Abstract
Purpose:
We aimed to assess whether the transcription factor PAX6 affects transcription of FMNL2. PAX6 is a transcription factor with significant roles in development of the eye and eye-related functions. FMNL2 encodes a member of the formin family of proteins and has roles in polymerization of actin and features of the cytoskeleton. The state of the cytoskeleton affects the flow of aqueous humor, disruption of which is a cornerstone of glaucoma pathology.
Methods:
Initially, bioinformatics were used extensively to identify FMNL2 as an appropriate candidate gene for possible targeting by PAX6. Subsequently, direct targeting of the promoter of FMNL2 by PAX6 was tested using the dual luciferase assay. The experiment was performed by cloning a promoter region of FMNL2 that contains PAX6 binding sitesupstream of a firefly luciferase gene and comparison of expression of luciferase in the presence and absence of PAX6 expression vectors in the HEK293T cell line. The effect of PAX6 on endogenous expression of FMNL2 in primary trabecular meshwork (TM) cells was assessed by real-time polymerase chain reaction.
Results:
Dual luciferase assays in HEK293T cells clearly demonstrated that PAX6 directly affects the FMNL2 promoter to increase expression of downstream sequences. However, overexpression of PAX6 in TM cells caused mild but statistically significant downregulation of endogenous FMNL2 as assessed by real-time polymerase chain reaction.
Conclusion:
It is concluded that PAX6 can indeed directly affect transcription of FMNL2. However, regulation of FMNL2 expression in TM cells is complicated and not limited to the direct effects of PAX6.
Keywords: FMNL2, FOXC1, Glaucoma, MEIS2, PAX6, Trabecular Meshwork
INTRODUCTION
Glaucoma comprises a group of neurodegenerative diseases accompanied by progressive loss of retinal ganglion cells, degeneration of the optic nerve, and characteristic visual field defects.[1,2] Glaucoma is the leading cause of irreversible blindness worldwide, and is expected to affect nearly 80 million people by 2020.[3] It is a complex disorder, the etiology of which is poorly understood. Elevated intraocular pressure is a major risk factor for glaucoma and is caused by impaired drainage of aqueous humor through the trabecular meshwork (TM) into Schlemm's canal and the venous system in the anterior chamber of the eye.[4] Primary open angle glaucoma (POAG) is the most prevalent type of glaucoma in Western populations. Five genes that cause POAG (MYOC, OPTN, WDR36, NTF4, and TBK1) have been identified, but mutations in these genes cause disease in less than 10% of cases with POAG.[5,6] The combined effect of several genes and gene-environment interactions is expected to be important in the etiology of POAG in patients without mutations in the above-mentioned genes.[7] Genome-wide association studies and genetic studies of traits relevant to glaucoma, such as central cornea thickness, are useful for identifying genes that contribute to glaucoma in a non-Mendelian fashion.[8,9,10] High throughput gene expression analysis by deep RNA sequencing or microarray protocols may also be used for identification of genes within gene networks with relevance to glaucoma. The contribution of each gene and even each network may be nominal, but their cumulative effects may culminate in glaucomatous disease. We have performed gene expression analysis with the aim of improving our understanding of the molecular components in the etiology of glaucoma.
FOXC1 (forkhead box c1) is a transcription factor that has a crucial role in differentiation of neural crest-derived ocular tissues.[11] Additionally, mutations in FOXC1 can cause Axenfeld-Rieger syndrome, a disorder characterized by anterior eye segment defects and systemic anomalies.[12,13] Approximately 50% of patients with Axenfeld-Rieger syndrome develop glaucoma, and patients with FOXC1 mutations are even more likely to develop glaucoma.[14] Because of the relevance of FOXC1 to eye development and glaucoma, we attempted to identify genes with transcript levels that are affected by FOXC1 by performing whole genome microarray gene expression analysis in primary human trabecular cell lines that had undergone FOXC1 knockdown.[15] We identified 849 genes with mRNA levels that were affected by FOXC1 knockdown.[15] It is expected that some of these genes have FOXC1 binding elements in their promoter regions and are direct targets of FOXC1, while others are indirectly affected by expression of FOXC1. MEIS2 is a homeobox transcription factor (homeobox protein Meis2) that is known to have multiple functions in the development and ocular processes of the vertebrate eye.[16] The gene that encodes this protein is among the genes shown to be directly affected by FOXC1 knockdown.[15] It is well known that MEIS2 controls the expression of PAX6 (paired box 6), another transcription factor that is considered to be a master regulator of eye development.[17,18,19] Heterozygous and homozygous mutations in the PAX6 gene in mice cause, respectively, the small eye (sey) phenotype and total absence of eye development.[20,21] In humans, mutations in PAX6 cause Peters anomaly and aniridia, both of which are anterior segment dysgenesis disorders.[22] It was thus considered that expression of some of the genes in the microarray analysis that were indirectly affected by FOXC1 was changed because of the effect of FOXC1 on MEIS2 and the subsequent effect of MEIS2 on PAX6 expression. In the present study, we attempted to expand the genetic network relevant to functions of the TM that include FOXC1 by identification of a potentially glaucoma-relevant gene that is indirectly affected by FOXC1 and directly affected by PAX6. The target gene studied was FMNL2, which encodesformin-like protein 2. Direct targeting of FMNL2 by PAX6 was demonstrated. The significance of the results is discussed.
