Abstract
While horseshoe crabs Limulus polyphemus from regions with two daily tides express endogenous circatidal (~ 12.4 h) activity rhythms, much less is known about locomotor rhythm expression in horseshoe crabs from other tidal regimes. This study investigated whether horseshoe crabs (1) always express activity rhythms consistent with their natural tides, and (2) can alter activity rhythm expression in response to novel tide cycles. Activity rhythms of animals from environments with two daily tides (Gulf of Maine, 43°6′ N/70°52′ W, and Massachusetts, 41°32′ N/70°40′W), one dominant daily tide (Apalachee Bay, Florida, 29°58′ N/84°20′ W), and microtides (Indian River Lagoon, Florida, 28°5′ N/80°35′ W) were recorded in 2011–2013 during three artificial tide conditions: no tides, a 12.4 h tidal cycle, and a 24.8 h tidal cycle. Interestingly, L. polyphemus from the microtidal site (n = 7) appeared “plastic” in their responses; they were able to express both bimodal and unimodal rhythms in response to different tide cycles. In contrast, the other two populations exhibited more fixed responses: regardless of the tides they were exposed to, animals from areas with one dominant daily tide (n = 18) consistently expressed unimodal rhythms, while those from areas with two daily tides (n = 28) generally expressed bimodal rhythms. Rhythms expressed by L. polyphemus thus appear to be a function of endogenous clocks, the tidal cues to which animals are exposed, and tidal cues that animals experience throughout ontogeny.
Keywords: American horseshoe crabs, Limulus polyphemus, circadian, circalunidian, circatidal, activity
Introduction
The American horseshoe crab Limulus polyphemus inhabits a wide range of coastal and estuarine habitats along the East and Gulf coasts of North America. As transient visitors to the intertidal zone, horseshoe crabs experience rhythmic tidal variations in water depth to which they may synchronize their behaviors (Watson and Chabot 2010; Brockmann and Johnson 2011). For example, horseshoe crabs from semidiurnal environments (two approximately equal tides per day, every 12.4 h) express tidal rhythms of locomotion that are synchronized to the tides in their native regions (Watson and Chabot 2010). Importantly, these tidal rhythms of activity persist in constant laboratory conditions (Chabot et al. 2007) and can be entrained by artificial tidal cycles (Chabot et al. 2008), indicating they are controlled by endogenous tidal oscillators (Chabot et al. 2007, 2008; Chabot and Watson 2010). These circatidal rhythms manifest themselves in the field both during the early summer (May – June), when animals appear in the intertidal zone during high tide to spawn (Barlow et al. 1986; Moore and Perrin 2007; Watson and Chabot 2010), as well as throughout the remainder of the summer (June – September) and fall (September – November), when animals in at least one northern population forage on tidal flats during high tides (Great Bay, NH; Watson and Chabot 2010; Lee 2010).
While tidal rhythms have been well described for L. polyphemus that inhabit environments with two daily tides, much less is known about the locomotor rhythms of individuals from areas with mixed semidiurnal tides (two tides of unequal amplitude per day, hereafter referred to as one dominant daily tide) and microtides (small-amplitude, primarily wind-driven, tides). These tidal regimes vary throughout the North American range of L. polyphemus’, along which at least four genetically distinct populations can be distinguished – Gulf of Maine (GM), Mid-Atlantic (MA), Atlantic Florida (AF), and Gulf of Florida (GF; King et al. 2005, 2015) – each of which may experience very different tidal periodicities. Whereas the GM and MA populations experience two daily tides, the GF population experiences either one dominant daily tide (Rudloe 1980; Lopez-Duarte and Tankersley 2007), or strictly diurnal tides (one tide every 24.8 h). Furthermore, the AF population in the Indian River Lagoon inhabits a variable microtidal environment (Ehlinger and Tankersley 2009): northward of Melbourne, Florida, tides are very small (1–2 cm) and primarily weather driven, while two daily small (10–20 cm) tides may occur in the immediate vicinity of coastal inlets (Woodward-Clyde 1994). Spawning patterns suggest that L. polyphemus from the one dominant daily tide region of the Gulf coast may express circatidal behavioral rhythms, as breeding occurs on both daily tides with spawning density correlated to tide height (Cohen and Brockmann 1983; Rudloe 1985). In contrast, L. polyphemus from the microtidal Indian River Lagoon appear arrhythmic: breeding occurs throughout the year, with no clear relationship to environmental cues (e.g., water depth, tide amplitude; Ehlinger et al. 2003).
Among some intertidal species that share a geographic distribution similar to that of L. polyphemus, individual populations express behavioral rhythms that match the periodicity of local tides (Stillman and Barnwell 2004; Lopez-Duarte and Tankersley 2007; Darnell et al. 2010). For example, female blue crabs Callinectes sapidus from environments with two daily tides express circatidal rhythms consisting of two bouts of swimming activity per day, while those from environments with a single daily tide express unimodal circalunidian rhythms (activity approximately every 24.8 h, the length of a lunar day), and those from a non-tidal environment express circadian rhythms of activity (Darnell et al. 2010). Further, while adult fiddler crabs Uca princeps from regions with one dominant daily tide express both tidal and daily activity patterns, the magnitudes of the activity bouts match the amplitudes of the tides (Stillman and Barnwell 2004). Interestingly, while fiddler crab (Uca pugilator) larvae from the microtidal Indian River Lagoon express circatidal activity patterns in constant conditions after hatching in the laboratory (Lopez-Duarte and Tankersly 2007), green crab (Carcinus maenas) larvae from other microtidal regions are arrhythmic (Queiroga et al. 2002). Across these species, differences in the rhythms expressed are hypothesized to be due to both intraspecific genetic variation and the influence of the environment during development (Queiroga et al. 2002; Stillman and Barnwell 2004; Lopez-Duarte and Tankersly 2007). In larval horseshoe crabs, the early environment can influence the type of rhythm expressed, as larvae from environments with two daily tides express circatidal rhythms only if exposed to periodic tidal (12.4 h) cues as embryos (Ehlinger and Tankersley 2006). This influence of the environment on circatidal rhythm expression in L. polyphemus suggests that, in adults of this species, populations from different tidal regimes will tend to express rhythms that correspond to the structure of the local tides.
However, even though the tidal history of a species might influence the behavioral rhythms it expresses when in constant water depth (i.e., no tides), the species might also have the ability to adapt to local tidal regimes. For example, when fiddler crabs Uca longisignalis are transplanted from an environment with one daily high tide to an environment with two daily high tides, their behavioral rhythms change to match the tidal periodicity in the environment. Moreover, the activity pattern persists upon I ntroduction to atidal conditions, suggesting their tidal clocks have become entrained to the two daily tides (Barnwell 1968). The ability of L. polyphemus to successfully inhabit separate tide environments suggests that horseshoe crabs may express similar plasticity in their expression of behavioral rhythms.
To investigate potential adaptations to different local tidal regimes, we evaluated the activity rhythms expressed by L. polyphemus obtained from three different tidal environments (“source tides”): (1) environments with two daily tides (GM and MA populations), (2) a microtidal environment (AF population), and (3) an environment with one dominant daily tide (GF population), during exposure to no tides (a constant water depth) and two different artificial tidal cycles. By exposing animals of each population to 12.4 h (twice daily) and 24.8 h (once daily) cycles of water depth changes, we assessed whether animals from these populations could (1) synchronize to artificial tidal cycles of any type, and (2) adjust their rhythms to be in synchrony with a tidal regime that was different from their natural regime.
