and was delivered on a fixed-ratio 10 (FR10) schedule. Each incorrect response reset the FR requirement.
Daily 15-min training sessions were performed Monday to Friday with dosing following a double alternation sequence of drug or vehicle (e.g. drug, drug, vehicle, vehicle) until mice met two criteria during 9 of 10 consecutive sessions: (criterion i) correct completion of the first FR10 (e.g. first 10 consecutive responses on condition appropriate aperture) and (criterion ii) > 80% of all responses were condition (i.e. drug or vehicle) appropriate. During test sessions, responses in either aperture delivered reinforcement according to an FR10 schedule. When these two criteria were met, acquisition of the discrimination was established, and substitution testing began. Stimulus substitution tests were conducted twice per week with a minimum of 72 h between the 15-min test sessions, though training continued between test days. Control tests were conducted with the training dose of the drug (5.6 mg/kg THC, s.c.) or vehicle. For substitution tests, CP47,497 was given via the s.c. route of administration (0.03–3.2 mg/kg) 30 min before the test session.
Tolerance experiments
The effectiveness of CP47,497 to produce tolerance and/or cross-tolerance to catalepsy, antinociception, and hypothermia was assessed by treating mice with CP47,497 (15 mg/kg, s.c.) or THC (50 mg/kg, s.c.) twice daily for 5.5 days, with a cumulative CP47,497 (1–56 mg/kg, i.p.) dose-response regimen administered 24 h after the last morning dose on day 6 (to allow drug washout). This dosing regimen was selected because our laboratory has previously demonstrated tolerance and dependence using a twice daily subchronic dosing regimen for 5.5 days with THC [40].
Behavioral evaluation of somatic withdrawal signs
For dependence experiments, mice received CP47,497 (15 mg/kg, s.c.) or THC (50 mg/kg, s.c.) twice daily (approx. 0900 and 1700 h) for 5.5 days. The THC dose was selected based upon our previous efforts [40], and the CP47,497 dose was calculated to be equipotent via linear regression in triad measures. The same acrylic boxes and cabinets described for locomotor assessment were utilized to assess dependence. After final drug administration (day 6, 0900 h injection), mice were acclimated for 30 min to behavioral chambers, which consisted of an opaque acrylic box (20 × 20 × 20 cm), equipped with a clear front panel and mirrored back panel, enclosed within the activity cabinets. After the 30-min period, mice were removed from the chambers, received rimonabant (10 mg/kg, i.p.), and were then immediately placed back into their boxes for a 1-h observation period. The acrylic boxes were cleaned with water after the acclimation but before the observation period to remove urine and feces present. Somatic withdrawal signs (paw flutters/tremors/clapping, headshakes, and jumping) were captured through the clear front acrylic panel (and by mirrored reflection of the backside of box) by Fire-i™ digital cameras and recorded by ANY-maze™ digital tracking software for 1 h. Somatic withdrawal signs were observed and scored by a researcher blinded to experimental treatment.
Data analysis and statistics
Unless specifically stated otherwise, presented data are reported as mean (±SEM). Data were analyzed by one- or two-way ANOVA and were further analyzed by Holm-Sidak post hoc test when appropriate. In drug discrimination studies, Dunnett’s post hoc analysis was used to make comparisons versus control. Overall, significance was denoted for p-values < 0.05 with all statistical analyses performed using GraphPad Prism® statistical software (Version 6.0; LA Jolla, CA, USA). ED50 values and 95% confidence limits (CL) were calculated by standard linear regression analysis of generated drug dose-response curves. Following the method described by Colquhoun et al. [41], potency ratio with 95% CL were determined by comparing potency between drug treatments groups, and ED50 and potency ratio for hypothermia data were calculated using an Emax of −6 °C, which represents the maximal mean decrease in temperature from the CP47,497-treated group. Agonist-stimulated [35S]-GTPγS binding data were analyzed utilizing nonlinear regression to determine maximum Emax (±standard error) EC50 values (with 95% CL) for CP55,940 and CP47,497. To verify apparent differences in Emax in agonist-stimulated [35S]-GTPγS binding, a t-test was used.
Results
CP47,497 and CP55,940 stimulate [35S]-GTPγS binding in whole brain homogenate
To assess functional activation of G-proteins by the cyclohexylphenol CP47,497, we employed the agonist-stimulated [35S]-GTPγS binding assay [36, 42]. Concentration-effect curves were generated using CP47,497 and CP55,940 for comparison. CP47,497 stimulated [35S] GTPγS binding with an Emax (±SEM) value of 62.1±2.1%, and CP55,940 produced an Emax value of 79.8±3.3% (Figure 1). The EC50 (±SEM) values of CP47,497 and CP55,940 were 135±25.2 SEM nM and 13.5±3.6 SEM nM, respectively. Thus, the relative efficacy of CP47,497 for G-protein activation in whole brain was approximately ~78% that of CP55,940, and CP47,497 was approximately 10-fold less potent than CP55,940. Student’s t-test confirmed a significant difference in Emax (p < 0.05).
