Abstract
Purpose:
To report a case of bilateral acute angle closure glaucoma after one dose of oseltamivir 75 mg.
Case Report:
A 37-year-old man with a history of influenza developed high intraocular pressure and uniformly shallow anterior chamber in both eyes, 5 hours after the first dose of oseltamivir 75 mg. The condition was managed successfully with topical cycloplegic and systemic/topical antiglaucoma medications.
Conclusion:
Since a presumed idiosyncratic reaction developed right after the first dose of the medication, it challenges the common concept of adaptive immune system involvement in this type of reaction in medication-related ciliochoroidal effusion.
Keywords: Ciliochoroidal Effusion, Idiosyncratic Reaction, Oseltamivir
INTRODUCTION
Oseltamivir is a commonly used antiviral drug for the prophylaxis and treatment of Influenza. Herein we present a case that developed bilateral acute angle closure glaucoma following one dose of oseltamivir. Patient had not received the medication before so it was impossible for him to have developed sensitization to medication. This clinical presentation challenges the common understanding of mechanism of idiosyncratic reaction in bilateral angle closure glaucoma.
CASE REPORT
A 37-year-old Caucasian man, with a 2-day history of influenza, presented to the emergency room complaining of bilateral ocular pain and blurred vision of 6 hours’ duration. Because of the viral infection, he had been prescribed oseltamivir (Tamiflu, F. Hoffmann-La Roche Ltd, Basel, Switzerland) 75 mg twice daily and no other medication. The patient reported the onset of symptoms 5 hours after the first dose of the above-mentioned medication.
On ophthalmic examination, visual acuity was 20/400 in both eyes. Refractive error of the right and left eyes were -6.5 sphere, and -7.00-1.00×123° respectively, which improved visual acuity to 20/80 and 20/160 respectively; his presenting glasses were -1.5 diopters/OU. Slit lamp examination revealed corneal stromal and epithelial edema with uniformly shallow anterior chamber (AC) and mid-dilated pupils, in both eyes [Figure 1]. Intraocular pressure (IOP) was 50 and 47 mmHg in the right and left eyes, respectively. On gonioscopy, the anterior chamber drainage angles were closed, with complete apposition of the iris root to the trabecular meshwork. Fundus examination revealed normal appearing optic nerve heads with no cupping, and there were chorioretinal folds in the macula of both eyes, but the retina was otherwise normal.
Figure 1.
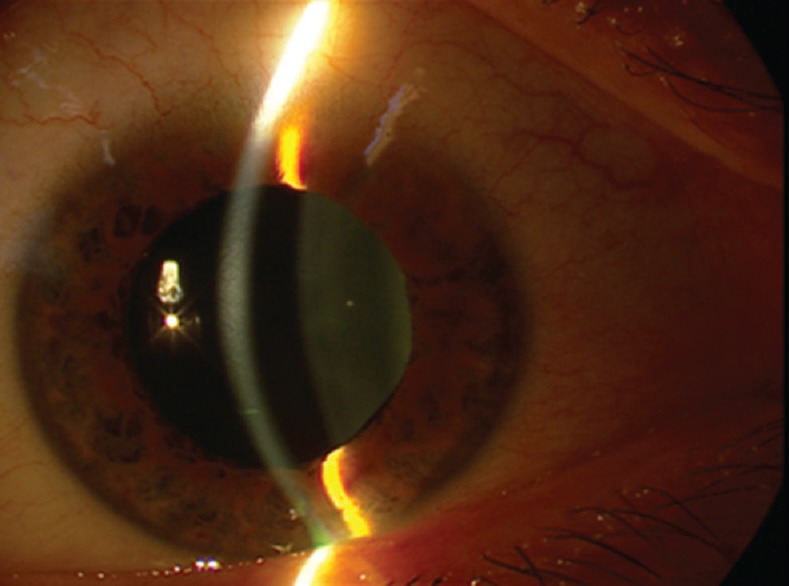
Uniformly shallow anterior chamber and mid-dilated pupil.
Based on the patient's history and ophthalmic examination, including uniformly shallow AC and dramatic myopic shift, a diagnosis of bilateral acute angle closure glaucoma (AACG) due to ciliochoroidal effusion secondary to medication was entertained, which was confirmed by 360-degree ciliochoroidal effusion and anteriorly displaced ciliary bodies on ultrasound biomicroscopy [Figure 2].
Figure 2.
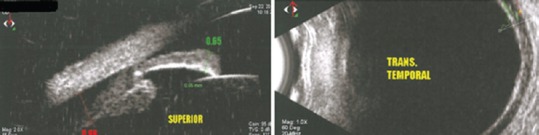
Ciliochoroidal effusion on ultrabiomicroscopic examination.
The patient was instructed to discontinue oseltamivir; cycloplegic and systemic/topical antiglaucoma medications were prescribed. IOP responded quickly to the above-mentioned medications, which were tapered over the next few days. Fifteen days after initial presentation, vision returned to 20/20 with his previous -1.5 diopters correction in both eyes. The anterior chambers were deep with open angles on gonioscopy and IOPs were 13 mmHg in both eyes [Figure 3].
Figure 3.