Methods
2.1 Animal Collection and Maintenance
Four experiments were conducted from December, 2011 through August 2013 to assess activity rhythms of male L. polyphemus from the three tidal environments (“source tides”). Animals from a site with two daily tides of the GM population (n = 21) were collected from breeding beaches in the vicinity of Adam’s Point, Durham, New Hampshire (43°6′ N, 70°52′ W; tide amplitude: 2.1 ± 0.3 m, mean ± SD; CO-OPS, 2013) around the time of the late afternoon high tide, and transported by van in polyurethane coolers to Plymouth State University (PSU, Plymouth, New Hampshire; 2 hours away). Animals from a second site with two daily tides of the MA population (n = 7) were obtained from Woods Hole Marine Biological Laboratory Animal Supply (Woods Hole, Massachusetts; 41°32′ N, 70° 40′W) and shipped overnight to PSU. Animals from the microtidal site of the AF population (n = 7) were collected from the Banana River in the Indian River Lagoon, in the vicinity of Melbourne, Florida (28°5′ N, 80°35′ W), and shipped overnight to PSU. Animals from the site with one dominant daily tide of the GF population (n = 28) were purchased from Gulf Specimen Marine Laboratories, Inc. (Panacea, Florida), and shipped overnight to PSU. These animals had been collected from Mashes Sands Beach in Apalachee Bay (Wakulla County, Florida; 29°58′ N, 84°20′ W), a mixed tide site where the mean ratio between two successive tides is 1.65 ± 0.3 (CO-OPS, 2013; Supplementary Table 1). Animals from all sites were freshly obtained for each of the four separate experiments described below, maintained in the laboratory for the duration of each experiment only (Table 1), and not re-used across separate experiments. Recording of activity patterns in each experiment began upon the animals’ arrival at the laboratory.
Table 1.
Sequence, timing, and duration of artificial tide conditions applied during each of four experiments designed to assess locomotor activity rhythms of L. polyphemus sourced from separate tide environments
| Experiment | Stage of experiment
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1. Animals from regions with one dominant daily tide (GF; n = 7) and two daily tides (GM; n = 7) | No tides 12/3/2011–12/20/2011 | 12.4-h tidal cycle 12/21/2011–1/19/2012 | 24.8-h tidal cycle 1/10/2012–2/4/2012 | No tides 2/5/2012–2/12/2012 | NA |
| 2 Animals from areas with one dominant daily tide (GF; n = 7) and two daily tides (MA; n = 7) | No tides 9/29/2012–10/15/2012 | 12.4-h tidal cycle 10/16/2012–11/4/2012 | 24.8-h tidal cycle 11/5/2012–11/24/2012 | No tides 11/25/2012–12/2/2012 | NA |
| 3. Animals from areas with one dominant daily tide (GF; n = 7) and microtides (AF; n = 7) | No tides 4/3/2013–4/17/2013 | 12.4-h tidal cycle 4/18/2013–5/2/2013 | No tides 5/3/2013–5/11/2013 | 24.8-h tidal cycle 5/12/2013–5/29/2013 | No tides 5/30/2013–6/4/2013 |
| 4. Animals from areas with two daily tides (GM; n = 14) | No tides 6/13/2013–6/27/2013 | 24.8-h tidal cycle 6/28/2013–7/12/2013 | No tides 7/13/2013–7/21/2013 | 12.4-h tidal cycle 7/22/2013–8/5/2013 | No tides 8/6/2013–8/18/2013 |
Upon arrival at PSU, any epibionts were first removed from the carapace of each animal. To enable video tracking of activity patterns, white duct tape was then adhered to the prosoma (between the two lateral eyes from the hinge to the medial eye) with cyanoacrylate glue. Animals were then placed in the experimental chamber (described below) and maintained under a 12:12 light/dark cycle (LD; lights-on: 8:00 am, lights-off: 8:00 pm) with instantaneous photic transitions, salinity between 25 and 30 psu, and temperature between 18–21°C for the duration of the experiments (Table 1; Exp. 1 – 72 d ; Exp. 2 – 65 d; Exp. 3 – 70 d ; Exp. 4 – 65 d). Temperature and lighting conditions were continuously recorded using Vernier Labquest data collection units (Vernier Software and Technology LCC, Beaverton, Oregon). Animals were not fed during each experiment (Table 1).
2.2 Experimental Chamber and General Procedures
Two 1136 L tanks (1.7 m L × 0.9 m W × 0.75 m D), each with a separate filter system, were subdivided by plastic eggshell grating (1 cm × 1 cm) into a total of 16 arenas (each 21 cm × 45 cm); the perforations of the eggshell grating allowed continuous water connection between all the arenas of each tank. A 4-m long waterproof red LED Ribbon Flex strip lighting (LED Liquidators, Inc., Westlake Village, California) was threaded through the eggshell grating in each arena to provide continuous illumination necessary for video tracking. A Rio+ Aqua 50 powerhead pump (262 L/h; Technological Aquatic Associated Manufacturing, Camarillo, California), connected to an automated timer, was mounted to the grating in an unoccupied arena in each tank. A 2-m long aquarium tubing was attached to the outflow of each pump and secured to the outflow of the filter of the opposite tank, allowing water from each tank to be emptied into the opposite tank. To generate the no tides condition, each tank was filled to a depth of 0.40 m, and this depth was maintained for the no tides phase in each experiment (Table 1). To generate the 12.4 h tidal cycle, one tank was filled to a depth of 0.70-m by emptying the opposite tank to a depth of 0.10-m. Every 6.2 h, water was pumped out of the “high tide” tank into the opposite “low tide” tank; complete exchange of water took 2 hours and 45 minutes, creating a “high tide” of 0.70-m every 12.4 h in each tank. To generate a 24.8 h tidal cycle, the same mechanism was employed except that the pumps turned on every 12.4 h to create a high tide every 24.8 h in each tank. The sequence and duration of tide conditions applied in each experiment are shown in Table 1.
Limulus polyphemus is capable of expressing circatidal behavioral rhythms year-round as long as the water temperature exceeds 11°C (Chabot et al. 2004, 2007), so experiments were conducted throughout the year (Table 1). In each experiment, animals of the two populations were obtained as described and distributed in the two identical separate tanks (with the seven animals of one population in one tank, and seven animals of the second population in the second tank). Each animal was placed in an individual 0.21 m L × 0.45 m W × 0.75 m D arena in each tank (seven animals per tank, for a total of 14 animals per experiment; Table 1; arenas were separated via plastic mesh, so water could circulate through all of them. While this might theoretically enable the animals to influence one another’s rhythms, we have never observed this in any of our previous experiments with horseshoe crabs. For example, in constant conditions, adjacent animals frequently express free-running rhythms that are unique compared to their neighbors and, when exposed to artificial tides, there are often unique phase angles of entrainment (Chabot et al., 2004; Chabot et al., 2007; Chabot et al., 2008; Watson et al., 2008; Chabot et al., 2011; Chabot et al., 2016).
An infrared video camera was suspended 1.2 meters above the tanks, and digital video recordings were taken at a rate of one frame per 20 seconds using Gawker software (Piwonka, Seattle, Washington). Animals of the two tanks were simultaneously exposed to a sequence of three artificial tides (no tides, a 24.8 h tide cycle, and a 12.4 h tide cycle); these sequences always began with a no tides stage, initiated on the first day the animals arrived at the laboratory, and concluded with a final no tides stage (Table 1). Each artificial tide condition was applied for at least 9 d; this duration was extended by up to 20 d when necessary to clarify behavioral responses (no tides [mean ± SEM] – 15.5 ± 2.1 d; 12.4 h tidal cycle – 17.0 ± 1.8 d; 24.8 h tidal cycle – 17.0 ± 1.6 d; Table 1).