Figure 1.
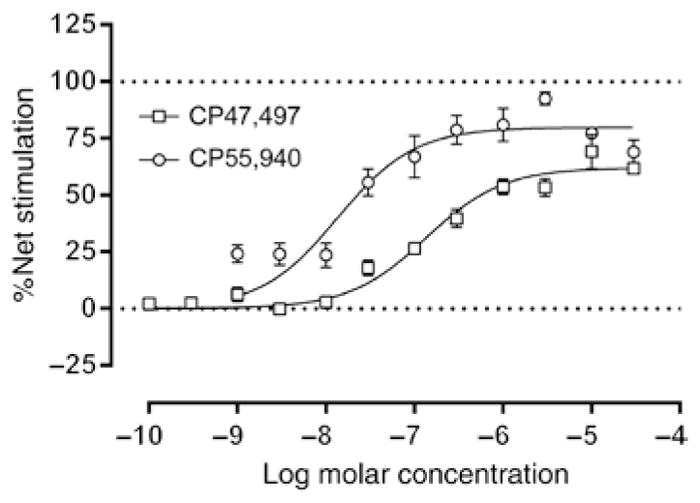
CP47,497 produces functional activity at the CB1 receptor.
Whole mouse brain membranes were incubated with cannabinoid agonist (CP55,940 or CP47,497) and [35S]GTPγS to assess functional activity of cannabinoids at the CB1 receptor. Each dose-response curve was performed in triplicate, and data are expressed as mean±standard error. CP47,497 (Emax±SE = 62.09±2.12% net stimulation above basal) exhibited relatively less metabotropic receptor activation compared to CP55,940 (Emax±SE = 79.81±3.26% net stimulation above basal) and was approximately 10-fold less potent [EC50 (with 95% CL) = 0.135 (0.084–0.187) μM for CP47,497 and 0.014 (0.006–0.021) μM for CP55,940].
CP47,497 elicits potent cannabimimetic effects in mice
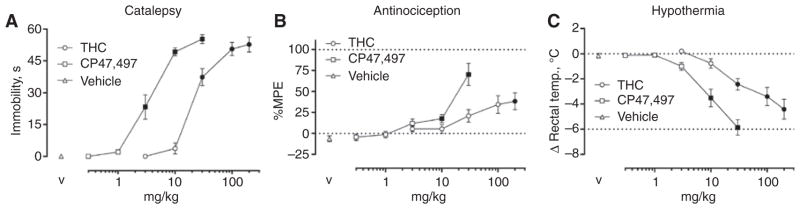
A cumulative dose-response study was used to compare the potency between CP47,497 and THC in producing acute cataleptic, antinociceptive, and hypothermic effects. Both CP47,497 [F(5,35) = 91.61, p < 0.0001; ED50 (95% CL) = 4.7 (3.8–5.9) mg/kg] and THC [F(5,35) = 113.8, p < 0.0001, ED50 = 33.8 (26.1–43.7) mg/kg] dose-dependently produced catalepsy (Figure 2A). CP47,497 was 7.2-fold (5.4–9.6; 95% CL) more potent than THC in eliciting catalepsy. Furthermore, CP47,497 (p < 0.0001) and THC (p < 0.0001) produced significant antinociceptive effects, with maximal effects of 70.3±13.2%MPE and 38.3±10.1%MPE (Figure 2B), respectively. In addition, CP47,497 [F(5,35) = 55.4, p < 0.0001; ED50 = 7.1 (4.7–10.6) mg/kg] and THC [F(5,35) = 29.8, p < 0.0001; ED50 = 66.6 (69.7–172.7) mg/kg] produced dose-dependent hypothermic effects (Figure 2C). CP47,497 was 8.1-fold (4.8–13.7) more potent than THC in producing hypothermia. Because the dose range evaluated for CP47,497 and THC did not completely capture the linear portion of the dose-response curve, ED50 and potency ratio values were not calculated for antinociception.
Figure 2.
THC and CP47,497 produce cannabimimetic effects in the cannabinoid tetrad.
(A) CP47,497 and THC produced dose-related catalepsy. (B) Increases in tail withdrawal were also observed for both CP47,497 and THC, though 100%MPE was not achieved in either case. (C) Dose-dependent decreases in body temperature were detected following administration of CP47,497 and THC. Filled symbols p < 0.05 vs. vehicle. Values represent means±SEM.