METHODS
This research was performed in accordance with the tenets of the Declaration of Helsinki. Eye globes used for isolation of TM for use in preparation of TM cell cultures were obtained from the Central Eye Bank of Iran.
Bioinformatics Protocols
DECODE (http://www.sabiosciences.com/chipqpcrsearch.php?app=TFBS) was used to identify genes with PAX6 binding sites in their promoter regions. DECODE combines text mining applications and data from the UCSC Genome Browser to compile lists of human genes with predicted binding sites for over 200 human transcription factors, including PAX6. Genes with PAX6 binding sites that were also included among 849 genes affected by FOXC1 levels in TM cells were selected. The genes affected by FOXC1 had been identified earlier by knockdown studies and microarray experiments in our laboratory.[15] Subsequently, genes with functions specifically relevant to the TM or to glaucoma were identified among the FOXC1-affected genes with putative PAX6 binding sites by use of three in silico bioinformatics tools, KEGG (Kyoto Encyclopedia of Genes and Genomes; www.genome.jp/kegg/), GeneCards (www.genecards.org/), and DisGeNET (www.disgenet.org/). KEGG and GeneCards provide information on the physical and functional properties of biological entities and interactions between these entities. DisGeNET integrates available data on gene-disease associations from several public data sources and the literature.[23] We then confirmed the presence of PAX6 binding sites in the significantly reduced number of genes by use of TRANSFAC (http://www.gene-regulation.com/pub/databases.html) and POSSUM (http://zlab.bu.edu/~mfrith/possum/) software, which is available in the public domain. TRANSFAC reported a matrix that reflected PAX6 binding sites based on experimental data. This matrix along with promoter sequences (obtained from NCBI; http://www.ncbi.nml.gov/) spanning -3000 with respect to A nucleotide of AUG translational initiation codon were submitted to POSSUM. Based on the inputs, POSSUM identified PAX6 binding sites in the promoter region. Having confirmed the presence of binding sites, the literature was reviewed and a single gene was selected for the purpose of performing empirical studies.
Empirical Verification of Targeting of FMNL2 by PAX6
Direct targeting of the promoter of FMNL2 by PAX6 was tested by the dual luciferase assay. The experiment was performed by cloning a promoter region of FMNL2 upstream of a firefly luciferase gene and comparison of expression of the luciferase in the presence and absence of PAX6 expression vectors in the HEK293T cell line. Initially, a 1912 base pair promoter fragment of FMNL2 that contained the predicted PAX6 binding site was amplified by polymerase chain reaction (PCR; Figure 1). The primer pairs used for its amplification created XhoI and ECORV restriction enzyme recognition sites at the 5′ and 3′ ends, respectively [Table 1]. The fragment was cloned upstream of the firefly luciferase reporter gene in the pGL4.14 vector (Promega Corporation, Madison, WI, USA) to create FMNL2 prom-pGL4.14. The cloned fragment was sequenced for verification of accuracy. Cultured cells were co-transfected with FMNL2 prom-pGL4.14 recombinant plasmid, pRL-TK (Promega Corporation), and empty pCMV-SPORT6 vector (Invitrogen, Carlsbad, CA, USA) or pCMV-SPORT6-PAX6 (Dharmacon, Lafayette, CO, USA). PRL-TK contains the renilla luciferase gene adjacent to the thymidine kinase promoter, and pCMV-SPORT6-PAX6 expresses PAX6 under the constitutively expressed cytomegalovirus promoter. Transfections were performed in 24-well plates. Additional control transfections included recombinant FMNL2 prom-pGL4.14 + pRL-TK, non-recombinant pGL4.14 + pRL-TK + pCMV-SPORT6-PAX6, and mock transfections without vector. All transfections were done in triplicate in HEK293T cells (National Institute of Genetic Engineering, Tehran, Iran). Transfection reactions were performed using 500 ng of plasmid DNAs, Lipofectamine LTX reagent (Invitrogen) and 2 × 105 cells. Forty-eight hours after transfection, firefly and renilla luciferase activity levels were measured using dual luciferase assays (Promega Corporation) according to the manufacturer's instructions.