2.3. Experiments 1 and 2: Animals from the sites with one dominant daily tide (GF population) and two daily tides (GM and MA populations)
These experiments were designed to assess the activity rhythms expressed by animals obtained from environments with two daily tides (GM and MA populations) and with one dominant daily tide (GF population) under three different artificial tide conditions: no tides (constant water depth), a 24.8 h tidal cycle, and a 12.4 h tidal cycle. In Experiment 1 (conducted December 2011 – February 2012; Table 1), seven male GF L. polyphemus and seven male GM L. polyphemus were obtained in December, 2011 and treated as described in 2.1 and 2.2. Animals were maintained from days 1–17 in no tides to assess endogenous behavioral rhythms, then exposed to the 12.4 h tidal cycle from days 17–36 to investigate whether animals from an environment with one dominant daily tide could synchronize behaviors to a 12.4 h tidal cycle, then exposed to a 24.8 h tidal cycle from days 37–62 to investigate whether animals from both environments could synchronize behaviors to a 24.8 h tidal cycle, and finally returned to no tides from days 63–70 to investigate whether their behavioral rhythms had been entrained by the 24.8 h tide cycle. In Exp. 2 (conducted over September – December 2012; Table 1), seven new male GF L. polyphemus and seven male MA L. polyphemus were obtained in September, 2012, and treated as described in 2.1 and 2.2. Animals were maintained from days 1–16 without tides, then exposed to a 12.4 h tidal cycle from days 16–37, then a 24.8 h tidal cycle from days 37–58, and finally returned to no tides from days 58–65. During 12.4 h and 24.8 h cycles, tidal exchange of water occurred by pumping water from one tank into the adjacent tank (as described in 2.2), and so water from the tanks housing each population was continually exchanged.
2.4. Experiment 3: Animals from the sites with one dominant daily tide (GF) and with microtidal regime (AF)
Seven males from the microtidal site (AF population) and seven males from the one dominant daily tide site (GF population) were obtained in March 2013, and treated as described in 2.1 and 2.2. Animals were kept without tides from days 1–14, and then exposed to a 12.4 h tidal cycle (the “novel” tide for both populations) from days 14–28. To assess entrainment to the 12.4 h tidal cycle, animals were then returned to the no tides condition from days 28–36. The 24.8 h tidal cycle was applied on days 37–55, and animals were returned to no tides for days 56–70. One GF animal died after the first stage in no tides, one died after the second stage in no tides, and one died after the 24.8 h tidal cycle stage. This experiment was conducted during March – May 2013 (Table 1).
2.5 Experiment 4: Animals from the site with two daily tides (GM population)
Though water temperature, and not time of year, appears to be the driving factor facilitating the expression of circatidal rhythms in L. polyphemus (Chabot and Watson 2010), animals display behavioral changes, such as increased excursions to the intertidal zone, during their spawning season (May – August). This experiment, conducted June – August 2013, was primarily designed to investigate whether during the spawning season animals from environments with two daily tides would respond similarly to the 24.8 h and 12.4 h tidal cycles as had the non-spawning animals (section 2.3). A second goal of this experiment was to investigate how the GM animals would respond to the 24.8 h tidal cycle (their “novel” tide) when it was presented first (before the 12.4 h tidal cycle) in the tide sequence. Fourteen male GM L. polyphemus obtained from spawning beaches in Great Bay, NH, in June of 2013, and treated as described in 2.1 and 2.2. Animals were maintained from days 1–14 without tides, and then exposed to a 24.8 h tidal cycle from days 14–28. To assess entrainment to the 24.8 h tidal cycle, animals were then returned to no tides from days 29–37. Animals were then exposed to the 12.4 h tidal cycle from days 38–52, and returned to no tides for days 53–65. Three animals died after the second stage in no tides.
2.6 Data Analysis
Video footage was analyzed for distance moved (cm) at 20 second intervals using Ethovision XT software (Noldus Information Technology Inc., Leesburg, Virginia). Distance was summed for each minute, and these values were used to generate actograms and Lomb-Scargle periodograms using ClockLab Data Analysis software (Actimetrics, Wilmette, Illinois). Lomb-Scargle periodogram analyses were used to determine whether animals expressed significant bimodal (approximately 12.4 h) or unimodal (approximately 24 h) rhythms for each tide condition. Peaks exceeding P = 0.001 in the 10.4–14.4 h range were classified as bimodal/tidal, and peaks in the 22.8 – 26.8 h range were classified unimodal/daily; animals with no significant peaks in either range were classified as arrhythmic. To clarify the relationship between activity and time of tide, parallelograms representing the time and duration of the high tide were drawn onto the actograms. These parallelograms were drawn such that the left-hand side of each indicated the time at which high tide began (the time at which the pump was turned on to add water to the tank), and the right-hand side indicated the time at which high-tide ended (the time at which the pump to empty the tank was turned on), allowing the width of the parallelogram to represent the approximate duration of the high tide. Visual inspection of these actograms was used to assess the relationship of animals’ behavioral patterns to tide cycles.
All subsequent analyses were performed using SAS Studio (SAS University Edition, SAS Institute Inc., Cary, North Carolina). For each experiment, the number of animals that expressed bimodal and unimodal rhythms during the first no tides stage, during a 12.4 h tidal cycle, and during a 24.8 h tidal cycle was counted for each population. Fisher’s exact tests (FET) were used to determine whether the proportion of animals from the environment with one dominant daily tide (GF population) that expressed bimodal and unimodal rhythms during the three different tide conditions was affected by time of year that the experiments had been conducted, and whether the proportions of animals from the environments with two daily tides (GM and MA population) that expressed bimodal and unimodal rhythms was affected by either population type (GM or MA) or time of year. Single factor ANOVAs were performed to assess effects of time of year on mean unimodal period length for animals of the environment with one dominant daily tide, and to assess effects of time of year and population type (GM, MA) on mean bimodal and unimodal period length during the three different tide conditions for animals of the environments with two daily tides. Significance of mean differences were determined through Tukey’s HSD multiple comparisons procedure (P = 0.05).
There were no significant effects of time of year on the proportions of animals from the environment with one dominant daily tide (n = 18) that expressed unimodal rhythms during each experimental tide condition (no tides – FET, P = 0.222; 24.8 h tidal cycle – FET, P = 0.999; 12.4 h tidal cycle – FET, P = 0.222) or on mean unimodal period length (ANOVA, F(2,45) = 3.06, P = 0.06), so the data from the three experiments were combined for analysis. Similarly, for the three experiments that used animals from the environments with two daily tides (GM and MA populations), period length and proportion of animals expressing bimodal rhythms were not affected by population type (period: ANOVA, F(1,56) = 0.57, P = 0.454; proportion: no tides – FET, P = 0.647; 24.8 h tidal cycle – FET, P = 0.318; 12.4 h tidal cycle – FET, P = 0.180) or time of year (period: ANOVA, F(1,43)= 0.71, P = 0.405; number – no tides: FET, P = 0.670; 24.8 h tidal cycle: FET, P = 0.382; 12.4 h tidal cycle: FET, P = 0.431), and data from these experiments were combined for analysis.
