Abstract
Purpose:
This study aimed to compare pattern visual evoked potential (PVEP) components in dyslexic and normal children.
Methods:
This cross-sectional analytic study recruited 72 children, including 36 dyslexic and 36 normal participants aged 8-12 years. Visual examinations included measurement of distance visual acuity, refraction, and PVEP components of amplitudes and latencies with two different check sizes of 15 and 60 minutes (min) of arc at two contrast levels of 25% and 100%.
Results:
Our results demonstrated significant differences between dyslexic and normal children in terms of P100 latency and amplitude of PVEP at 25% contrast, with check sizes of 15 and 60 min of arc. However, there were no significant differences between the two groups regarding P100 latency and amplitude at 100% contrast with check sizes of both 15 and 60 min of arc.
Conclusion:
Dyslexic participants showed reduced amplitude and prolonged latency in most PVEP components at low-contrast levels. These findings may support the magnocellular deficit hypothesis in dyslexic participants, even though the parvocellular pathway remains intact.
Keywords: Dyslexia, Magnocellular Pathway, Parvocellular Pathway, Visual Evoked Potential
INTRODUCTION
Low intelligence, behavioral or motivational problems, or lack of reading ability cannot explain the selective impairment of reading skills in individuals with dyslexia.[1] Dyslexia is the most common learning disability, found in 5–17% of school-age children.[2,3] However, dyslexia involves issues that are more profound than problems in reading alone. Indeed, a neurological syndrome can better explain this condition, which involves many acquired skills.[4] Symptoms and signs found in dyslexia include a strong genetic predisposition, brain involvement (documented with both structural and functional imaging), slow visual and auditory processing (which may explain the visual and phonological reading problems), attention deficit, poor sequencing, impaired short-term memory and timing skills, and left-right confusion.[5] It has been suggested that deficits in timing and visual information processing while reading are secondary to a disorder in the magnocellular pathway,[6] whereas other cerebral deficits originate from a congenital disorder in the cerebellum, which is the head ganglion of the magnocellular system.[7]
Various aspects of visual function in dyslexic persons have been investigated to validate the magnocellular deficit theory; however, the results of visual evoked potential (VEP) testing in dyslexic participants have been inconsistent.[1,8,9,10,11,12,13,14,15,16] Researchers modified specific stimulus parameters in order to achieve dominant activation of either parvocellular or magnocellular subsystems. The outcomes of these studies are conflicting, as several studies[1,8,11,12,13,14,15,16] found differences between dyslexics and controls, while others[9,10] did not.
The present study applied different pattern VEP (PVEP) parameters to examine 36 dyslexic children and 36 age- and sex-matched controls. A previous study[17] did not find any significant difference in PVEP components between dyslexic and control groups. In that study, two check sizes of 15 and 60 min of arc, with temporal frequencies of 1.5 Hz for transient and 6 Hz for steady-state methods, and contrast of 100% were employed. Therefore, in the current study, PVEP parameters (constant temporal frequency, 1.5 Hz, but at 25 and 100% contrast) were changed to selectively activate the magnocellular system for potential deficits.