CP47,497 and THC have a similar duration of action
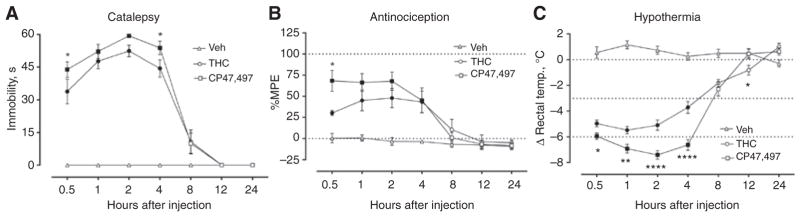
Next, we sought to examine the time course of the pharmacological effects of CP47,497, with particular interest in comparison to the temporal effects of THC. In these studies, mice received a single bolus dose of CP47,497 (30 mg/kg), THC (100 mg/kg), or vehicle with measurements taken at 0.5, 1, 2, 4, 8, 12, and 24 h. These doses were selected because they reliably produced measurable in vivo effects, as shown in Figure 2.
The cataleptic effects of CP47,497 (30 mg/kg) and THC (100 mg/kg) persisted for up to 8 h and returned to baseline levels by 12 h [Figure 3A, F(12, 144) = 29.3, p < 0.0001]. CP47,497-induced catalepsy was greater in magnitude than that by THC at 0.5 and 4 h after injection (Figure 3A). The time course of antinociceptive effects of CP47,497 and THC lasted up to 8 h, similar to those of catalepsy [Figure 3B, F(12, 146) = 4.2, p < 0.0001] but only differed at the initial 0.5 h time point (Figure 3B). CP47,497- and THC-induced hypothermia was longer in duration than the other measures, lasting 8 and 12 h, respectively [Figure 3C, F(12, 146) = 35.4, p < 0.0001]. In addition, CP47,497-treated mice exhibited statistically significant decreases in body temperature from 0.5 to 4 h versus THC-treated mice (Figure 3C).
Figure 3.
CP47,497 (30 mg/kg) and THC (100 mg/kg) produced long-lasting cannabimimetic effects.
(A) The cataleptic effects of CP47,497 and THC differed from vehicle up to 8 h, returning to baseline levels at 12 h. CP47,497 evoked a greater magnitude of catalepsy at 0.5 and 4 h. (B) CP47,497- and THC-induced antinociception persisted for up to 4 h after injection, and only at 0.5 h did the magnitude of the effect differ between drugs. (C) Cannabinoid-induced hypothermia was observed up to 8 h and 12 h for CP47,497 and THC, respectively, with CP47,497 producing greater reductions in body temperature relative to THC from 0.5 to 4 h. Filled symbols indicate p < 0.05 vs. vehicle, *p < 0.05, **p < 0.01, ****p < 0.0001 CP47,497 vs. THC. Values represent means±SEM.
CP47,497-induced catalepsy, antinociception, hypothermia, and hypolocomotor effects are mediated by CB1 receptors
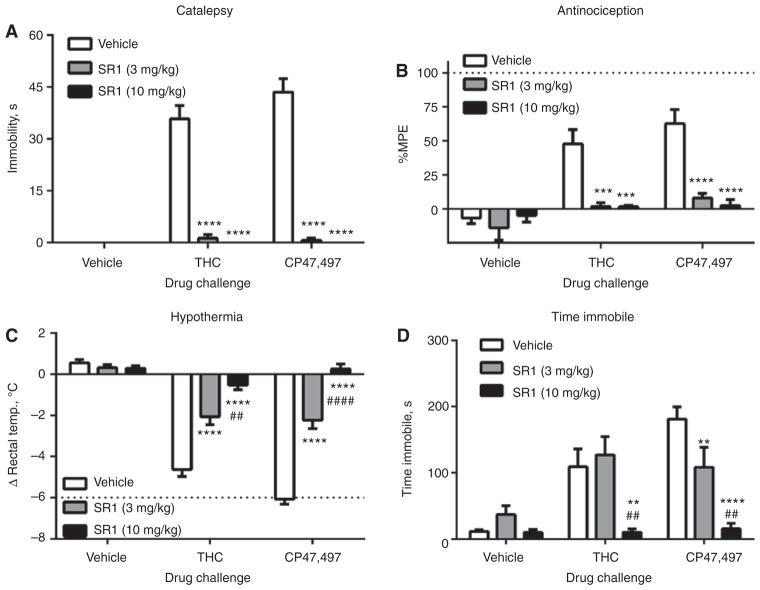
Rimonabant (3 and 10 mg/kg) blocked the cataleptic [Figure 4A, F(4, 69) = 33.4, p < 0.0001], antinociceptive [Figure 4B, F(4, 73) = 4.9, p < 0.01], and hypothermic [Figure 4C, F(4, 75) = 36.9, p < 0.0001] effects of both THC and CP47,497 but had no effects on these measures when given alone. Pretreatment with rimonabant prevented locomotor depression elicited by THC and CP47,497 [Figure 4D, F(4, 75) = 4.8, p < 0.01].
Figure 4.
Cannabinoid tetrad effects of CP47,497 and THC are mediated by CB1 receptors.