Figure 1.

PAX6 binding site within FMNL2 promoter fragment. The fragment of FMNL2 amplified and cloned upstream of the luciferase gene in the pGL4.14 vector to create FMNL2prom-pGL4.14 is indicated by the bold line. Nucleotide positions are with respect to A of AUG translation initiation site. The bar indicates the position of the PAX6 binding site in this fragment.
Table 1.
Primer sequences

Endogenous regulation of FMNL2 expression by PAX6 was tested in two human primary TM cell lines. The TM cell lines were developed from donors without a history of eye disease and aged 50 years (female; TM1) and 12 years (male; TM2) at the time of death as described previously.[24] The cells were transfected with pCMV-SPORT6 control vector or pCMV-SPORT6-PAX6 that overexpressed PAX6. Transfections were performed as described for HEK293T cells. Total RNA was isolated 48 h after the transfections, and cDNAs were synthesized by standard procedures. Real-time PCR for PAX6 and FMNL2 was performed on a Corbett 65H0 instrument (Corbett Research, Sidney, Australia) using the QuantiFast SYBR Green PCR Kit (Qiagen, Germantown, MD, USA). GAPDH was used as the control gene. The experiments were performed in triplicate. The primers used in the real-time PCR experiments are presented in [Table 1]. The statistical analysis was performed using the Relative Expression Software Tool (REST).[25]
RESULTS
DECODE identified 6013 genes with PAX6 binding sites, and 225 of these were among the genes that had been observed to be affected by FOXC1 knockdown in the microarray experiments.[15] These were candidate genes for which transcription may be directly affected by binding of PAX6 protein to their promoters. Based on information derived from KEGG and GeneCards, we identified that at least 11 genes were associated with TM-specific and/or glaucoma-associated functions [Table 2]. Based on the PAX6 binding sequences predicted by TRANSFAC, POSSUM confirmed that the promoter regions of all 11 genes described above that were affected by FOXC1 knockdown and that also had TM/glaucoma-relevant functions contained at least one PAX6 binding site. For reasons described in the Discussion section, FMNL2 was selected for the purpose of performing empirical studies. There was one PAX6 binding site (5′-CATTTGTCTGCTCCAGGTGCT-3′) in the promoter region of this gene. The 1912 base pair promoter fragment of FMNL2 that was cloned into the pGL4.14 vector for performance of dual luciferase assays contained this binding site [Figure 1].
Table 2.
Genes with PAX6 binding sites in promoter Regions, affected by FOXC1 knock down, and with TM/Glaucoma related functions*

The results of the dual luciferase assay demonstrated that the presence of pCMV-SPORT6-PAX6 caused increased expression of firefly luciferase when the enzyme's encoding sequence was placed under the regulation of promoter fragments of FMNL2 in HEK293T cells [P ≤ 0.01; Figure 2]. PAX6 also affected the levels of FMNL2 endogenous transcripts in TM cells as assessed by real-time experiments, but the effect was to decrease the level of the transcripts [Figure 3]. The effect was small but statistically significant (P < 0.05). Decreased FMNL2 transcript levels were observed in two independent TM cell cultures, adding support to the validity of the observation.
Figure 2.
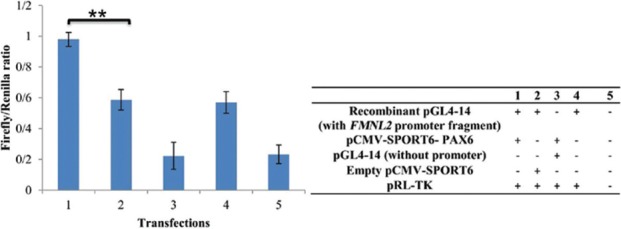
Dual luciferase assays in HEK293T cells in the presence of various combinations of vectors. Experiments were performed under 5 conditions (conditions 1–5) that are described in the panel. Standard deviations based on three replicate transfection experiments are shown. **P ≤ 0.01 for comparisons of firefly/renilla ratios of conditions 1 and 2.
Figure 3.