METHODS
The Ethics Committee of Mashhad University of Medical Sciences approved the study protocol. The procedure was explained to all participants and their parents, and informed consent was obtained before initiating the examination. This study recruited 72 children, including 36 dyslexic (21 girls and 15 boys, aged 8 to 12 years, mean: 8.72 ± 1.44) and 36 normal participants (19 girls and 17 boys, aged 8 to 12 years, mean: 9.16 ± 1.23). None of the children was diagnosed with ophthalmic, neurologic, emotional, or behavioral disorders, or unusual educational circumstances that could result in poor reading and spelling[18,19] or low intelligence quotient scores.[20,21] A psychiatrist and a speech therapist examined all participants by using the National Anger Management Association (NAMA), a reading and dyslexia test. A standard Snellen distance vision chart demonstrated visual acuity of 6/6 or better in each eye in all cases. Participants underwent refraction to ensure precise optical correction, and none of the participants had strabismus. Ophthalmoscopic and biomicroscopic examination demonstrated no pathological abnormality. The VEP recording equipment included a Roland RETI (model ISCEV60, Roland Consult, Brandenburg an der Havel, Germany) signal-averaging system with 2-8 channels and an amplifier in order to store and summate the waves.[22] The amplifier band-pass filters were set at 1-50 Hz with a sensitivity of 100 μv. The mean screen luminance was 100 cd/m2 at contrasts of 100% and 25% in full-field display. The mean luminance in the test room was kept at 80 cd/m2, and recording conditions were in accordance with International Society of Clinical Electrophysiology of Vision standards. According to the international method of PVEP electrode placement (10-20 method), the examiner placed the active electrode one inch above the inion (Oz). The center of the forehead was considered as a reference, with a ground electrode on the vertex (Cz). At a viewing distance of 1 m, two check sizes of 15 and 60 min of arc were used. The small 15 min of arc stimulus promotes a response largely from the central section of the visual field (macular area).[23] It was found that most of the responses recorded from the scalp at Oz were apparently foveal responses, because of cortical magnification. In each recording, 200 sweeps were averaged, and monocular PVEP was recorded. The pattern reversal variation rate was 1.5 Hz (3 reversals/s). The inter-electrode impedance was maintained below 5 Ω in each recording.
For statistical analysis, SPSS software version 16 (SPSS Corporation, Chicago, Il, USA) was used. Pearson's or Spearman's correlation tests were used according to normal or abnormal distribution of data, respectively.
RESULTS
Figures 1 and 2 represent a typical set of results obtained from participants in each of the dyslexic and normal children at two different contrasts of 100% and 25%. The PVEP waves commonly consisted of a negative trough (N1) of around 75 ms, followed by a positive peak (P1) of around 100 ms, and then a negative trough (N2) of around 135 ms.
Figure 1.

Waveforms 1 and 2 are the VEP recordings obtained at contrast of 100% in normal and dyslexic children for 60 min arc check sizes. Waveforms 3 and 4 are the VEP recordings obtained at contrast of 25% in normal and dyslexic children for 60 min arc check sizes.
Figure 2.
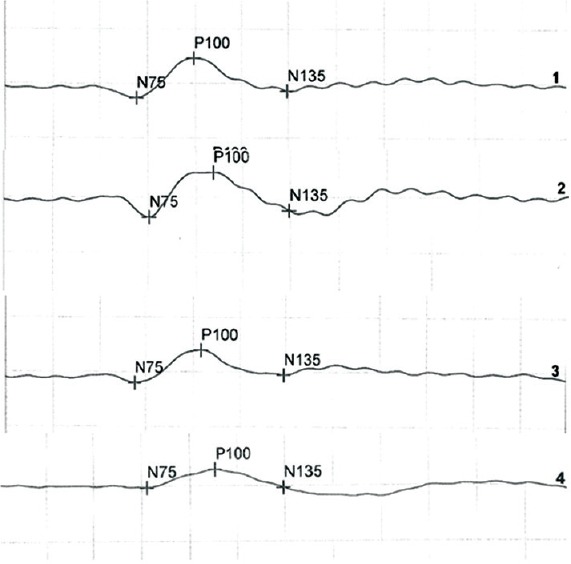
Waveforms 1 and 2 are the VEP recordings obtained at contrast of 100% in normal and dyslexic children for 15 min arc check sizes. Waveforms 3 and 4 are the VEP recordings obtained at contrast of 25% in normal and dyslexic children for 15 min arc check sizes.
In Figures 1 and 2, waveforms (1) and (2) are the PVEP recordings obtained at contrast of 100% in non-dyslexic and dyslexic children for 15 and 60 min of arc check sizes. Waveforms (3) and (4) are the PVEP recordings obtained at contrast of 25% in normal and dyslexic children for 15 and 60 min of arc check sizes.
Table 1 presents the mean (±SD) P100 latency and amplitude of N75P100 and P100N135 for two check sizes of 15 and 60 min of arc at contrast of 100% in dyslexic and normal children. In comparing the level of variability, P100 latency and amplitude of N75P100 and P100N135 showed no significant differences between the two groups (P ≥ 0.05).
Table 1.
