Abstract
Oncological management of skeletal metastases has changed dramatically in the last few decades. A significant number of patients survive for many years with their metastases.
Surgeons are more active and the technical repertoire is broader, from plates to intramedullary devices to (tumour) endoprostheses.
The philosophy of treatment should be different in the case of a trauma-related fracture and a pathological fracture. A proper algorithm for establishing a diagnosis and evaluation of prognostic factors helps in planning the surgical intervention.
The aim of palliative surgery is usually to eliminate pain and to allow the patient to regain his/her mobility as well as to improve the quality of life through minimally invasive techniques using life-long durable devices.
In a selected group of patients with an oncologically controlled primary tumour site and a solitary bone metastasis with positive prognostic factors, which meet the criteria for radical excision (approximately 10% to 15% of the cases), a promising three to five years of survival may be achieved, especially in cases of metastases from breast and kidney cancer.
Spinal metastases require meticulous evaluation because decisions on treatment mostly depend on the tumour type, segmental stability, the patient’s symptoms and general state of health.
Advanced radiotherapy combined with minimally invasive surgical techniques (minimally invasive stabilisation and separation surgery) provides durable local control with a low complication rate in a number of patients.
Cite this article: EFORT Open Rev 2017;2:372-381.
Keywords: bone metastases, prognostic factors, diagnostic algorithm, surgery, long bones, spine
Introduction
Cancer is the second most frequent cause of death. According to the data of the Scandinavian Skeletal Metastasis Registry,1 the incidence of cancer has increased by 18% during the last decade, but due to improved treatment, cancer mortality rates have remained nearly constant (+2%).
The third most important filter for cancer metastases after the lungs and the liver is the skeletal system. There has been a change in paradigm in the treatment of metastatic disease of the bone. In the past, mostly palliative treatments, radiotherapy and pain relief were favoured. Nowadays, modern diagnostic tools (PET-CT, whole body MRI, etc) are included in the follow-up protocols allowing for early detection of bony metastases. Besides improved chemotherapy and radiotherapy, new targeted therapy such as bisphosphonates and denosumab (antigen against RANKL) reduce skeletal-related events (SREs). A broad spectrum of surgical options is available for reconstruction of defects. Many of the osteosytheses may be performed by minimally invasive techniques. All these factors have resulted in a significantly longer survival for metastatic patients, even with multiple metastases allowing for an increase in SREs, e.g. fracture, spinal cord compression, hypercalcaemia, etc, which lead to a substantial burden for both the patients and financially for society.
This review discusses the diagnostic work-up, prognostic factors, survival and surgical management of the metastatic lesions affecting the spine and long bones of the extremities.
Metastatic diseases of the long bones
Diagnostic algorithm
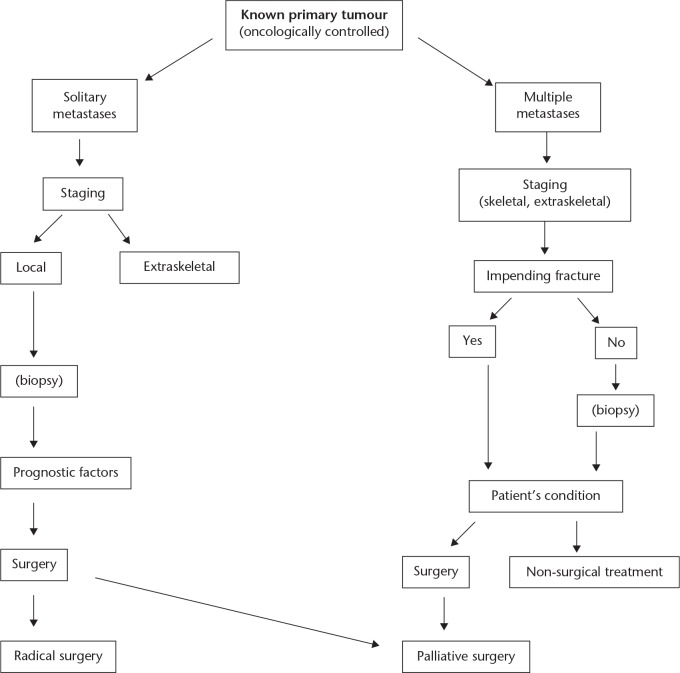
Many authors2-7 recommend rather similar diagnostic work-up protocols for potential metastatic diseases. These flow charts help orthopaedic surgeons and oncologists to establish the correct diagnosis and plan the most favourable treatment accordingly. Our own protocol is presented in Figure 1.
Fig. 1.
Diagnostic algorithm at impending fracture (known primary tumour).