The (A) cataleptic, (B) antinociceptive, (C) hypothermic, and (D) immobility effects of CP47,497 (30 mg/kg) and THC (100 mg/kg) are blocked by the CB1 receptor antagonist rimonabant (SR1, 3 or 10 mg/kg), **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. vehicle/vehicle, ##p < 0.01, ####p < 0.0001 vs. 3 mg/kg SR1. Values represent means±SEM.
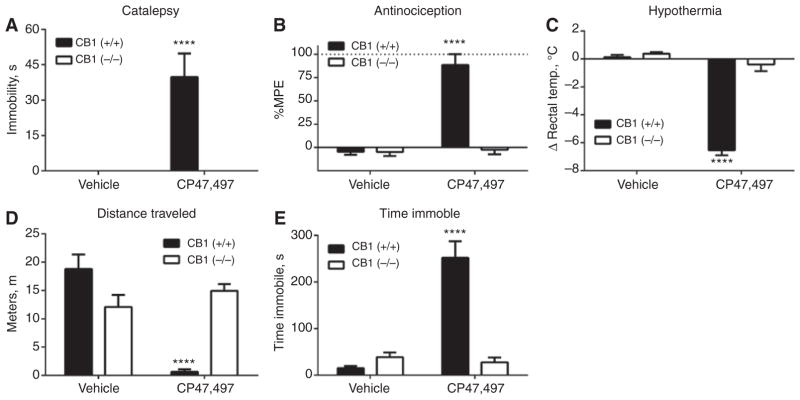
To further assess whether CB1 receptors mediate the pharmacological effects of CP47,497, age-matched CB1 (−/−) mice and wild type CB1 (+/+) mice were assessed in the tetrad assay after receiving an acute dose of CP47,497 (30 mg/kg, i.p.). CP47,497-treated CB1 (+/+) mice showed significant increases in catalepsy [Figure 5A, F(1,13) = 34.7, p < 0.0001], antinociception [Figure 5B, F(1,16) = 36.9, p < 0.0001], hypothermia [Figure 5C, F(1,16) = 142.3, p < 0.0001], and both measures indicative of locomotor depression – distance traveled [Figure 5D, F(1,16) = 16.5, p < 0.001] and immobility time [Figure 5E, F(1,16) = 24.4, p = 0.0001]. In contrast, CP47,497 lacked activity in CB1 (−/−) mice. Also, during catalepsy testing, 67% of CP47,497-treated CB1 (+/+) mice displayed hyper-reflexia, whereas this behavior was not exhibited by CP47,497-treated CB1 (−/−) mice.
Figure 5.
Mice lacking CB1 receptors did not display tetrad effects following CP47,497 (30 mg/kg) administration.
Age-matched CB1 (+/+) and (−/−) mice were treated with either vehicle or CP47,497 (30 mg/kg). CP47,497-treated CB1 (+/+) mice displayed (A) catalepsy, (B) antinociception, (C) hypothermia, and locomotor immobility as indicated by decreases in (D) distance traveled and (E) time spent immobile. In contrast, CP47,497-treated CB1 (−/−) mice did not differ from vehicle-treated CB1 (+/+) and (−/−) mice, implying CB1 mediation of these effects. Values represent means±SEM. ****p < 0.0001 vs. vehicle-treated mice.
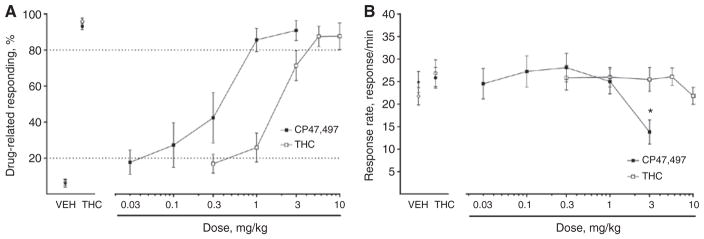
CP47,497 produces THC-like subject effects in the drug discrimination assay
The data in Figure 6 show that CP47,497 substitutes for THC in the drug discrimination assay. CP47,497 (0.03–3.2 mg/kg, ED50 = 0.25 (0.14–0.44) and THC [3.2–10 mg/kg, ED50 = 1.47 (1.11–1.96)], respectively substituted and generalized for the training dose of THC (Figure 6A). CP47,497 was 6.3-fold (3.5–10.8) more potent than THC in this assay. Post hoc analyses indicated that response rates were reduced at the highest dose of CP47,497 (3.2 mg/kg) tested when compared to mice that received vehicle (Figure 6B, p < 0.001).
Figure 6.
CP47,497 substituted for THC in a dose-related manner. C57BL6/J mice trained to discriminate THC (5.6 mg/kg) from vehicle were administered THC (0.3–10 mg/kg) or CP47,497 (0.03–3 mg/kg).
(A) THC showed dose-related generalization and CP47,497 elicited dose-related substitution for the training dose of THC. (B) Only the highest dose of CP47,497 (3 mg/kg) suppressed rates of operant responding, while THC did not suppress response rates. Values represent means±SEM. *p < 0.05 vs. vehicle.