Mean and (±SD) of P100 latency and amplitude of N75P100 and P100N135 for check sizes of 15 and 60 min of arc at contrast of 100% for the right eye in dyslexic and normal children
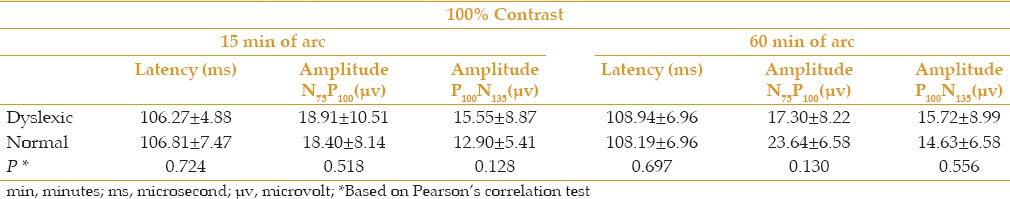
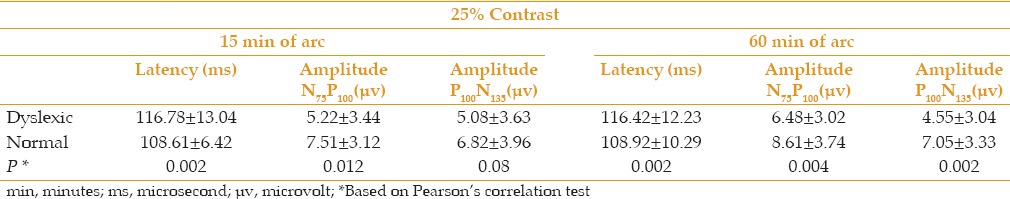
Table 2 presents the mean (±SD) P100 latency and amplitude of N75P100 and P100N135 for 2 check sizes of 15 and 60 min of arc at contrast of 25% in dyslexic and normal children. In comparing the level of variability, P100 latency and amplitude of N75P100 and P100N135 showed significant differences between the two groups (P < 0.05), except for P100N135 in a target size of 15 min of arc (P = 0.08).
Table 2.
Mean (±SD) of P100 latency and amplitude of N75P100 and P100N135 for check sizes of 15 and 60 min of arc at contrast of 25% in dyslexic and normal children
DISCUSSION
The current study confirmed the findings of the previous study that investigated PVEP in dyslexic children and demonstrated no significance difference between dyslexic and normal children in high-contrast PVEP components.[17] There were also no significant differences between the two groups at 100% contrast, which is in accordance with the previous study. These results indicate that dyslexic and normal groups showed similar results for high-contrast components; therefore, the parvocellular subsystem is not involved in dyslexic children. Moreover, our results at 25% contrast revealed that under specific stimulation conditions, PVEP varies significantly between dyslexics and normal controls. As in the visual pathway, the magnocellular subsystem carries low-contrast visual information; therefore, it can be inferred that the magnocellular pathway is involved in dyslexic children. Several other studies also claimed that the magnocellular deficit was involved in dyslexic participants.[1,8,11,12,13,14,15,16]
Our findings for P100 amplitude and PVEP latency revealed significant differences between dyslexic and normal children for both check sizes. These results are consistent with previous findings. Romani et al[1] compared dyslexic and normal participants with PVEP. Reversal frequencies ranged between 2.1 and 8 HZ at constant contrast (50%), and target sizes were large (0.50 cpd) and small (2 cpd). They did not find any significant differences at 2 cpd, but at 0.50 cpd and reversal frequency of 8 HZ, amplitude and latency of N70 were decreased. As the presentation of large stimuli or high stimulation frequency are representative of magnocellular activity, these data support the theory of selective magnocellular dysfunction in dyslexics. Accordingly, these data are in agreement with our results regarding the of magnocellular deficit hypothesis in dyslexic children. In another study, Brannan et al[15] changed temporal frequency and luminance to verify any possible deficit in the magnocellular system in dyslexic children. Two groups of children were recruited for binocular and monocular single channel PVEP testing utilizing a sinusoidal checkerboard pattern with spatial frequency of 14 min of arc at three different temporal frequencies (8, 4, and 1 Hz), and an 8-Hz flicker fusion stimulus. The stimuli were submitted under high and low luminance circumstances. Significant effects were found for monocular versus binocular viewing, high versus low luminance, and temporal frequencies. Equivalent analysis of latencies revealed no significant differences. The significant difference in PVEP amplitudes between the two reading groups provided an objective measure of a deficit in the magnocellular pathway. In our study, we did not change the luminance or temporal frequency, but both studies supported the same theory of magnocellular impairment. In a study by Kobayashi et al[14] reversed patterns of white and black sinusoidal gratings recorded at a low spatial frequency, high reversal frequency of 7.5 Hz, and low contrast were used to stimulate the magnocellular system. They found lower peak PVEP amplitude in dyslexic children. These results confirmed our findings. However, Kobayashi used different methods, sinusoidal gratings and parameters, and high reversal frequency (7.5 Hz).