In cases of an aggressive bone lesion, in a patient with a history of oncologically controlled cancer, the basic question is the number of the metastases. Plain radiographs, isotope bone scan, MRI,4,5 or in certain cases PET-CT, should be used for staging. When we are sure that the lesion is solitary, we should evaluate patient- and tumour-related prognostic factors (see ‘Prognostic factors and survival’ below). Biopsy is only necessary when other diseases, i.e. tumour-like lesions, raise a differential diagnostic problem or if ablative surgery is planned. Evaluating laboratory findings, the patient’s condition and the local stage of the tumour, we can decide whether to perform radical or palliative surgery.
In cases with multiple bone metastases, surgery is always palliative. The question here is the local status of the involved bone regardless of the patient’s condition. The fracture risk can be determined according to Mirel’s score system.8 Biopsy (fine needle aspiration biopsy or core) is only necessary when there are two different types of primary cancers present in the clinical history of the patient. In cases with impending fracture, a careful evaluation of all circumstances is necessary, e.g. the type of the primary tumour, the chemo-sensitivity, the radiosensitivity, the metastatic load and the general status of the patient, available and effective drugs, etc. Not all impending fractures, especially not those of non-weight-bearing bones of the upper extremity, must be operated on as prophylaxis.9
When the site and type of the primary cancer is unknown, routine laboratory tests should be complemented by tumour markers: prostate specific antigen; thyroid function tests; and myeloma screen. The orthopaedic surgeon should keep in mind that breast, thyroid, lung, kidney and prostate cancer present as the primary type of more than 70% of all bone metastases.1 Whole-body MRI, CT of the chest, abdomen and pelvis, bone scintigraphy and PET-CT are helpful in locating the primary tumour. PET-CT has high sensitivity but low specificity. It is, however, useful in cancers with a high risk of bone metastases, e.g. breast cancer, kidneys, etc.6 Biopsy is often required for histological examination, which is also helpful in finding the primary tumour site, but should be the last procedure in the diagnostic work-up because it weakens the affected bone and can lead to a pathological fracture.
Approximately 20% of patients with bone metastases are referred with an actual pathological fracture to the trauma unit. It is important to recognise the pathological nature of the fracture. The treatment policy should differ in pathological and trauma-related fractures.2 While in trauma cases a rapid osteosynthesis is required for union of the fracture, treatment of the pathological fracture is usually less urgent. It is more important to assess the patient’s general condition, the primary tumour site and prognostic factors than to determine the precise surgical procedure, e.g. plating with cement augmentation/prosthesis, etc. In this way, irredeemable failures, such as intramedullary nailing spanning the entire length of the bone in cases of primary bone sarcoma, can be avoided. Even though patients with a pathological fracture of the extremity are very difficult, examination by endoscopy for gastrointestinal malignancies, by MRI or bone scan, clinical history and lab tests, and CT for the chest, abdomen and pelvis, as well as radiographs, are helpful to screen the five most frequent primary tumours.
Prognostic factors and survival
Potential prognostic factors have been extensively investigated by several authors.1,2,10-14
Willeumier et al,2 Katagiri et al,11 Bollen et al,14 Toyoda et al15 and others also presented a predictive model for patient survival based on scoring systems. Forsberg et al10,16 applied the Bayesian Belief Network for estimating the survival in patients with skeletal metastases and found this model accurate and robust.
It is, however, very difficult to estimate the actual patient’s survival in terms of months or years with bone metastatic disease.17 Different factors play a role if the long bones or spine are affected. Most authors17-19 still regard the type of primary tumour as one of the most predictive prognostic survival factors. Based on large population studies, Cox regression analysis indicated the following further important factors: the Karnofsky/Eastern Co-operative Oncology Group performance status; the presence of visceral metastases; the haemaglobin count; and number of metastases. Pathological fracture as a prognostic factor is a controversial issue. Some authors did not find a statistically proven relevant correlation in this regard,1,20-22 while others,19,23 including Kirkinis et al18 in thier meta-analysis of the literature, found a worse outcome if pathological fracture occurred. In the Kaplan-Meier univariate analysis, the axial location, the time interval between the diagnosis of cancer and that of the metastases (more than three years), additional conservative treatments and type of surgery were also significant factors in cases with solitary metastases.20,24,25 In another study,19 age, gender, method of surgical fixation and location in different long bones did not play a significant role.