Fisher’s exact tests were used on the combined data to determine significant relationships (P = 0.05) between source tide environments (two daily tides, one dominant daily tide, and microtides) and proportions of animals that expressed unimodal and bimodal rhythms during the three tide conditions (no tides, 24.8 h tidal cycle, and 12.4 h tidal cycles), and to determine significant effects of artificial tide cycles on proportions of animals of each tide environment that expressed unimodal and bimodal rhythms. Significant differences were determined by pairwise Fisher’s exact tests, with the level of significance (P = 0.017) determined through the Bonferroni correction.
3. Results
3.1 Animals from areas with one dominant daily tide (GF population)
Most animals collected from areas with one dominant daily high tide expressed unimodal activity patterns during all artificial tide conditions. Specifically, most (20/21) of these animals expressed unimodal activity patterns during the initial stage in no tides (Fig. 1; Table 2), and most (19/20) animals continued to express unimodal activity patterns during the 12.4 h tidal cycle (Fig. 1, Table 2). During the 24.8 tidal cycle, all of the remaining animals (18/18) expressed unimodal activity patterns. Overall, the three artificial tide conditions did not affect the proportions of animals that expressed unimodal and bimodal rhythms (FET, P = 0.999).
Figure 1.
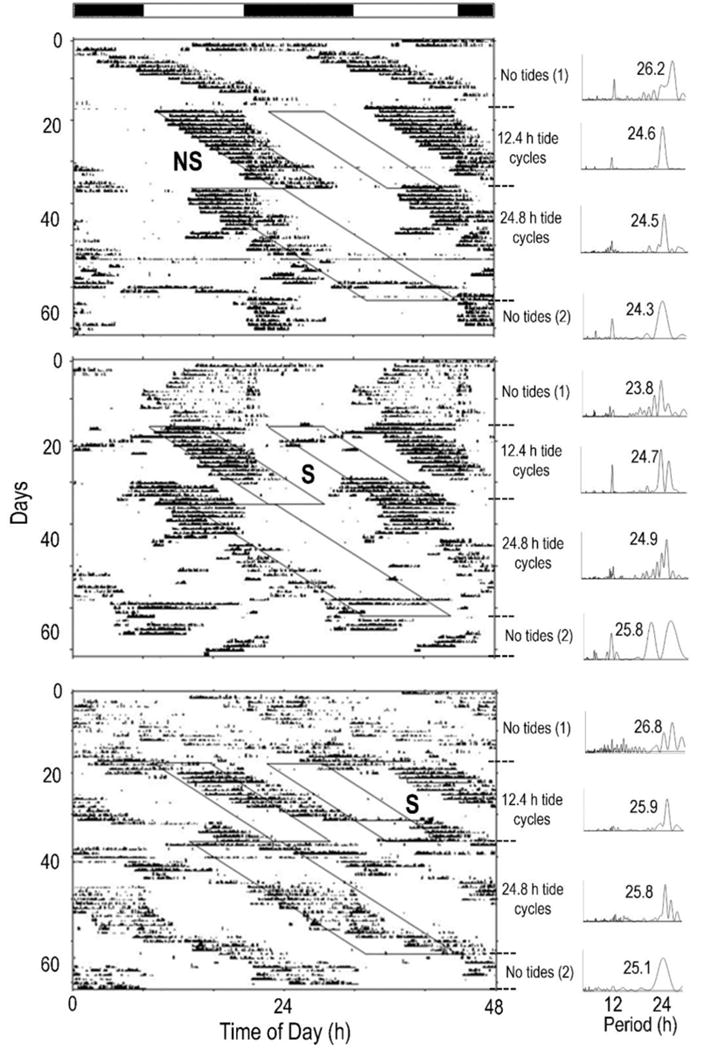
Table 2.
Percent of L. polyphemus collected from areas with one dominant daily tide, two daily tides, and microtides that expressed bimodal and unimodal tides during artificial tide cycles. Bold indicates the apparent higher percentage between “Unimodal” and “Bimodal” categories for animals of each source environment during each artificial tide.
| Rhythm type | Artificial tide
|
|||||
|---|---|---|---|---|---|---|
| No tides
|
12.4-h tidal cycle
|
24.8-h tidal cycle
|
||||
| Unimodal | Bimodal | Unimodal | Bimodal | Unimodal | Bimodal | |
| One dominant daily tide | 95% | 5% | 95% | 5% | 100% | 0% |
| n = 21 | ||||||
| τ± SEM | 25.1 ±0.2 | 12.5 | 25.0 ± 0.2 | 12.3 | 24.6 ± 0.2 | NA |
| Two daily tides n = 28 | 29% | 68% | 12% | 88% | 25% | 75% |
| τ± SEM | 25.0 ± 0.4 | 12.3 ±0.1 | 24.2 ± 0.6 | 12.2 ±0.1 | 24.6 ± 0.2 | 12.3±0.1 |
| Microtides | 100% | 0% | 14% | 71% | 100% | 0% |
| n = 7 | ||||||
| τ± SEM | 25.7 ± 0.5 | NA | 24.5 ± 0.6 | 12.6 ± 0.2 | 24.3 ±0.1 | NA |
During the no tides stage, most (16/21) animals appeared to express unimodal activity patterns that drifted relative to the light-dark cycle and thus had a period (tau) > 24 h (Fig. 1-top, “No tides (1)”; bottom, “No tides (1)”). Four animals appeared to be primarily active during the lights-on period (Fig. 1-middle, “No tides (1)”).
During the 12.4 h tidal cycle, 15 of the animals were initially active when they encountered the first artificial high tide of the lights-on period (Fig. 1-top, “12.4 h tidal cycle”), and 10 of them appeared to synchronize to this tide, and continued to be active with this tide, as it ran into the lights-off period (Fig. 1-top, “12.4 h tidal cycle”; bottom- “12.4 h tidal cycle”). However, five animals appeared to switch their time of greatest activity to the lights-on period high tide (Fig. 1-middle, “12.4 h tidal cycle”) a few days after the first tide began extending into the lights-off period. During the 24.8 h tidal cycle, 14/18 animals appeared to synchronize their activity to the tide (Fig 1 – “24.8 h tidal cycle”). During the final stage in no tides, 7/18 animals appeared to express activity at the anticipated time of the tide (Fig 1 – bottom, “No tides (2)”), and thus appeared to have been entrained by the tidal cycle.