Livingstone et al[12] applied variable stimulus contrast, ranging between 2% and 20%. In all conditions, parameters were lower in dyslexics, but the difference was significant for rapid stimulation only at 1% and 2% contrast. These results were convincingly stated by the authors, and supported the magnocellular deficit hypothesis of dyslexia. In contrast with our study, Livingstone used a small sample size (5 dyslexics and 7 normal). We did not change reversal frequency in our study, but the reduction in amplitude and latency along with changing contrast is consistent with Livingstone's findings and supportive of the magnocellular pathway deficit in dyslexic children.
Our findings are in contrast to those in some of the other studies. Brecel et al[8] investigated the simultaneous pattern electroretinogram (PERG) and PVEP in dyslexics and normal controls. They used various check sizes (24, 49, and 180 degrees) at different contrasts (5%, 42%, and 100%). The main finding of this study was prolonged P100 at 100% contrast and a 24-degree target. Therefore, they concluded that the magnocellular deficit exists in dyslexic cases. However, we observed significant differences at lower contrasts and no difference at higher contrasts, which could be attributed to the use of different methods (PERG and PVEP) and parameters (different sample size and temporal frequency) employed in the two studies.
Although our results support the magnocellular deficit hypothesis in dyslexic children, we should consider other factors such as color, movement, and temporal stimulus modulation to clearly demonstrate that the selective magnocellular pathway is affected. However, several previous studies claimed that the motion-onset PVEP is better for studying the magnocellular system in dyslexia.[24,25] Additionally, our findings suggest that for clinical evaluation of dyslexic participants, comparative tests such as visual acuity should be set at lower contrast thresholds for better investigation of visual problems.
In conclusion, in the present study, we found no difference in PVEP responses at high contrast in terms of amplitude and latency between dyslexic and normal children, which indicated an intact parvocellular system in the dyslexic group. However, dyslexic participants showed diminished responses in most PVEP components of latency and amplitude at low contrast, which might support a defect in the magnocellular pathway in dyslexia.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgement
This article was adapted from a dissertation by S. Shojaei to fulfill the requirements for an M.Sc. degree. The authors thank the department of Research and Technology of Mashhad University of Medical Sciences, Mashhad, Iran, for their cooperation and financial support.