The highest ratio of solitary metastases, 38.8%, was observed in the Scandinavian Skeletal Metastasis Registry (n = 1195) among patients with renal cell cancer.1 Logically, one would assume that the best five-year survival is also in this group following complete surgical resection, but this is not the case. This may be due to the fact that in most cases there is an acceleration of the process in a few years and additional conservative treatments (radiotherapy, interferon, etc) have little influence on overall survival. Lin et al26 found that the clear-cell histological sub-type was associated with better survival. In this study, the Fuhrman grade27 of the initial tumour was not a predictive factor. Our data supported this20 and we could, however, demonstrate a good correlation between the grade of the metastatic lesion and survival. This may be explained by our finding that when comparing the Fuhrman grade of the initial tumour, progression in the grade of metastases was found in 40%, downgrading in 30% and the same grade in 30%. This explains that the final outcome for the patient depends more on the new cell population of the metastases than on the grade of the original renal cell cancer. Toyoda et al15 and Szendrői et al20 found a significant difference between synchron and metachronbone metastases and survival. When the interval between the diagnosis of the first tumour and metastasis was 24 months or more, the ratio of survival was higher.
According to data from a large patient population (n = 7064), about 22% of women with breast cancer will develop metastases in the bone.28 Risk factors for developing a bone metastasis were tumour size (> 5 cm), higher tumour grade, sub-type of the tumour (lobular carcinoma) and number of positive lymph nodes. For those patients with metastases in the bone, factors influencing survival were the extent of the disease (multiple locations, visceral metastases) and duration of the symptoms in the series from Dürr et al.21 Other authors,29 in a multivariate Cox regression model, found oestrogen receptor positivity, solitary bone metastasis and biphosphonate treatment positive prognostic factors. Weiss et al23 reported age over 60 years and a haemaglobin level less than 110 g/L as negative factors.
Bone metastases from lung cancer and melanoma have the worst outcomes in terms of survival (Table 1).1,15,19,20,21,23,26,29-37 The former mostly presents in multiple forms and the mean survival is under one year.32 Sugiura et al32 found the histological sub-type adenocarcinoma, solitary lesions, lack of metastases to the appendicular bone, good patient general health status and the use of chemotherapy and targeted therapy (epithelial growth factor receptor inhibitor) as factors influenced survival in a positive way.
Table 1.
Survival rates (%) of cancer patients with operated skeletal metastases
| Reference | Patients | Primary tumour | Median survival (mths) | 6 mths (%) | 1 yr (%) | 2 yrs (%) | 3 yrs (%) | 5 yrs (%) | 10 yrs (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lin et al 200726 | 295 | Renal cell cc | 47 | 30 | 11 | ||||
| Hwang et al 201430 | 135 | Renal cell cc | 72 | 45 | 28 | ||||
| Toyoda et al 200715 | 50 | Renal cell cc | 12 | 37 | |||||
| Szendrői et al 201020 | 64 | Renal cell cc | 58 | 39.5 | 30 | 19.2; solitary 35.5 | |||
| Dürr et al 200221 | 70 | Breast cc | 59 | 36 | 13; solitary 39 | 7; | |||
| Ahn et al 201329 | 110 | Breast cc | 55 | solitary 34.9 | |||||
| Weiss et al 201423 | 301 | Breast cc | 45 | 27 | 8 | ||||
| Oster et al 201331 | 621 | Breast cc | 66.3 | 32.8 | |||||
| Sugiura et al 200832 | 118 | Lung cc | 9.7 | 59.9 | 36 | 11 | |||
| Weiss and Wedin 201133 | 98 | Lung cc | 3 | 24 | 13 | 6 | |||
| Oster et al 201331 | 477 | Lung cc | 19 | 2.5 | |||||
| Ratasvuori et al 20131 | 1107 | All types of primary tumour | 58 | 41 | 2 | ||||
| Harvey et al 201234 | 158 | All types of primary tumour | 51 | 29 | |||||
| Mavrogenis et al 201235 | 110 | All types of primary tumour | 54 | 30 | 20 | 16 | |||
| Wedin et al 201236 | 208 | All types of primary tumour | 40 | 21 | 16 | ||||
| Hansen et al 200419 | 474 | All types of primary tumour | 39 | 26 | 18 | ||||
| Nakayama et al 201437 | 40 | Thyroid cc (differentiated) | 77 | 64 | 45 |
cc, cell carcinoma
Some data on recent studies of the survival rates of cancer patients with operated skeletal metastases are summarised in Table 1.1,15,19,20,21,23,26,29-37 In studies where all types of primary tumour were included, the one-year survival rates were in the range of 40% to 50%, with significantly lower rates than in cases of breast, kidney, prostate and differentiated thyroid cancer metastases but higher than in lung cancer metastases. The one-year survival rates in breast and kidney cancers (between 45% and 59%) are high and promising, but these decrease within the first five years to a range of 8% to 20%. The best five-year results were achieved when solitary metastases were operated on extensively.