3.2 Animals from areas with two daily tides (GM and MA population)
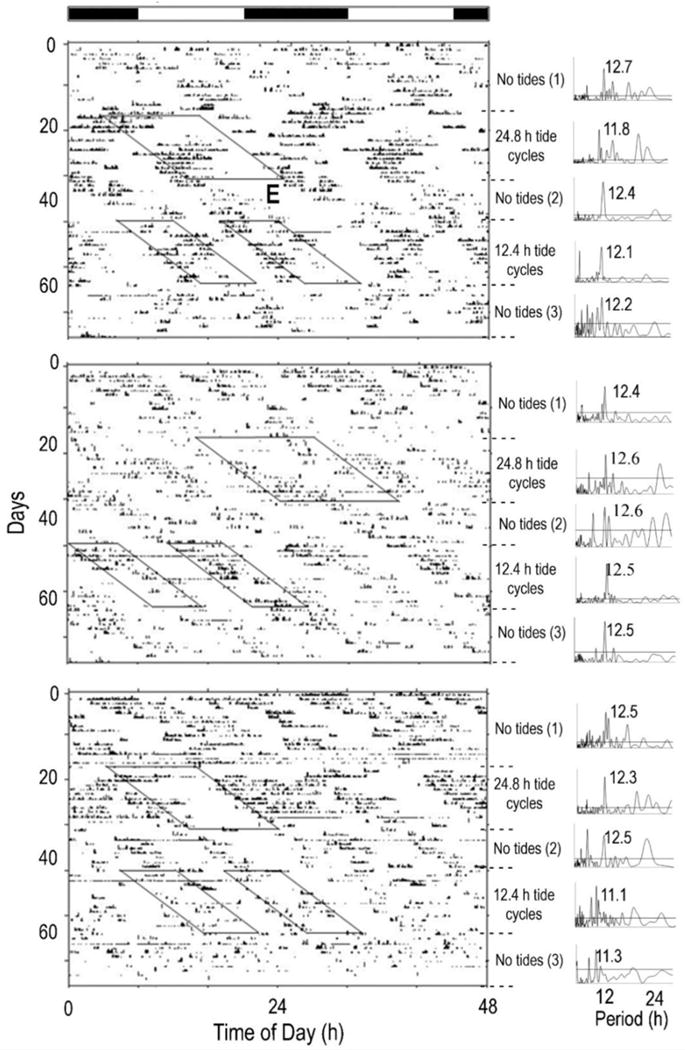
Most animals from areas with two daily tides expressed bimodal activity rhythms throughout the three artificial tide conditions. During no tides, 68% (19/28) of the horseshoe crabs exhibited bimodal activity patterns (Table 2; Fig. 2, “No Tides (1)”), while 29% (8/28) expressed unimodal activity patterns. When exposed to 12.4 h tidal cycles, most (22/25) of the animals expressed bimodal activity patterns (Table 2; Fig. 2- “12.4 h tidal cycle”), while the remainder (3/25) of them expressed a unimodal rhythm (data not shown). The majority (21/28) of the animals expressed bimodal rhythms during the 24.8 h tidal cycle (Fig. 2, “24.8 h tidal cycle”), with synchronization of one of the two activity bouts to the single daily tide in 43% (12/28; Fig. 2) of the animals. While 25% (7/28) of the animals expressed unimodal rhythms during the 24.8 h tidal cycle, synchronization to the tide was only apparent in 11% (3/28; data not shown). Over all stages of the experiment, type of artificial tide cycle had no effect on the proportions of animals that expressed unimodal and bimodal rhythms (FET, P = 0.2842).
Figure 2.
3.3. Animals from the microtidal environment
While animals from the microtidal environment expressed unimodal rhythms during the no tides stages and during the 24.8 h tidal cycle, most (5/7) expressed bimodal activity rhythms during the 12.4 h tidal cycle. When maintained without tides, all (7/7) animals exhibited significant unimodal activity; five animals appeared to express activity rhythms that drifted relative to the LD cycle (Fig. 3-top, “No tides (1)”), while two animals appeared to express activity patterns in synchrony with the LD cycle (Figure 3 – bottom, “No tides (1)”) with the majority of their activity during the “lights-on” period. When exposed to the 12.4 h tidal cycle, most (5/7) animals displayed bimodal activity patterns (Fig. 3-top, middle – “12.4 h tidal cycle”), one animal expressed a unimodal rhythm (Fig. 3-bottom, “12.4 h tidal cycle”), and one animal did not express a significant rhythm. Upon suspension of the tides and return to the no tide condition, all seven animals expressed unimodal rhythms: 3 animals expressed rhythms that drifted relative to the light-dark cycle (Fig. 3 – top, middle), while 4 animals expressed rhythms that appeared synchronized to the light-dark cycle (Fig. 3 – bottom). During the 24.8 h tidal cycle, 7/7 animals expressed unimodal rhythms (Fig. 3, “24.8 h tidal cycle”), and during the following week in no tides, all animals continued to express unimodal rhythms; of these, 2 animals expressed rhythms that appeared to drift relative to the light-dark cycle (Fig. 3 – middle), while 5 animals expressed rhythms that appeared synchronized to the light-dark cycle (Fig. 3 – top, bottom).
Figure 3.
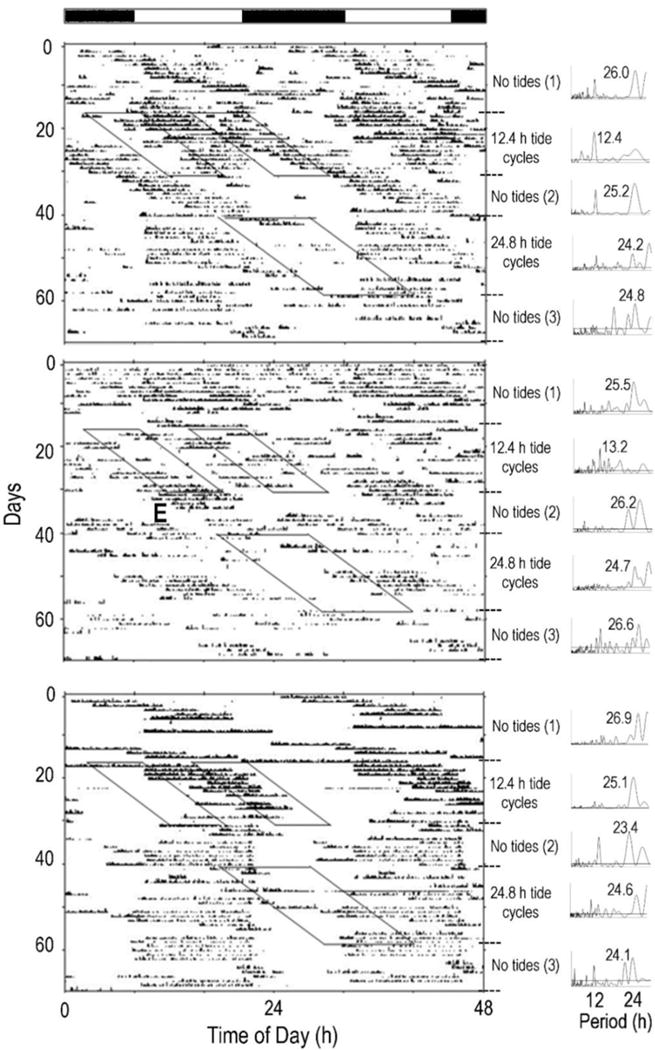
Artificial tide conditions significantly affected the proportions of animals from the microtidal environment that expressed unimodal and bimodal rhythms (FET, P = 0.0031). The proportion of animals that expressed bimodal rhythms was significantly greater during the 12.4 h tidal cycle than during no tides (FET, P = 0.011) and during the 24.8 h tidal cycle (FET, P = 0.011), while proportions of animals expressing bimodal and unimodal rhythms during no tides and the 24.8 h tidal cycle were similar (FET, P = 0.999).