Assessment of repeated administration of CP47,497
Separate groups of mice were given two daily s.c. injections per day for 5.5 days of vehicle, CP47,497 (15 mg/kg), or THC (50 mg/kg). On the test day (i.e. day 7), the cumulative dose-response relationship of CP47,497 was evaluated in each mouse. Mice in the vehicle group displayed dose-dependent catalepsy following cumulative dosing with CP47,497, whereas mice treated repeatedly with THC or CP47,497 displayed diminished cataleptic responses [Figure 7A, F(12, 112) = 14.1, p < 0.0001]. Neither THC-nor CP47,497-treated groups exhibited increases in tail withdrawal latency after cumulative CP47,497 challenge, while the vehicle group showed significant dose-related antinociceptive effects [Figure 7B, F(12, 112) = 10.4, p < 0.0001]. Mice treated repeatedly with CP47,497 displayed diminished hypothermia compared to vehicle-treated mice, with THC-treated mice remaining near baseline levels throughout dosing [Figure 7C, F(12, 112] = 20.4, p < 0.0001).
Figure 7.
Mice repeatedly treated with THC or CP47,497 displayed tolerance to the cannabimimetic effects of CP47,497.
(A) THC- and CP47,497-treated mice exhibited marked tolerance to the cataleptic effects of CP47,497. (B) The antinociceptive effects of CP47,497 underwent tolerance following repeated drug administration and cross-tolerance to repeated THC administration. (C) The hypothermic effects of CP47,497 underwent tolerance following repeated drug administration and cross-tolerance to repeated THC administration. Values represent means±SEM. Filled symbols indicate p < 0.05 vs. vehicle, **p < 0.01 vs. THC.
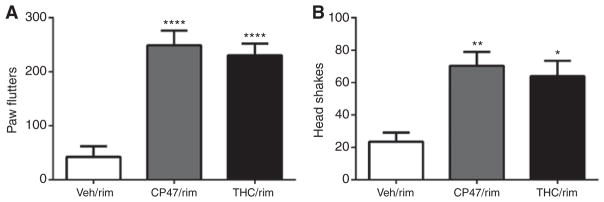
The final experiment investigated whether repeated administration of CP47,497 would lead to physical dependence. Rimonabant challenge to groups treated repeatedly with THC or CP47,497 precipitated significant increases in paw flutters [Figure 8A, F(2, 13) = 8.8, p < 0.01] and head shakes [Figure 8B, F(2, 13) = 5.9, p < 0.05].
Figure 8.
Rimonabant (10 mg/kg) precipitated somatic withdrawal signs in mice given repeated injections of CP47,497 (CP47) or THC.
(A) significant increases in paw flutters relative to vehicle controls; and (B) significantly more head shakes than the vehicle-treated group. Values represent means±SEM. *p < 0.05, **p < 0.01, ****p < 0.0001 vs. vehicle.
Discussion
Previous agonist-stimulated [35S]GTPγS binding studies showed that THC is a low-efficacy agonist, whereas CP55,940 is a high-efficacy agonist [43]. As CP47,497 and CP55,940 belong to the bicyclic family of synthetic cannabinoids [44], it is not surprising that both of these compounds stimulated [35S]GTPγS binding, though CP47,497 (i.e. 62.1% above basal activity) produced a significantly lower maximal stimulation of G-protein activity than CP55,940 (i.e. 79.8% above basal activity). CP47,497 has also been shown to inhibit neurotransmission via CB1 receptors in hippocampal cultures, an effect elicited by JWH018 and other synthetic cannabinoids [45]. These results support the idea that CP47,497 produces its central effects via activation of G-proteins and subsequent inhibition of neurotransmission in the brain.
The tetrad assay has historically been used to assess cannabimimetic activity [9]. Here, we compared the potency between THC and CP47,497 to elicit catalepsy, hypothermia, and antinociception. Route of administration plays an important role in potency and efficacy of cannabinoids in this assay, as intravenous injection of THC has been shown to be 45-fold more potent than subcutaneous THC administration in producing antinociception in ICR mice [46]. Regardless, CP47,497 produced significant increases in tail withdrawal latency at much lower doses than THC (10 mg/kg vs. 200 mg/kg, respectively) via the i.p. route of administration, indicating a large difference in potency, concordant with previous reports showing that CP47,497 is far more potent than THC [9, 13, 30, 35, 47, 48]. Time course studies revealed similar durations of action for THC- and CP47,497-induced catalepsy, hypothermia, and antinociception. Although the doses selected were intended to be equipotent based upon triad measures in ICR mice, the differences in magnitude may reflect an imperfect estimation of potency or potential pharmacokinetic differences between CP47,497 and THC. Nonetheless, THC and CP47,497 readily enter the CNS [49, 50] which is consistent with their in vivo effects. Whereas CP47,497 possesses higher efficacy than THC in activating Gαi/o proteins, both drugs produce similar magnitudes of effects in the in vivo assays assessed in the present study. Given the high expression levels of CB1 receptors in the CNS [2], it is plausible that sufficient CB1 receptor reserve obfuscates the impact of efficacy in the whole animal.