Surgical treatment
Surgery is usually not the primary choice of treatment in bone metastases. In multiple bone metastases in particular, treatment starts with palliative chemo-, radio- or hormone therapy, isotopes or new targeted therapies according to the nature of the primary tumour. SREs can be effectively reduced by administration of bisphosphonates and denosumab.
The main goals of surgical treatment are to alleviate the pain, to prevent an imminent fracture, to perform an osteosynthesis in cases of a pathological fracture, to restore patient mobility and to improve the patient’s quality of life.
Intolerable pain and the presence of a pathological fracture are clear indications for surgery. It is, however, more contradictory in cases withan impending fracture. Many aspects, such as patient general health, the primary tumour and its histology and the effectiveness of other non-surgical treatments for this special kind of metastasis, should be evaluated before deciding to operate on an impending fracture. On the one hand, surgical intervention carries risks for the patient and also affects immune status. On the other hand, it is not certain that pathological fractures really occur during an effective non-surgical treatment or if the patient survives this event. This concern is also reflected in the statistical data: in the 1195 patients operated on for metastases, 74.2% had a pathological fracture and 18.3% had an impending fracture.1
The metastatic load is different in the different skeletal areas. According to the large statistics of the Skandinavian Skeletal Metastasis Registry,1 the femur is affected in 64%, the humerus in 21% and the pelvis in 9%. Less than 1% occurred in the region of the hands and feet. Three-quarters of the lesions appear in the proximal part of the femur, whereas in the humerus the diaphysis is the most frequently affected area.
Today, there are many different surgical tools for osteosynthesis and reconstruction of bony defects. The type of the primary (oncologically controlled or not) tumour, the patient’s general health status, other prognostic factors for expected survival and the local extent of the metastasis all play a role in planning the surgery. On rare occasions, e.g. solitary metastasis, a small lesion or when the tumour can be excised without a demanding procedure, the tumour should be excised completely to avoid further local complications. In most cases, however, an intralesional approach with minimally invasive technique is justified for an end-of-life solution to avoid re-operations for complications.2,4
More than two-thirds of the femoral metastases appear in the proximal epi-metaphysis. Most authors favour normal long-stem cemented endoprosthesis designs or modular tumour endoprostheses in these cases, which allow the patient immediate mobility and are associated with fewer complications than intramedullary nails or plates.34,38,39 In cases of acetabular involvement, the classification according to Harrington40 is useful. In this the treatment options are adapted to the severity and location of destruction.41,42 In the diaphysis of long bones, a plate, an intramedullary nail or a prosthesis may be implanted. All of these methods have their advantages and disadvantages.43,44 For short-term life expectancies, intramedullar nailing with locking screws introduced by minimally invasive technique and augmented by bone cement is optimal. The patient may load the extremity immediately; post-operative radiotherapy, if necessary, may be started early on. The incidence of fatigue fracture, however, increases with time.9,25,34,45 For the treatment of metastases located near the knee joint, intramedullary nails and angulated plates with screws augmented by bone cement are good options for patients with short-term life expectancies, whereas endoprosthesis should be used for patients with better prognosis.22 The relatively high price of these types of tumour endoprostheses should, however, be taken into consideration.
Good results are described by Weiss et al,46 with a cemented plate technique for fixation of proximal pathological fractures of the humerus, whereas Wedin et al36 recommended a hemi-prosthesis for more destructive proximal humeral lesions and interlocking intramedullar nailing for the treatment of pathological fractures of the diaphyseal segment.
The incidence of complications associated with surgery of metastatic lesions of the bone is rather high, with authors reporting a range of 9% to 22% for the humerus36,46,47 and 10% to 30% for the femur.1,4,22,24,39 These are mostly related to the poor quality of bone, failed implant selection, progression of the disease, the condition of the patient, but also dislocation of the prosthesis, loosening, periprosthetic infection and fracture of the implant.
Spinal metastases
Skeletal metastases most frequently occur in the vertebral column.48 The incidence of symptomatic spinal metastases is continuously growing with the increasing incidence of cancer and the associated survival.49 Due to the particular anatomy and biomechanics of the spine, the early diagnosis and adequate management of a spinal metastasis is crucial in the patient’s quality of life. The diagnostic evaluation of a suspicious spinal secondary lesion contains some recently developed steps which are major cornerstones of the therapeutic planning. The indication for surgery is a key issue and it is strongly related to the clinical appearance and the overall prognosis. In the last few years, new techniques have been introduced, such as minimally invasive and stereotactic body radiotherapy, to achieve long-term local control with reduced morbidity.