3.4. Population Comparison
The source locations significantly affected the proportions of animals that expressed unimodal and bimodal rhythms during the three tide conditions (no tides: FET, P = 0.0001; 24.8 h tidal cycle: FET, P = 0.0001; 12.4 h tidal cycles: FET,P = 0.0001; Table 2). In all tidal conditions, almost all animals from the three environments expressed either a significant unimodal or bimodal rhythm (Table 2); the one exception was an animal from the microtidal environment that lacked expression of a significant rhythm during the application of the 12.4 h tidal cycle. During no tides and during the 24.8 h tidal cycle, significantly more animals from one dominant daily tide and microtidal environments expressed unimodal rhythms than did L. polyphemus from areas with two daily tides (no tides: one dominant daily tide and two daily tides – FET, P = 0.0001; microtide and two daily tides – FET, P = 0.0017; one dominant daily tide and microtide – FET, P = 0.999; 24.8 h tidal cycle: one dominant daily tide and two daily tides – FET, P = 0.0001; microtide and two daily tides – FET, P = 0.0005; one dominant daily tide and microtide- FET, P = 0.999; Table 2). However, with application of the 12.4 h tidal cycle, the proportion of animals that expressed unimodal rhythms was significantly higher in the population from the site with one dominant daily tide than in those from the sites with two daily tides (FET, P = 0.0001) and microtides (FET, P = 0.0012), while the proportions of animals that expressed unimodal and bimodal rhythms were similar in the populations with two daily tides and microtides (FET, P = 0.2964)
4. Discussion
4.1 Limulus polyphemus populations differ in expression of activity rhythms
Our findings show, for the first time, that L. polyphemus collected from three distinct tidal regimes differ significantly in their expression of behavioral rhythms. Specifically, animals from environments with one dominant daily tide and with microtides typically expressed unimodal, non-light-dark cycle synchronized, activity patterns when no tidal cues were available, which contrasts with the behavior of animals collected from environments with two daily tides (Chabot et al. 2004, 2007) and suggests endogenous unimodal rhythms in these animals. The apparent inability of L. polyphemus from the GF population to express bimodal rhythms when exposed to a 12.4 h tidal cycle suggests that bimodal circatidal rhythm expression is not a characteristic of all L. polyphemus. This persistence of unimodal activity in animals from areas with one dominant daily tide is consistent with the unimodal behavioral patterns expressed by populations of L. polyphemus larvae from the same site when they are held in constant conditions in the laboratory (Apalachee Bay, Florida; Rudloe 1979).
While animals from the microtidal environment expressed bimodal rhythms in response to the 12.4 h tidal cycle, the lack of persistence of this bimodal pattern during subsequent exposure to no tides suggests these bimodal rhythms were driven by the exogenous tide cues. Interestingly, some animals from the microtidal environment also exhibited activity patterns that appeared to transition from synchronization to the light-dark cycle to the tidal cycle and vice versa, further suggesting rhythms in animals from the microtidal environment may be strongly regulated by exogenous factors. Similarly, the microtidal isopod Excirolana braziliensis expresses variation in response to light and tide cues (Yannicelli et al. 2001), and individual estuarine mud crabs Rhithropanopeus harrissi vary in their rhythm expression (daily or circatidal; Cronin and Forward 1983). Such behavioral flexibility has been proposed to increase fitness in microtidal environments (Cronin and Forward 1983; Yannicelli et al. 2001), in which tidal cues may be less regular and predictable than in other tidal environments. In L. polyphemus, these behavioral changes in response to external cues may be beneficial in the dynamic tide environment of the 156 mile long Indian River Lagoon, where tides range from primarily wind-driven microtides in the northern portion of the lagoon to two 0.1 – 0.2 m tides per day (Woodward-Clyde 1993) in the southern portion (south of Melbourne, FL, our source site), and may facilitate synchronization of activities (such as foraging) to the environmental variables of separate locales.
Unlike animals from the microtidal environment, L. polyphemus from the sites with one dominant daily tide and with two daily tides appeared fixed in their behavioral repertoires. While animals of both environments appeared unable to adjust the structure of their activity rhythms in response to novel tide cycles, they demonstrated both synchronization of their respective activity patterns to tidal cycles and persistence of activity patterns upon removal of the tides, suggesting endogenous rhythms of animals from both environments were entrained by the tidal cycles. However, the apparent inability of these animals to alter the structure of their activity patterns (from bimodal to unimodal or vice versa) in response to novel tide cycles differs from behavioral responses of fiddler crabs Uca minax and Uca longisignalis, which express unimodal activity patterns in a single daily tide environment but bimodal activity patterns when transplanted to regions with two daily tides (Barnwell, 1968). The discrepancies between these two species may suggest differences in the composition, inputs, or outputs of the underlying oscillator(s) controlling behavioral rhythm expression in Uca crabs and in the horseshoe crab L. polyphemus.
4.2 Circalunidian rhythms appear present in all populations
All populations examined appeared capable of expressing circalunidian (~24.8 h) behavioral rhythms. Circalunidian control of tidal rhythms, first proposed by Williams and Palmer (1986), suggests that circatidal rhythms are generated by two circalunidian oscillators locked in antiphase so that each oscillator drives one of the bouts of activity associated with one of the two tides that occur each day (Williams and Palmer 1986). Extensions of this theory suggest that circalunidian oscillators may receive light input in addition to tidal cues (Darnell et al. 2009), and that each oscillator may be selectively inhibited by such cues or by the second oscillator (Lopez-Duarte and Tankersley 2007; Dugaw et al. 2009). This circalunidian hypothesis contrasts two other models of tidal rhythm generation: (1) the “circatidal/circadian clock hypothesis,” which proposes that the interaction of a circadian oscillator and at least one circatidal oscillator (an oscillator with 12.4 h period) drives rhythm expression (Harris and Morgan, 1984; Naylor, 1996) and (2) the hypothesis that the circatidal and circadian rhythm are jointly driven by a single bimodal circadian oscillator receptive to light and tide cues (Enright, 1976) or dual coupled circadian oscillators (Saigusa 1986, 1988). We observed four characteristics of activity patterns in L. polyphemus that suggest control by a circalunidian timing mechanism: (1) the activity patterns of most animals from the one dominant daily tide site and microtidal site “drifted” (exhibited free-running rhythms) relative to the light-dark cycle, a finding that would not be expected if this behavior was controlled by a circadian clock; (2) the periods of these rhythms more closely and consistently approximated the lunar day period (24.8 h) than the daily, light-dark cycle (24 h) period used in these experiments; (3) unimodal activity usually synchronized to at least one of the two daily tides during the 12.4 h tidal cycle and to the single tide during the 24.8 h tidal cycle; and (4) the timing of activity patterns sometimes appeared to persist after cessation of the tidal cycles, suggesting entrainment of the tidal oscillators. Previous field and laboratory studies of L. polyphemus from environments with two daily tides had suggested that dual circalunidian oscillators control their behavioral rhythms as well (Chabot and Watson 2010; Chabot et al. 2016). Likewise, the expression of apparent circalunidian rhythms in L. polyphemus from the other two environments suggests that circalunidian oscillators may be widespread in this species.
While animals from the site with one dominant daily tide expressed solely unimodal rhythms, their ability to switch the timing of activity bouts between the two tides in apparent response to the light – dark cycle may further suggest the presence of two functioning circalunidian oscillators (one of which may be inhibited at any given time by either a circadian oscillator or the second circalunidian oscillator; Lopez-Duarte and Tankersley 2007; Dugaw et al. 2009). Similar phase shifts in the timing of larval release activity have been observed in Sesarma haematocheir (Saigusa 1986), and these activity patterns have previously been explained as the output of two coupled circadian oscillators, one of which is responsive to the daily light – dark cycle and the second of which is phased by the first (Saigusa 1986, 1988). However, molecular studies in Eurydice pulchra (Zhang et al. 2009) and Apteronemobius asahinai (Takekata et al. 2014) have since demonstrated that the circadian and tidal timekeepers are distinct entities, and have further suggested that a functioning circadian system is not necessary for the generation of tidal rhythms (Zhang et al. 2009), findings which lend support to the explanation of coupled circalunidian oscillators, rather than circadian oscillators, as drivers of this phase shifting ability. Alternatively, such a behavioral pattern may arise through the interaction of the circadian and circatidal oscillators (Naylor, 1996). However, this hypothesis predicts activity only at the times in which promotion by the circadian oscillator overcomes inhibition by the circatidal oscillator, and thus suggests predictable modulation of activity patterns by the light-dark cycle. Among animals from the site with one dominant daily tide some animals exhibited no apparent modulation of activity by the light cycle (i.e, activity ran through the LD cycle during the “no tides” stage, and activity persisted with one of the two tides as the tide ran through the light-dark cycle during the 12.4 h tidal cycle stage), suggesting a lack of the influence of a circadian oscillator on the behaviors of some animals.