Complementary genetic and pharmacological approaches were utilized to determine whether CB1 receptors mediate the pharmacological effects of CP47,497 and THC in the tetrad. Pretreatment with the CB1-selective antagonist rimonabant (3 or 10 mg/kg) blocked the cataleptic, hypothermic, antinociceptive, and locomotor depressive effects of CP47,497 (30 mg/kg) and THC (100 mg/kg). Similarly, CP47,497 (30 mg/kg) produced robust catalepsy, hypothermia, antinociception, and hypomotility in CB1 (+/+) mice but not in CB1 (−/−) mice. These complementary approaches indicate that the pharmacological effects of CP47,497 in the tetrad require CB1 receptors. These findings are consistent with studies demonstrating that a structural variant of CP47,497, CP47-497-C8, elicits inhibition of neurotransmission in a CB1 receptor-dependent manner [45].
To assess the internal stimulus properties of CP47,497 relative to THC, dose-response studies were conducted in mice trained to discriminate THC (5.6 mg/kg). Consistent with both in vitro functional activity assessment and cannabinoid tetrad measures, CP47,497 substituted for the training dose of THC. CP47,497 was 6.3-fold more potent than THC in eliciting responses in the drug-associated aperture, a finding that is consistent with previous studies indicating a 3- to 14-fold difference in potency in drug discrimination studies in rats [13] and 5.6-fold more potent than THC in another mouse drug discrimination study [51]. Previous work has shown that CB1 receptor agonists (e.g. CP55,940 and WIN55,212-2) substitute for THC [52] and that THC substitutes for other CB1 receptor agonists (JWH-018) [53], corroborating our findings. Likewise, the C8 homolog of CP47,497 fully substituted for THC in a rat drug discrimination study, though it displayed a relatively slow onset and considerably long duration of action, which was interpreted as its possessing the potential of increased hazardous effects [54]. As the present study did not assess time course in the mouse drug discrimination paradigm, it is unclear whether equivalent doses of CP47,497 and THC would have similar durations of discriminative stimulus effects, as they did in the tetrad assay. Nonetheless, the present findings indicate that CP47,497 possesses similar subjective effects as THC, perhaps conferring a similar abuse liability.
Repeated administration of cannabinoid agonists produces adaptive changes in CB1 receptors that occur concomitantly with tolerance and dependence [55]. Tolerance develops following repeated administration of several synthetic cannabinoids, including CP55,940 [56]. Similarly, the pharmacological effects of CP47,497 underwent tolerance following repeated administration and showed cross-tolerance to repeated injections of THC. Moreover, the degree of tolerance was similar across catalepsy, antinociception, and hypothermia between both THC- and CP47,497-treated mice. The expression of cross-tolerance further supports a common mechanism of action involving CB1 receptors. Although the consequences of repeated CP47,497 treatment on CB1 receptor expression and function in brain have not been investigated, other studies demonstrated that repeated administration of the structurally related CP55,940 produces CB1 receptor desensitization and downregulation [57]. Studies in cell models showed that treatment with CP47,497-C8, as well as JWH-018 and JWH-073, promoted CB1 receptor internalization [45]. The findings that CP47,497 variants produce tolerance and CB1 receptor internalization suggest similar adaptive mechanisms in response to repeated treatment as other synthetic cannabinoids and THC [55].
Cannabis-dependent humans display a well-recognized withdrawal syndrome with a characteristic set of criteria including anxiety, gastrointestinal complications, malaise, and sleep disturbances [58]. In order to investigate dependence liability of CP47,497, we assessed cannabinoid withdrawal signs in mice treated repeatedly with THC or CP47,497 and then challenged with rimonabant [59]. Rimonabant (10 mg/kg) challenge precipitated robust expression of head twitches and paw tremors in THC- and CP47,497-treated mice. These findings suggest that repeated administration of THC or CP47,497 leads to adaptive changes in the CNS that subserve dependence [60]. Together, these data suggest that repeated CP47,497 exposure leads to a similar dependence spectrum as THC, suggesting a potential risk for dependence in humans repeatedly using CP47,497.
In conclusion, the present study demonstrates that the abused synthetic cannabinoid CP47,497 produces in vivo tetrad and discriminative stimulus effects similar to THC and other cannabinoid receptor agonists. These effects are mediated by CB1 receptors, supporting the idea that THC and abused synthetic cannabinoids share a common mechanism of action. In addition, CP47,497 possesses increased potency compared with THC and acts as a high-efficacy partial agonist for G-protein activation. Finally, the finding that rimonabant precipitated withdrawal signs in mice given repeated injections of CP47,497, suggests that this drug poses a risk of dependence. The results of the present study expand the body of knowledge regarding the pharmacology of abused synthetic cannabinoids and provides an experimental strategy that can be used to test acute and chronic effects of emerging abused synthetic cannabinoids.