Diagnostic cornerstone: stability
In addition to the diagnostic process applicable for any skeletal metastases, the evaluation of a spinal metastasis has to be completed by the determination of the biomechanical stability of the spine. Instability is associated with consequent pathological fracture and neurological impairment which significantly worsen the patient’s quality of life as well as survival. Early recognition of unstable lesions is crucial in the treatment choice, but the evaluation of stability is challenging because both radiological and clinical findings have to be considered. Tumorous spinal instability can be defined as “the loss of spinal integrity as a result of a neoplastic process that is associated with movement-related pain, symptomatic or progressive deformity, and/or neural compromise under physiological loads”.50 The first evidence-based, comprehensive and easy-to-use system for the evaluation of the stability of the spinal metastases was published in 2010 by the Spine Oncology Study Group (SOSG).50 The Spinal Instability Neoplastic Score (SINS) is a scoring system based on six features of the metastasis (Table 2). The sum of these parameters gives the SINS score (0 to 18), where the higher score indicates the more instable lesion. The SINS was developed to help the primary healthcare provider to decide whether the patient has to be referred to a spine surgeon or not. Consultation of a spine surgeon is advised for lesions with a SINS score of 7 or more (potentially unstable and unstable lesions). The reliability and validity of SINS were tested in different, independent studies. The SOSG members published a near-perfect intra- and interobserver reliability for the total SINS score and high sensitivity (95.7%) and moderate specificity (79.5%) for the binary SINS score (stable versus (potentially) unstable lesions) comparing the binary score with consensus opinion (benchmark).51 The multidisciplinary study group of the AOSpine Knowledge Forum on Tumors (AOSpine KF) conducted two studies to determine the properties of the SINS among radiologists and radiation oncologists,52,53 where substantial intra- and interobserver reproducibility of the binary SINS score was found in both clinician groups. Three independent studies published further evidence on the clinical use of the SINS score.54-56 The SINS has been adopted in several guidelines and clinical studies so far.57,58 The SINS was also included in two clinical decision frameworks for patients with spinal metastasis. The Neurologic, Oncologic, Mechanical stability, Systemic disease criteria (NOMS)59 as well as the Location, Mechanical instability, Neurological status, Oncological history, Physical status framework (LMNOP)60 advise the use of the SINS for the assessment of the stability of the lesion. A number of studies have been published concerning the clinical prognostic value of the SINS in the last few years. A higher SINS score was significantly associated with the need for re-irradiation61 and with occurrence of spinal adverse events62 after radiation therapy of vertebral metastases. Survival after surgical management was found not to be associated with SINS score, but consequent vertebral compression fracture in cases with a higher SINS score significantly reduced quality of life.63-65
Table 2.
Spinal Neoplastic Instability Score (SINS)
| Score | |
|---|---|
|
Location
Junctional (occiput-C2, C7T2, T11-L1, L5-S1) Mobile spine (C3-C6, L2-L4) Semirigid (T3-T10) Rigid (S2-S5) |
3 2 1 0 |
|
Pain
Yes Occasional pain but not mechanical Pain-free lesion |
3 1 0 |
|
Bone lesion
Lytic Mixed Blastic |
2 1 0 |
|
Radiographic spinal alignment
Subluxation/translation present De novo deformity (kyphosis/scoliosis) Normal alignment |
4 2 0 |
|
Vertebral body collapse
>50% collapse <50% collapse No collapse with >50% body involved None of the above |
3 2 1 0 |
|
Posterolateral involvement of spinal elements
Bilateral Unilateral None of the above |
3 1 0 |
|
Total score
Stable Potentially unstable Unstable |
0-6 7-12* 13-18* |
SINS score of 7 or higher requires consultation with a spine surgeon
Prognostic factors and survival
A number of high quality studies have recently been published about the use of different prognosis systems in case of spinal metastases. Bollen et al66 compared six prognostic scoring systems on survival after the diagnosis of a spinal metastasis. They analysed the clinical data of 1379 patients. Overall median survival was 5.1 months with a high range (0.8 to 18.6) depending on the histology of the primary tumour. There was also a big difference in the expected survival among the three most common histological types. Since a long survival can be expected in the case of the most common (28%) breast cancer metastasis (median 18.6 months), the second most common type, lung cancer, showed a short survival (median 2.0 months), while the third most common histology (prostate) showed a medium-long survival (median 7.4 months). This large study underlies the most important oncological principle in survival estimation: ‘tissue is the issue’, i.e. the patient’s life expectancy is mostly determined by the histology of the primary tumour. Not surprisingly, this factor is the primary prognostic factor in all of the previously published prognostic systems for spinal metastasis. Bollen et al67 reported that consideration of the general health status of the patient and the presence of visceral metastases combined with the primary tumour profile has given a simple scoring system (Table 2); its performance in survival estimation is better compared with the formerly published systems.66 The Bollen-score forms four categories (A to D) where survival dramatically decreased (29.8, 16.5, 4.9 and 1.7 months median survival in categories A, B, C and D, respectively). The patient’s general health status characterised by the Karnofsky score is also a strong predictor for the quality of life in surgicallytreated patients.68 Choi et al68 clearly showed that patients with a low Karnofsky score (< 60) before the surgery could not benefit from the surgery regarding their quality of life independent of the pre-operative neurological status. Verlaan et al69 analysed the characteristics of the patients who survived less than three months or more than two years after the surgery for spinal metastases in a large cohort (n = 1266). They found that increased age and Karnofsky score were associated with short survival, while lower number of levels included in the spinal surgery and primary tumour type were related to long survival.