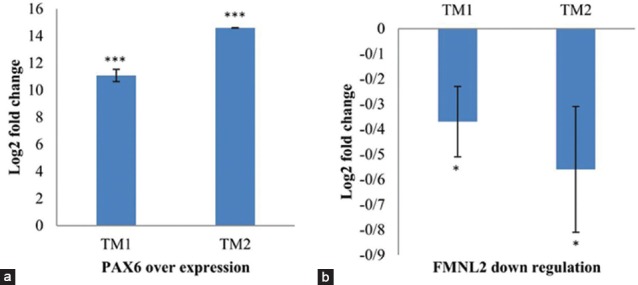
Effect of PAX6 overexpression on endogenous expression of FMNL2 in two human primary trabecular meshwork cell cultures as assessed by real-time polymerase chain reaction. (a) Fold increase of PAX6 expression in each of the trabecular meshwork cells in the presence of pCMV-SPORT6-PAX6 as compared with the empty vector is shown. (b) Decreased expression of FMNL2 in each of the trabecular meshwork cells in the presence of pCMV-SPORT6-PAX6 as compared with the empty vector is shown. Standard deviations based on three replicate transfection experiments are shown. *P < 0.05; ***P < 0.001.
DISCUSSION
The most important finding of this study was that PAX6 affects FMNL2 gene expression in TM cells. The effect of PAX6 on endogenous FMNL2 expression and the overall regulation of FMNL2 transcription in TM cells are clearly complicated. The results of our earlier FOXC1 knockdown microarray analysis and information provided by the literature were consistent with a regulatory pathway in which expression of FOXC1 causes increased expression of MEIS2 that in turn causes increased expression of PAX6 and eventually causes increased expression of FMNL2. The results of the luciferase assays are consistent with this because they show that PAX6 can act directly on the FMNL2 promoter sequence to increase expression of downstream coding sequences. The fact that overexpression of PAX6 in TM cells did not result in increased levels of FMNL2 mRNAs suggests that PAX6 additionally affects other regulatory molecules that directly or indirectly affect mRNA levels of FMNL2. It is possible that analysis of FMNL2 expression at time intervals shorter than 48 hours after transfection would have been informative in this regard. It can also be considered that the effect of FOXC1 on mRNA levels of FMNL2 evidenced by the microarray experiments may reflect the effects of multiple pathways, only one of which would be the proposed MEIS2/PAX6 pathway. The contribution of PAX6 to expression of FMNL2 in TM cells may be better clarified by experiments in which PAX6 is knocked down.
The protein encoded by FMNL2 is a member of the evolutionarily conserved formin family of proteins. These proteins are regulators of the cytoskeleton.[26] FMNL2 has actin binding domains that affect polymerization of actin and formation of actin filaments.[27] Among the 11 genes described above that were identified to have TM/glaucoma-related functions, FMNL2 was selected for empirical studies because of its role in formation of actin filaments and organization of the cytoskeleton. Actin filaments in the cytoskeleton are responsible for the contractile properties of the TM and for modulation of resistance to aqueous humor outflow in this tissue, so are very relevant in the pathogenesis of glaucoma.[28,29,30]
Furthermore, FMNL2 is a downstream effector of Rho GTPases such as Cdc42 and Rac1.[31,32] Members of the Rho family of proteins are involved in modulation of TM contraction and outflow of aqueous humor.[33,34,35] It has been shown that FMNL2 is present at cell-cell junctions and has roles in cell-cell adhesion and epithelial integrity; this role is likely related to its effects on actin dynamics.[26,32,33,34,35] Cell junction functions may be affected as part of the pathology of glaucoma because of differences in levels of proteins involved in maintenance of intracellular adhesions in the aqueous humor of glaucomatous eyes and that in normal eyes.[36,37] In short, functions attributed to FMNL2 partially overlap with TM/glaucoma-relevant functions. The evidence for existence of a PAX6-FMNL2 component in a regulatory pathway that includes FOXC1, MEIS2, PAX6, and FMNL2 has been strengthened in this study. Future research will expand this pathway, and query the possible involvement of its various components in the etiology of glaucoma.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta N, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 2.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15–19. doi: 10.1136/bjophthalmol-2013-304326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace DM, Murphy-Ullrich JE, Downs JC, O’Brien CJ. The role of matricellular proteins in glaucoma. Matrix Biology. 2014;37:174–182. doi: 10.1016/j.matbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Fingert JH. Primary open-angle glaucoma genes. Eye (Lond) 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou T, Sigss OM, Landers J, Mills R, Leo P, Ellis J, et al. Contribution of mutations in known mendelian glaucoma genes to advanced early-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2017;58:1537–1544. doi: 10.1167/iovs.16-21049. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Amero K, Kondkar AA, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci. 2015;16:28886–28911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loomis SJ, Kang JH, Weinreb RN, Yaspan BL, Bailey JN, Gaasterland D, et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology. 2014;121:508–516. doi: 10.1016/j.ophtha.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabuchi F, Sakurada Y, Kashiwagi A, Yamagata Z, Lijima H, Tsukahara S. Association between SRBD1 and ELOVL5 Gene Polymorphisms and Primary Open-Angle Glaucoma. Investig Ophthalmol Vis Sci. 2011;52:4626–4629. doi: 10.1167/iovs.11-7382. [DOI] [PubMed] [Google Scholar]