Acknowledgments
Research funding: Funding for this study was provided by the NIH grants R01DA032933, F31DA033183, and T32DA007027.
Footnotes
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. Travis Grim prepared and analyzed the data and composed the final draft of the manuscript. Kimberly Samano designed and performed the in vivo tetrad, tolerance, and dependence studies; analyzed the data; prepared the first draft of the manuscript; and made significant contributions to the final composition of the manuscript. Bogna Ignatowska-Jankowska designed and executed the drug discrimination studies. Qing Tao performed the in vitro studies assessing CB1 functional activity. Laura Sim-Selley contributed to the experimental design and analysis of in vitro studies. Dana Selley provided valuable input on the analysis and interpretation of in vitro studies. Laura Wise contributed to the design of experiments, analysis, and composition of the manuscript. Alphonse Poklis contributed to the experimental design of the experiments. Aron Lichtman contributed to the design of experiments, analysis, data interpretation, and composition of the manuscript.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Travis W. Grim, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA.
Kimberly L. Samano, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA.
Bogna Ignatowska-Jankowska, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA.
Qing Tao, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA.
Laura J. Sim-Selly, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA
Dana E. Selley, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA
Laura E. Wise, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA
Alphonse Poklis, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA, USA; Department of Pathology, Virginia Commonwealth University, Richmond, VA, USA; and Department of Forensic Science, Virginia Commonwealth University, Richmond, VA, USA.
Aron H. Lichtman, Department of Pharmacology and Toxicology, Virginia Commonwealth University, PO Box 980613, Richmond, VA 23298-0613, USA.
References
- 1.Schultes RE. Hallucinogens of plant origin. Science. 1969;163:245–54. doi: 10.1126/science.163.3864.245. [DOI] [PubMed] [Google Scholar]
- 2.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devane WA, Dysarz FAI, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- 4.EMCDDA. European Monitoring Centre for Drugs and Drug Addiction. Thematic paper – Understanding the “Spice” phenomenon. 2009:1–34. Available at: http://www.emcdda.europa.eu/publications/thematic-papers/spice.
- 5.Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, et al. Spice: a never ending story? Forensic Sci Int. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Ogata J, Uchiyama N, Kikura-Hanajiri R, Goda Y. DNA sequence analyses of blended herbal products including synthetic cannabinoids as designer drugs. Forensic Sci Int. 2013;227:33–41. doi: 10.1016/j.forsciint.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull (Tokyo) 2009;57:439–41. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- 8.The Drug Enforcement Administration. CP-47,497 seized in Wisconsin. Microgram Bull. 2009;42:89–102. [Google Scholar]
- 9.Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–9. [PubMed] [Google Scholar]
- 10.Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–26. [PubMed] [Google Scholar]
- 11.Melvin LS, Johnson MR, Harbert CA, Milne GM, Weissman A. A cannabinoid derived prototypical analgesic. J Med Chem. 1984;27:67–71. doi: 10.1021/jm00367a013. [DOI] [PubMed] [Google Scholar]
- 12.Melvin LS, Milne GM, Johnson MR, Subramaniam B, Wilken GH, Howlett AC. Structure-activity relagionships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs. Mol Pharmacol. 1993;44:1006–15. [PubMed] [Google Scholar]
- 13.Weissman A, Milne GM, Melvin LS. Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol. J Pharmacol Exp Ther. 1982;223:516–23. [PubMed] [Google Scholar]
- 14.Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57:1168–80. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- 15.Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3:466–78. doi: 10.1002/dta.268. [DOI] [PubMed] [Google Scholar]
- 16.Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, et al. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol. 2010;34:252–60. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- 17.Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. “Spice” and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–7. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin WJ, Patrick SL, Coffin P, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–9. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- 20.Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, et al. Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology (Berl) 2006;186:226–34. doi: 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 21.Wiley JL, Smith FL, Razdan RK, Dewey WL. Task specificity of cross-tolerance between Delta9-tetrahydrocannabinol and anandamide analogs in mice. Eur J Pharmacol. 2005;510:59–68. doi: 10.1016/j.ejphar.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR. The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend. 2001;63:107–16. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- 23.Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–6. [Google Scholar]
- 24.Little J, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–51. [PubMed] [Google Scholar]
- 25.Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, et al. Structure-activity relationships of indole- and pyrrole- derived cannabinoids. J Pharmacol Exp Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- 26.Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct for delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–26. [PubMed] [Google Scholar]
- 27.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–96. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 28.Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, et al. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9- tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–77. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macfarlane V, Christie G. Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 2015;34:147–53. doi: 10.1111/dar.12225. [DOI] [PubMed] [Google Scholar]