Surgical treatment
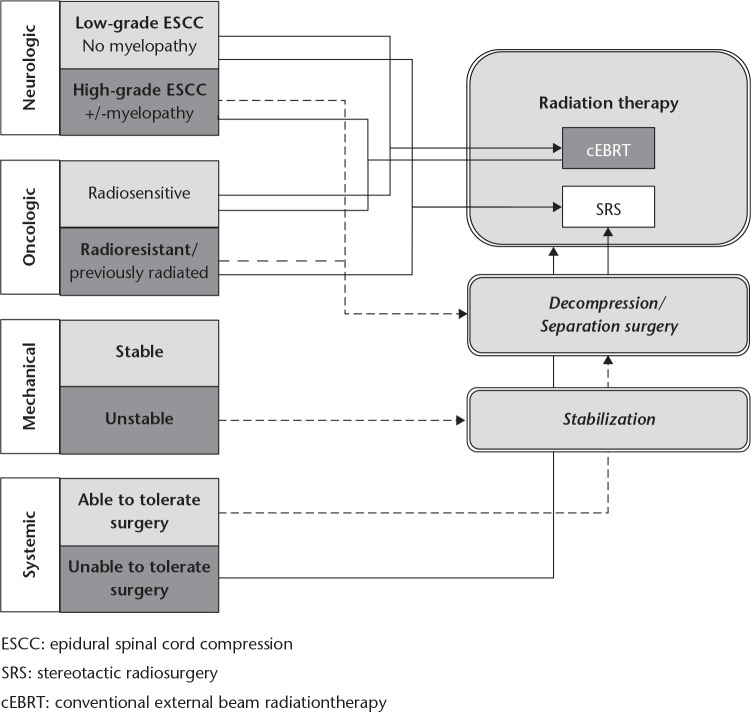
Radiation and surgical therapy are the two most effective options for the local control of spinal metastases. Both therapeutic modalities have been developed significantly over the past ten years, so the proper indication, treatment choice and timing can give significantly longer local control with fewer complications for the patients than before. A few algorithms for the indications and treatment choice have been recently published; nevertheless, none of them has been validated in a randomised prospective study. The multidisciplinary team of the Memorial Sloan-Kettering Cancer Center has developed and published the NOMS decision framework, which is a comprehensive combination of the previously described significant factors, resulting in a clinically applicable and clear system (Fig. 2).59 Parts of this decision-supporting framework have to be individually assessed for all patients diagnosed with spinal metastases, however, the access to the different treatment options show a huge diversity nationally and internationally. Neurologic assessment indicates the degree of the spinal cord/nerve root compromise and the associated neurological deficit. Degree of epidural spinal cord compression (ESCC) is the key element in treatment choice, but the timing and progression of the neurological symptoms specifies the indications for an emergency treatment. A strong recommendation as a result of a recent systematic review of the AOSpine KF has been published concerning the need and urgency of the surgical decompression. According to this study, a patient with neurologic deficit from a ESCC resulting in loss of ability to ambulate requires urgent surgical decompression if there is no oncological or medical contra-indication.70 Diagnosis should be expeditious and surgery should be prompt to improve the probability of neurological recovery.
Fig. 2.
The Neurological, Oncological, Mechanical stability, Systemic disease (NOMS) decision framework for the treatment of spinal metastases (reproduced with permission from Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors.Oncologist 2013;18:744-751).59
Oncological assessment mostly means the consideration of the possibility for radiotherapy. Radiosensitivity of the tumour, previous radiotherapy and availability for radiotherapy have to be taken into account during the decision process. Solid tumours show a wide range of radiosensitivity. Breast, prostate and ovarian carcinomas are usually sensitive to radiotherapy, while renal, thyroid, colon and non-small-cell lung carcinomas, sarcoma and melanoma exhibit less, total or partial radioresistency.