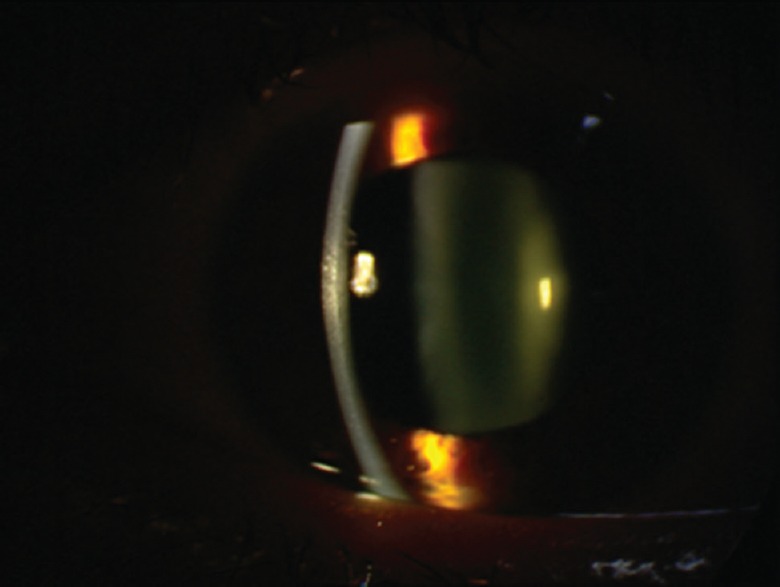
Deep anterior chamber and well-controlled IOP, five days after treatment.
DISCUSSION
Bilateral AACG and myopic shift have been reported to be associated with several types of medications, the prototype of which is topiramate, a sulfa-based anticonvulsant compound.[1]
Oseltamivir is an oral antiviral medication used for management of influenza A and B infections.[2]
It is a prodrug that once metabolized to its active form, oseltamivir carboxylate, inhibits the preserved active site of the neuraminidase enzymes on all influenza viruses and reduces viral replication. Since its introduction into the market in the year 2000, the patient reported herein is the second case of AACG following its administration. In an earlier report, Lee et al described a 27-year-old woman who developed acute angle closure attack, myopic shift, and ciliochoroidal effusion 4 days after treatment with oseltamivir 75 mg twice daily.[3]
Several mechanisms have been proposed for drug-induced ciliochoroidal effusion, but the most plausible mechanism seems to be idiosyncratic drug reaction (IDR).[4] These are rare and unpredictable adverse reactions that do not occur in most people at any given dose. These reactions are more frequently seen with anticancer, anticonvulsant, antipsychotic, antidepressant, and non-steroidal anti-inflammatory medications.
Several indicators suggest the role of the adaptive immune system in IDRs, such as the presence of fever, anaphylaxis, and skin rash; the delay observed between starting a drug and the onset of an IDR; and the decreased time to onset of the reaction on re-challenge.[4] Since drug molecules are too small to be immunogenic, they attach to specific proteins in the body and stimulate the immune system, a mechanism that forms the basis of the hapten theory. History, presentation, and the course of most reported cases of topiramate-induced AACG can be attributed to an adaptive immune system reaction. In contrast, our case developed AACG shortly after the first dose of the medication and had not received this medication before, which cannot be explained by the adaptive immune system mechanism (hapten theory).
We believe this behavior can be explained with a different and relatively new mechanism: the danger theory.[5] According to this hypothesis, covalent binding of drug metabolites and proteins is not sufficient to stimulate the immune system and cause IDRs. In these cases, simultaneous activation of the immune system by “danger signals” released from damaged or stressed cells are required to boost the reaction. These signals activate local antigen presenting cells, which up-regulate co-stimulatory molecules needed to activate the immune system. We believe in our case, the innate immune system played a critical role in causing cell damage and the observed clinical manifestation; in other words, the innate immune system had already been activated and “alerted” by the viral infection and acted through co-stimulatory molecules leading to IDRs; in the absence of such stress, tolerance would have resulted.
Oseltamivir has been administered for almost 15 years and this is only the second report of its implication in ciliochoroidal effusion and AACG, stressing the role of individual susceptibility to IDR reactions and also suggesting that, in contrast to topiramate-induced AACG, the incidence of acute angle closure with ciliochoroidal effusion due to oseltamivir administration is very rare.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Postel EA, Assalian A, Epstein DL. Drug-induced transient myopia and angle-closure glaucoma associated with supraciliary choroidal effusion. Am J Ophthalmol. 1996;122:110–112. doi: 10.1016/s0002-9394(14)71972-5. [DOI] [PubMed] [Google Scholar]
- 2.Davies BE. Pharmacokinetics of oseltamivir: An oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(Suppl 2):ii5–ii10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JW, Lee JE, Choi HY, Lee JS. Oseltamivir (Tamiflu)-induced bilateral acute angle closure glaucoma and transient myopia. Indian J Ophthalmol. 2014;62:1165–1167. doi: 10.4103/0301-4738.109531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieder MJ. Mechanisms of unpredictable adverse drug reactions. Drug Saf. 1994;11:196–212. doi: 10.2165/00002018-199411030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]