- 30.Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–8. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 31.Järbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (delta 8-THC and delta 9-THC), and other drugs. Psychopharmacologia. 1974;40:1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- 32.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–40. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 33.Wiley JL, Matthew Walentiny D, Vann RE, Baskfield CY. Dissimilar cannabinoid substitution patterns in mice trained to discriminate Δ(9)-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol. 2011;22:480–8. doi: 10.1097/FBP.0b013e328348eced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldi-Carmona M. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–4. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 35.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selley DE, Stark S, Sim LJ, Childers SR. Cannabinoid receptor stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding in rat brain membranes. Life Sci. 1996;59:659–68. doi: 10.1016/0024-3205(96)00347-5. [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 38.Wiley J. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471:185–93. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- 39.Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, et al. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol. 2014;25:182–5. doi: 10.1097/FBP.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, et al. FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology. 2010;35:1775–87. doi: 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colquhoun D. Lectures on biostatistics: an introduction to statistics with applications in biology and medicine. Oxford, UK: Clarendon Press; 1971. pp. 327–32. [Google Scholar]
- 42.Burkey TH, Quock RM, Consroe P, Roeske WR, Yamamura HI. delta 9-Tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. Eur J Pharmacol. 1997;323:R3–4. doi: 10.1016/s0014-2999(97)00146-5. [DOI] [PubMed] [Google Scholar]
- 43.Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of cannabinoid receptor agonists and antagonists using the guanosine-5′-O-(3-[35S]thio)-triphosphate binding assay in rat cerebellar membranes. J Pharmacol Exp Ther. 1998;285:553–60. [PubMed] [Google Scholar]
- 44.Melvin LS, Johnson MR. Structure-activity relationships of tricyclic and nonclassical bicyclic cannabinoids. NIDA Res Monogr. 1987;79:31–47. [PubMed] [Google Scholar]
- 45.Atwood BK, Lee D, Straiker A, Widlanski TS, Mackie K. CP47, 497-C8 and JWH073, commonly found in “Spice” herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur J Pharmacol. 2011;659:139–45. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin BR. Structural requirements for cannabinoid-induced antinociceptive activity in mice. Life Sci. 1985;36:1523–30. doi: 10.1016/0024-3205(85)90376-5. [DOI] [PubMed] [Google Scholar]
- 47.Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141617A): inhibition of delta-9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–94. [PubMed] [Google Scholar]
- 48.Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alozie SO, Martin BR, Harris LS, Dewey WL. 3H-delta 9- Tetrahydrocannabinol, 3H-cannabinol and 3H-cannabidiol: penetration and regional distribution in rat brain. Pharmacol Biochem Behav. 1980;12:217–21. doi: 10.1016/0091-3057(80)90359-7. [DOI] [PubMed] [Google Scholar]
- 50.Samano KL, Poklis JL, Lichtman AH, Poklis A. Development of a high-performance liquid chromatography-tandem mass spectrometry method for the identification and quantification of CP-47,497, CP-47,497-C8 and JWH-250 in mouse brain. J Anal Toxicol. 2014;38:307–14. doi: 10.1093/jat/bku043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, et al. AB-CHMINACA, AB-PINACA, and FUBIMINA: affinity and potency of novel synthetic cannabinoids in producing Δ9-tetrahydrocannabinol-like effects in mice. J Pharmacol Exp Ther. 2015;354:328–39. doi: 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMahon LR, Ginsburg BC, Lamb RJ. Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 2008;198:487–95. doi: 10.1007/s00213-007-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiley JL, Lefever TW, Cortes RA, Marusich JA. Cross-substitution of Δ9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014;124:123–8. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gatch MB, Forster MJ. Δ9-Tetrahydrocannabinol-like discriminative stimulus effects of compounds commonly found in K2/Spice. Behav Pharmacol. 2014;25:750–7. doi: 10.1097/FBP.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–30. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Rubino T, Patrini G, Parenti M, Massi P, Parolaro D. Chronic treatment with a synthetic cannabinoid CP-55,940 alters G-protein expression in the rat central nervous system. Brain Res Mol Brain Res. 1997;44:191–7. [PubMed] [Google Scholar]
- 57.Rubino T, Viganò D, Massi P, Parolaro D. Changes in the cannabinoid receptor binding, G protein coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic cannabinoid compound CP55,940. J Neurochem. 2000;75:2080–6. doi: 10.1046/j.1471-4159.2000.0752080.x. [DOI] [PubMed] [Google Scholar]
- 58.Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7:e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai S, Nikas SP, Shukla VG, Vemuri K, Makriyannis A, Järbe TUC. Cannabinoid withdrawal in mice: inverse agonist vs neutral antagonist. Psychopharmacology (Berl) 2015;232:2751–61. doi: 10.1007/s00213-015-3907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wise LE, Varvel SA, Selley DE, Wiebelhaus JM, Long KA, Middleton LS, et al. delta(9)-Tetrahydrocannabinol-dependent mice undergoing withdrawal display impaired spatial memory. Psychopharmacology (Berl) 2011;217:485–94. doi: 10.1007/s00213-011-2305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]