Stereotactic radiosurgery (SRS) has to be used in cases with radio-resistant histology to achieve durable local control.71,72 The SINS is advised to assess the mechanical stability. Unstable lesions require stabilisation surgery. Use of minimally invasive surgical techniques, such as percutaneous stabilisation, use of tubular retractors and mini-open approaches as well as percutaneous cement augmentation of pathological, stable but painful vertebral body fractures, are strongly advised to reduce the peri-operative complication rate.73 The last element of the NOMS framework is the systemic assessment which means the prediction of the patient’s ability to tolerate the proposed intervention. Comorbidities, general health status and tumour burden have to be assessed. The minimisation of the surgical intervention can make the surgery safer and feasible for the patients. Therefore, the minimally invasive separation of the tumour tissue from the dural sac (separation surgery) with post-operative SRS radiotherapy would be the optimal treatment choice in a high number of cases, providing safe and effective local control.73
Footnotes
ICMJE Conflict of interest statement:None declared.
Funding: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol 2013;22:132-138. [DOI] [PubMed] [Google Scholar]
- 2. Willeumier JJ, van der Linden YM, van de Sande MAJ, Dijkstra PDS. Treatment of pathological fractures of the long bones. EFORT Open Rev 2017;1:136-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biermann JS, Holt GE, Lewis VO, Schwartz HS, Yaszemski MJ. Metastatic bone disease: diagnosis, evaluation, and treatment. J Bone Joint Surg [Am] 2009;91-A:1518-1530. [PubMed] [Google Scholar]
- 4. Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg [Am] 2009;91-A:1503-1516. [DOI] [PubMed] [Google Scholar]
- 5. Ashford RU, Pendlebury S, Stalley PD. Management of metastatic disease of the appendicular skeleton. Curr Orthop 2006;20:299-315. [Google Scholar]
- 6. Shibata H, Kato S, Sekine I, et al. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open 2016;1:e000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruggieri P, Mavrogenis AF, Casadei R, et al. Protocol of surgical treatment of long bone pathological fractures. Injury 2010;41:1161-1167. [DOI] [PubMed] [Google Scholar]
- 8. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 1989;249:256-264. [PubMed] [Google Scholar]
- 9. Laitinen M, Ratasvuori M, Pakarinen T-K. The multi-modal approach to metastatic diseases. In: Bentley G, ed. European Intructional Lectures, vol. 12 Berlin, Heidelberg: Springer, 2012:35-44. [Google Scholar]
- 10. Forsberg JA, Eberhardt J, Boland PJ, Wedin R, Healey JH. Estimating survival in patients with operable skeletal metastases: an application of a bayesian belief network. PLoS One 2011;6:e19956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Westhoff PG, de Graeff A, Monninkhof EM, et al. An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys 2014;90:739-747. [DOI] [PubMed] [Google Scholar]
- 13. Janssen SJ, van der Heijden AS, van Dijke M, et al. 2015. Marshall Urist Young Investigator Award: prognostication in patients with long bone metastases: does a boosting algorithm improve survival estimates? Clin Orthop Relat Res 2015;473:3112-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bollen L, van der Linden YM, Pondaag W, et al. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1,043 patients. Neuro Oncol 2014;16:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toyoda Y, Shinohara N, Harabayashi T, et al. Survival and prognostic classification of patients with metastatic renal cell carcinoma of bone. Eur Urol 2007;52:163-168. [DOI] [PubMed] [Google Scholar]
- 16. Forsberg JA, Wedin R, Bauer HCF, et al. External validation of the Bayesian Estimated Tools for Survival (BETS) models in patients with surgically treated skeletal metastases. BMC Cancer 2012;12:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nathan SS, Healey JH, Mellano D, et al. Survival in patients operated on for pathologic fracture: implications for end-of-life orthopedic care. J Clin Oncol 2005;23:6072-6082. [DOI] [PubMed] [Google Scholar]
- 18. Kirkinis MN, Lyne CJ, Wilson MD, Choong PFM. Metastatic bone disease: A review of survival, prognostic factors and outcomes following surgical treatment of the appendicular skeleton. Eur J Surg Oncol 2016;42:1787-1797. [DOI] [PubMed] [Google Scholar]
- 19. Hansen BH, Keller J, Laitinen M, et al. The Scandinavian Sarcoma Group skeletal metastasis register. Survival after surgery for bone metastases in the pelvis and extermities. Acta Orthop Scand 2004;75:11-15 [DOI] [PubMed] [Google Scholar]
- 20. Szendrői A, Dinya E, Kardos M, et al. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol Oncol Res 2010;16:29-38. [DOI] [PubMed] [Google Scholar]