- 10.Scheetz TE, Fingert JH, Wang K, Alward WL, Russell ER, Stone EM, et al. A genome-wide association study for primary open angle glaucoma and macular degeneration reveals novel Loci. PloS One. 2013;8:e58657. doi: 10.1371/journal.pone.0058657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann OJ, Sowden JC, Carlsson P, Jordan T, Bhattacharya SS. Fox's in development and disease. Trends Genet. 2003;19:339–44. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 12.Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi: 10.1016/s0002-9394(00)00525-0. [DOI] [PubMed] [Google Scholar]
- 13.Strungaru MH, Dinu I, Walter MA. Genotype-Phenotype Correlations in Axenfeld-Rieger Malformation and Glaucoma Patients with FOXC1 and PITX2 Mutations. Invest Ophthalmol Vis Sci. 2007;48:228–237. doi: 10.1167/iovs.06-0472. [DOI] [PubMed] [Google Scholar]
- 14.Tumer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009;17:1527–1539. doi: 10.1038/ejhg.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paylakhi SH, Yazdani S, Fan JB, April C, Amin S, Suri F, et al. FOXC1 in human trabecular meshwork cells is involved in regulatory pathway that includes miR-204, MEIS2, and ITGbeta1. Exp Eye Res. 2013;111:112–121. doi: 10.1016/j.exer.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Conte I, Carrella S, Avellino R, Karali M, Bovolenta P, Banfi S, et al. miR-204 is required for lens and retinal development via Meis2 targeting. Proc Natl Acad Sci U S A. 2010;107:15491–15496. doi: 10.1073/pnas.0914785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Friedman A, Heaney S, Purcell P, Maas RL. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 2002;16:2097–2107. doi: 10.1101/gad.1007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baulmann DC, Ohlmann A, Flugel-Koch C, Goswami S, Cvekl A, Tamm ER. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev. 2002;118:3–17. doi: 10.1016/s0925-4773(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Shan X, Gregory-Evans CY. A mouse model of aniridia reveals the in vivo downstream targets of Pax6 driving iris and ciliary body development in the eye. Biochim Biophys Acta. 2016;1863:60–67. doi: 10.1016/j.bbadis.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Hogan BL, Hirst EM, Horsburgh G, Hetherington CM. Small eye (Sey): A mouse model for the genetic analysis of craniofacial abnormalities. Development. 1998;103:115–119. doi: 10.1242/dev.103.Supplement.115. [DOI] [PubMed] [Google Scholar]
- 21.Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): A homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- 22.Ito YA, Walter MA. Genomics and anterior segment dysgenesis: A review. Clin Exp Ophthalmol. 2014;42:13–24. doi: 10.1111/ceo.12152. [DOI] [PubMed] [Google Scholar]
- 23.Pinero J, Bravo A, Deu-Pons J, Baron M, Sanz F, Furlong LI, et al. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford) 2015 doi: 10.1093/database/bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–350. [PubMed] [Google Scholar]
- 25.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grikscheit K, Grosse R. Formins at the Junction. Trends Biochem Sci. 2016;41:148–159. doi: 10.1016/j.tibs.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Schonichen A, Geyer M. Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian B, B’Ann TG, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88:713–717. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- 31.Block J, Kuhn S, Winterhoff M, Kage F, Rohn JL, Geyer M, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grikscheit K, Frank T, Wang Y, Grosse R. Junctional actin assembly is mediated by Formin-like 2 downstream of Rac1. J Cell Biol. 2015;209:367–376. doi: 10.1083/jcb.201412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honjo M, Inatani M, Kido N, Sawamura T, Yue BY, Honda Y, et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001;119:1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 34.Honjo M, Tanihara H, Inatani M, Kido N, Swamura T, Yue BY, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 35.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–63. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izzotti A, Longobardi M, Cartiglia C, Sacca SC. Proteome alterations in primary open angle glaucoma aqueous humor. J Proteome Res. 2010;9:4831–4838. doi: 10.1021/pr1005372. [DOI] [PubMed] [Google Scholar]
- 37.Sacca SC, Pulliero A, Izzotti A. The dysfunction of the trabecular meshwork during glaucoma course. J Cell Physiol. 2015;230:510–525. doi: 10.1002/jcp.24826. [DOI] [PubMed] [Google Scholar]