REFERENCES
- 1.Romani A, Conte S, Callieco R, Bergamaschi R, Versino M, Lanzi G, et al. Visual evoked potential abnormalities in dyslexic children. Funct Neurol. 2001;16:219–229. [PubMed] [Google Scholar]
- 2.Shaywitz SE. Dyslexia. N Engl J Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Zou L, Zhang J, Mo S, Shao S, Zhong R, et al. Prevalence and associated risk factors of dyslexic children in a middle-sized city of China: A cross-sectional study. PloS One. 2013;8:e56688. doi: 10.1371/journal.pone.0056688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miles T, Miles E. Dyslexia: A hundred years on. Oxford: Oxford University Press; 1990. [Google Scholar]
- 5.Stein J. Dyslexia: The role of vision and visual attention. Curr Dev Disord Rep. 2014;1:267–280. doi: 10.1007/s40474-014-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demb JB, Boynton GM, Best M, Heeger DJ. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vision Res. 1998;38:1555–1559. doi: 10.1016/s0042-6989(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 7.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 8.Brecelj J, Strucl M, Raic V. Simultaneouspattern electroretinogram and visualevoked potential recordings in dyslexic children. Doc Ophthalmol. 1997;94:355–364. doi: 10.1007/BF02580860. [DOI] [PubMed] [Google Scholar]
- 9.Johannes S, Kussmaul CL, Muente TF, Mangun R. Developmental dyslexia: Passive visual stimulation provides no evidencefor a magnocellular processing defect. Neuropsychologia. 1996;34:1123–1127. doi: 10.1016/0028-3932(96)00026-7. [DOI] [PubMed] [Google Scholar]
- 10.Victor JD, Conte MM, Burton L, Nass R. Visual evoked potentials in dyslexics andnormals: Failure to find a difference intransient or steady-state responses. Vis Neurosci. 1993;10:939–946. doi: 10.1017/s0952523800006155. [DOI] [PubMed] [Google Scholar]
- 11.Kubova Z, Kuba M, Peregrin J, Novakova V. Visual evoked potential evidence formagnocellular system deficit in dyslexia. Physiol Res. 1996;45:87–89. [PubMed] [Google Scholar]
- 12.Livingstone MS, Rosen GD, Drislane FW, Galaburda A. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May JG, Lovegrove WJ, Martin F, Nelson P. Pattern-elicited visual evoked potentials in good and poor readers. Clinical Vision Sciences. 1991;6:131–136. [Google Scholar]
- 14.Kobayashi T, Inagaki M, Yamazaki H, Kita Y, Kaga M, Oka A. Relationship between magnocellular function and reading skills in children: A study using visual evoked potentials. No To Hattatsu. 2014;46:424–428. [PubMed] [Google Scholar]
- 15.Brannan JR, Solan HA, Ficarra AP, Ong E. Effect of luminance in visual evoked potential amplitudes in normal and disabled readers. Optom Vis Sci. 1998;75:279–283. doi: 10.1097/00006324-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Kuba M, Szanyi J, Gayer D, Kremlacek J, Kubova Z. Electrophysiological testing of dyslexia. Acta Medica (Hradec Kralové) 2001;44:131–134. [PubMed] [Google Scholar]
- 17.Heravian J, Sobhani-Rad D, Lari S, Khoshsima MJ, Azimi A, Ostadimoghaddam H, et al. Pattern visual evoked potentials in dyslexic versus normal children. J Ophthalmic Vis Res. 2015;10:274–278. doi: 10.4103/2008-322X.170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- 19.Hutzler F, Kronbichler M, Jacobs AM, Wimmer H. Perhaps correlation but not causal: No effect of dyslexic readers’ magnocellular system on their eye movements during reading. Neuropsychologia. 2006;44:637–648. doi: 10.1016/j.neuropsychologia.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield- Gabrieli S, et al. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol Sci. 2011;22:1442–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingeesson SG. Stability of IQ measures in teenagers and young adults with developmental dyslexia. Dyslexia. 2006;12:81–95. doi: 10.1002/dys.306. [DOI] [PubMed] [Google Scholar]
- 22.Heravian J, Daneshvar R, Dashti F, Azimi A, Ostadi Moghadam H, Yekta AA, et al. simultaneously pattern evoked potential and pattern and electroretinogram in strabismic and anisometropic amblyopia. Iran Red Crescent Med J. 2011;13:21–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Heravian Shandiz J, Nourian A, Bahrhosseini M, Ostadi Moghaddam H, Yekta AA, Sharifzadeh L, et al. Contrast sensitivity versus Visual Evoked Potentials in Multiple Sclorosis. J Ophthalmic Vis Res. 2010;5:175–181. [PMC free article] [PubMed] [Google Scholar]
- 24.Kuba M, Szanyi J, Gayer D, Kremlácek J, Kubová Z. Electrophysiological testing of dyslexia. Acta Medica (Hradec Kralove) 2001;44:131–134. [PubMed] [Google Scholar]
- 25.Schulte-Körne G, Bartling J, Deimel W, Remschmidt H. Motion-onset VEPs in dyslexia. Evidence for visual perceptual deficit. Neuroreport. 2004;15:1075–1078. doi: 10.1097/00001756-200404290-00029. [DOI] [PubMed] [Google Scholar]