4.3 Genetic or environmental factors may contribute to population differences
Whether environmental influences, genetic factors, or a combination of both are responsible for the phenotypic behavioral differences among L. polyphemus populations remains to be determined. Both the GF and AF populations exhibit a major genetic break from the MA and GM populations (Saunders et al. 1986), and the GF population has further diverged from the AF population (King et al. 2005, 2015). The lack of entrainable bimodal circatidal rhythms in both Floridian populations may suggest a degree of genetic differentiation in tidal clock inputs, mechanisms, or outputs that separates them from the northern populations. Alternatively, environmental influences during ontogeny may be partially responsible for the persistence of unimodal behavioral patterns in GF animals, as exposure to two daily tides during embryonic development is necessary for expression of circatidal behavioral rhythms in L. polyphemus larvae (Ehlinger and Tankersley 2006). The mixed tide regime of the Gulf of Mexico, with unequal amplitudes between successive tides, may be insufficient to facilitate the expression of twice daily circatidal activity patterns. Similarly, depending on nest location in the Indian River Lagoon, embryos may receive cues with one daily tide, two daily tides, or constant immersion during development. Whether such environmental factors or genetic factors perpetuate the behavioral differences observed in this study is an intriguing question given both the degree of genetic separation among the populations and the disparate environments inhabited by each.
Supplementary Material
Acknowledgments
This project could not have been completed without the assistance of Steven Simpson, Katherine Fondo, Alexandria Santry, Kyle Kenyon, Tyler Remillard, Jonathan Reider, and Albert Lamonda, who helped with animal care. Special thanks to Stephanie Kronstadt for collection of IRL animals, to Christopher Freeman for construction of the experimental apparatus, to Meghan Owings for feedback on earlier drafts, and to the anonymous reviewers, who provided very helpful and constructive comments and suggestions. Financial support for this project was provided by NSF (IOS 0920342 to CCC and WHW III), Plymouth State University College of Graduate Studies, and the New Hampshire IDeA Network of Biological Research Excellence with grants from the National Center for Research Resources (5P20RR030360-03) and the National Institute of General Medical Sciences (8P20GM103506-03), National Institutes of Health.
Abbreviations
- IRL
Indian River Lagoon
- GM
Gulf of Maine
- MA
Mid-Atlantic
- AF
Atlantic Florida
- GF
Gulf Florida
Literature Cited
- Barlow RB, Powers MK, Howard H, Kass L. Migration of Limulus for mating: Relation to lunar phase, tide height, and sunlight. Biol Bull. 1986;171:310–329. doi: 10.2307/1541674. [DOI] [Google Scholar]
- Barnwell FH. The role of rhythmic systems in the adaptation of fiddler crabs to the intertidal zone. Am Zoologist. 1968;8:569–583. doi: 10.1093/icb/8.3.569. [DOI] [Google Scholar]
- Brockmann HJ, Johnson SL. A long-term study of horseshoe crabs in Florida. Estuaries Coasts. 2011;34:1049–1069. doi: 10.1007/s12237-011-9419-1. [DOI] [Google Scholar]
- Chabot CC, Kent J, Watson WH., III Circatidal and circadian rhythms of activity in Limulus polyphemus. Biol Bull. 2004;207:72–75. doi: 10.2307/1543630. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Betournay SH, Braley NR, Watson WH., III Endogenous rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. J Exp Mar Biol Ecol. 2007;345:79–89. doi: 10.1016/j.jembe.2007.01.009. [DOI] [Google Scholar]
- Chabot CC, Skinner SJ, Watson WH., III Rhythms of Locomotion Expressed by Limulus polyphemus, the American Horseshoe Crab: I. Synchronization by Artificial Tides. Biol Bull. 2008;215:34–45. doi: 10.2307/25470681. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Watson WH., III Circatidal rhythms of locomotion in the American horseshoe crab Limulus polyphemus: Underlying mechanisms and cues that influence them. Curr Zool. 2010;56:499–517. [Google Scholar]
- Chabot CC, Yelle JF, O’Donnell CB, Watson WH., III The effects of water pressure, temperature, and current cycles on circatidal rhythms expressed by the American horseshoe crab, Limulus polyphemus. Mar Freshw Behav Physiol. 2011;44:43–60. doi: 10.1080/10236244.2010.541992. [DOI] [Google Scholar]
- Chabot CC, Ramberg-Pihl N, Watson WH., III Circalunidian clocks control tidal rhythms of locomotion in the American horseshoe crab, Limulus polyphemus. Mar Fresh Behav Physiol. 2016;49:75–91. doi: 10.1080/10236244.2015.1127679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CO-OPS (Center for Operational Oceanographic Services and Products) NOAA Tide Predictions. 2013 Retrieved from http://tidesandcurrents.noaa.gov/tide_predictions.html.
- Cohen JA, Brockmann JH. Breeding activity and mate selection in the horseshoe crab Limulus polyphemus. Bull Mar Sci. 1983;33:274–281. [Google Scholar]
- Cronin TW, Forward RB. Vertical migration rhythms of newly hatched larvae of the estuarine crab, Rhithropanopeus harrisii. Biol Bull. 1983;165:139–153. doi: 10.2307/1541360. [DOI] [Google Scholar]
- Darnell MZ, Rittschof D, Forward RB. Endogenous swimming rhythms underlying the spawning migration of the blue crab, Callinectes sapidus: ontogeny and variation with ambient tidal regime. Mar Biol. 2010;157:2415–2425. doi: 10.1007/s00227-010-1506-5. [DOI] [Google Scholar]
- Dugaw CJ, Honeyfield R, Taylor CM, Verzi DW. Modeling activity rhythms in fiddler crabs. Chronobiol Int. 2009;26:1355–1368. doi: 10.3109/07420520903421872. [DOI] [PubMed] [Google Scholar]
- Ehlinger GS, Tankersley RA. Endogenous rhythms and entrainment cues of larval activity in the horseshoe crab Limulus polyphemus. J Exp Mar Biol Ecol. 2006;337:205–214. doi: 10.1016/j.jembe.2006.06.035. [DOI] [Google Scholar]
- Ehlinger GS, Tankersley RA. Ecology of horseshoe crabs in microtidal lagoons. In: Tanacredi JT, Botton ML, Smith D, editors. Biology and Conservation of Horseshoe Crabs. Springer Science+Business Media, LLC; New York, NY: 2009. pp. 149–162. [DOI] [Google Scholar]
- Ehlinger GS, Tankersley RA, Bush MB. Spatial and temporal patterns of spawning and larval hatching by the horseshoe crab, Limulus polyphemus, in a microtidal coastal lagoon. Estuaries. 2003;26:631–640. doi: 10.1007/BF02711975. [DOI] [Google Scholar]
- Enright JT. Plasticity in an isopods clockworks: shaking shapes form and affects phase and frequency. J Comp Physiol. 1976;107:13–37. [Google Scholar]
- Fraser PJ. Review: depth, navigation and orientation in crabs: angular acceleration, gravity, and hydrostatic pressure sensing during path integration. Mar Freshw Behav Physiol. 2006;39:87–97. doi: 10.1080/10236240600708439. [DOI] [Google Scholar]
- Fraser PJ, Macdonald AG. Crab hydrostatic pressure sensors. Nat. 1994;371:383–384. doi: 10.1038/371383b0. [DOI] [Google Scholar]
- Harris G, Morgan E. Entrainment of the circatidal rhythm of the estuarine amphipod Corophium volutator (Pallas) to non-tidal cycles of inundation and exposure in the laboratory. J Exp Mar Biol Ecol. 1986;80:235–245. [Google Scholar]
- King TL, Eackles MS, Spidle AP, Brockmann HJ. Regional differentiation and sex-biased dispersal among populations of the horseshoe crab Limulus polyphemus. Trans Am Fish Soc. 2005;134:441–465. doi: 10.1577/T04-023.1. [DOI] [Google Scholar]
- King T, Eackles M, Aunins AW, Brockmann HJ, Hallerman EM, Brown BB. Conservation genetics of the horseshoe crab (Limulus polyphemus): allelic diversity, zones of genetic discontinuity, and regional differentiation. In: Carmichael RH, Botton ML, Shin PKS, Cheung SG, editors. Changing Global Perspectives on Biology, Conservation and Management of Horseshoe Crabs. Springer; International Publishing, Switzerland: 2015. pp. 65–96. [DOI] [Google Scholar]
- Lee WJ. Intensive use of an intertidal mudflat by foraging adult American horseshoe crabs Limulus polyphemus in the Great Bay estuary, New Hampshire. Curr Zool. 2010;56:611–617. [Google Scholar]
- Lopez-Duarte PC, Tankersley RA. Circatidal swimming behaviors of fiddler crab Uca pugilator larvae from different tidal regimes. Mar Ecol Prog Ser. 2007;343:207–220. doi: 10.3354/meps06926. [DOI] [Google Scholar]
- Lopez-Duarte PC, Christy JH, Tankersley RA. A behavioral mechanism for dispersal in fiddler crab larvae (genus Uca) varies with adult habitat, not phylogeny. Limnol Oceanogr. 2007;56:1879–1892. doi: 10.4319/lo.2011.56.5.1879. [DOI] [Google Scholar]
- Moore S, Perrin S. Seasonal movements and resource-use patterns of resident horseshoe crab (Limulus polyphemus) populations in a Maine, USA estuary. Estuaries Coasts. 2007;30:1016–1026. doi: 10.1007/BF02841392. [DOI] [Google Scholar]
- Naylor E, Atkinson RJ, Williams BG. External factors influencing the tidal rhythm of shore crabs. J Interdiscip Stud. 1971;2:173–180. [Google Scholar]
- Naylor E. Crab clockwork: the case for interactive circatidal and circadian oscillators controlling rhythmic locomotor activity in Carcinus maenas. Cronobiol Int. 1996;13:153–161. doi: 10.3109/07420529609012649. [DOI] [PubMed] [Google Scholar]
- Queiroga H, Moksnes P, Meireles S. Vertical migration behaviour in the larvae of the shore crab Carcinus maenas from a mictrotidal system (Gullmarsfjord, Sweden) Mar Ecol Prog Ser. 2002;237:195–207. doi: 10.3354/meps237195. [DOI] [Google Scholar]
- Rudloe A. Locomotor and light responses of larvae of the horseshoe crab, Limulus polyphemus. Biol Bull. 1979;157:494–501. doi: 10.2307/1541033. [DOI] [PubMed] [Google Scholar]
- Rudloe A. The breeding behavior and patterns of movement of horseshoe crabs, Limulus polyphemus, in the vicinity of breeding beaches in Apalachee Bay, Florida. Estuaries. 1980;3:177–183. doi: 10.2307/1352067. [DOI] [Google Scholar]
- Rudloe A. Variation in the expression of lunar and tidal behavioral rhythms in the horseshoe crab, Limulus polyphemus. Bull Mar Sci. 1985;36:388–395. [Google Scholar]
- Saigusa M. The circa-tidal rhythm of larval release in the incubating crab Sesarma. J Comp Physiol. 1986;159:21–31. [Google Scholar]
- Saigusa M. Entrainment of tidal and semilunar rhythms by artificial moonlight cycles. Biol Bull. 1988;174:126–138. [Google Scholar]
- Saunders NC, Kessler LG, Avis JC. Genetic variation and geographic differentiation in mitochondrial DNA of the horseshoe crab, Limulus polyphemus. Genetics. 1986;112:613–627. doi: 10.1093/genetics/112.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman JH, Barnwell FH. Relationship of daily and circatidal activity rhythms of the fiddler crab, Uca princeps, to the harmonic structure of semidiurnal and mixed tides. Mar Biol. 2004;144:143–482. doi: 10.1007/s00227-003-1213-6. [DOI] [Google Scholar]
- Takekata H, Numata H, Shiga S, Goto SG. Silencing the circadian clock gene Clock using RNAi reveals dissociation of the circatidal clock from the circadian clock in the mangrove cricket. J Insect Physiol. 2014;68:16–22. doi: 10.1016/jinsphys.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Thurman CL. Unravelling the ecological significance of endogenous rhythms in intertidal crabs. Biol Rhythm Res. 2004;35:43–67. doi: 10.1080/09291010412331313232. [DOI] [Google Scholar]
- Vasquez MC, Johnson SL, Brockmann HJ, Julian D. Nest site selection minimizes environmental stressor exposure in the American horseshoe crab, Limulus polyphemus (L.) J Exp Mar Biol Ecol. 2015;463:105–114. doi: 10.1016/j.jembe.2014.10.028. [DOI] [Google Scholar]
- Watson WH, Bedford LB, Chabot CC. Rhythms of locomotion expressed by Limulus polyphemus, the American horseshoe crab: II. Relationship to circadian rhythms of visual sensitivity. Biol Bull. 2008;215:46–56. doi: 10.2307/25470682. [DOI] [PubMed] [Google Scholar]
- Watson WH, III, Chabot CC. High resolution tracking of adult horseshoe crabs Limulus polyphemus in a New Hampshire estuary using fixed array ultrasonic telemetry. Curr Zool. 2010;56:599–610. [Google Scholar]
- Williams BG, Palmer JD. Comparative study of tidal rhythms: the dual clock control of the locomotor rhythms of two decapod crustaceans. Mar Behav Physiol. 1986;12:269–278. doi: 10.1080/10236248609378653. [DOI] [Google Scholar]
- Woodward-Clyde Consultants, McCully Marshall, Associates, Inc., and Natural Systems Analysts, Inc . Final technical report: Physical features of the Indian River Lagoon. Woodward-Clyde Consultants; Tallahassee, FL: 1994. [Google Scholar]
- Yannicelli B, Palacios R, Gimenez L. Activity rhythms of two cirolanid isopods from an exposed microtidal sandy beach in Uruguay. Mar Biol. 2001;138:187–197. doi: 10.1007/s002270000451. [DOI] [Google Scholar]
- Zhang L, Hastings MH, Green EW, Tauber E, Sladek M, Webster SG, Kyriacou CP, Wilcockson DC. Dissociation of circadian and circatidal timekeeping in the marine crustacean Eurydice pulchra. Curr Biol. 2009;23:1863–1873. doi: 10.1016/j.cub.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