- 21. Dürr HR, Müller PE, Lenz T, et al. Surgical treatment of bone metastases in patients with breast cancer. Clin Orthop Relat Res 2002;396:191-196. [PubMed] [Google Scholar]
- 22. Henrichs M-P, Krebs J, Gosheger G, et al. Modular tumor endoprostheses in surgical palliation of long-bone metastases: a reduction in tumor burden and a durable reconstruction. World J Surg Oncol 2014;12:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiss RJ, Tullberg E, Forsberg JA, Bauer HC, Wedin R. Skeletal metastases in 301 breast cancer patients: patient survival and complications after surgery. Breast 2014;23:286-290. [DOI] [PubMed] [Google Scholar]
- 24. Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg [Br] 2002;84-B:521-529. [DOI] [PubMed] [Google Scholar]
- 25. Bauer HC. Controversies in the surgical management of skeletal metastases. J Bone Joint Surg [Br] 2005;87-B:608-617. [DOI] [PubMed] [Google Scholar]
- 26. Lin PP, Mirza AN, Lewis VO, et al. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg [Am] 2007;89-A:1794-1801. [DOI] [PubMed] [Google Scholar]
- 27. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655–663. [DOI] [PubMed] [Google Scholar]
- 28. Harries M, Taylor A, Holmberg L, et al. Incidence of bone metastases and survival after a diagnosis of bone metastases in breast cancer patients. Cancer Epidemiol 2014;38:427-434. [DOI] [PubMed] [Google Scholar]
- 29. Ahn SG, Lee HM, Cho SH, et al. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med J 2013;54:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hwang N, Nandra R, Grimer RJ, et al. Massive endoprosthetic replacement for bone metastases resulting from renal cell carcinoma: factors influencing patient survival. Eur J Surg Oncol 2014;40:429-434. [DOI] [PubMed] [Google Scholar]
- 31. Oster G, Lamerato I, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer 2013:21:3279-3286. [DOI] [PubMed] [Google Scholar]
- 32. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res 2008;466:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weiss RJ, Wedin R. Surgery for skeletal metastases in lung cancer. Acta Orthop 2011;82:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey N, Ahlmann ER, Allison DC, Wang L, Menendez LR. Endoprostheses last longer than intramedullary devices in proximal femur metastases. Clin Orthop Relat Res 2012;470:684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mavrogenis AF, Pala E, Romagnoli C, et al. Survival analysis of patients with femoral metastases. J Surg Oncol 2012;105:135-141. [DOI] [PubMed] [Google Scholar]
- 36. Wedin R, Hansen BH, Laitinen M, et al. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J Shoulder Elbow Surg 2012;21:1049-1055. [DOI] [PubMed] [Google Scholar]
- 37. Nakayama R, Horiuchi K, Susa M, et al. Clinical outcome after bone metastasis (BM) surgery in patients with differenciated thyroid carcinoma (DTC): a retrospective study of 40 cases. Jpn J Clin Oncol 2014;44:918-925. [DOI] [PubMed] [Google Scholar]
- 38. Liska F, Schmitz P, Harraser N, et al. Metastasen der Extremitäten. DerUnfallchirurg 2016:1-10. (In German: ) [Google Scholar]
- 39. Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur: endoprosthesis or reconstruction nail? J Bone Joint Surg [Br] 2005;87-B:1653-1657. [DOI] [PubMed] [Google Scholar]
- 40. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg [Am] 1981;63-A:653-664. [PubMed] [Google Scholar]
- 41. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer 1997;80:1614-1627. [DOI] [PubMed] [Google Scholar]
- 42. Tillman RM, Myers GJC, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg [Br] 2008;90-B:84-87. [DOI] [PubMed] [Google Scholar]
- 43. Sarahrudi K, Greitbauer M, Platzer P, et al. Surgical treatment of metastatic fractures of the femur: a retrospective analysis of 142 patients. J Trauma 2009;66:1158-1163. [DOI] [PubMed] [Google Scholar]
- 44. Alvi HM, Damron TA. Prophylactic stabilization for bone metastases, myeloma, or lymphoma: do we need to protect the entire bone? Clin Orthop Relat Res 2013;471:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller BJ, Soni EE, Gibbs CP, Scarborough MT. Intramedullary nails for long bone metastases: why do they fail? Orthopedics 2011;34:274. [DOI] [PubMed] [Google Scholar]
- 46. Weiss KR, Bhumbra R, Biau DJ, et al. Fixation of pathological humeral fractures by the cemented plate technique. J Bone Joint Surg [Br] 2011;93-B:1093-1097. [DOI] [PubMed] [Google Scholar]
- 47. Piccioli A, Maccauro G, Rossi B, et al. Surgical treatment of pathologic fractures of humerus. Injury 2010;41:1112-1116. [DOI] [PubMed] [Google Scholar]
- 48. Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus 2001;11:e10. [DOI] [PubMed] [Google Scholar]
- 49. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758-2765. [DOI] [PubMed] [Google Scholar]
- 50. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-E1229. [DOI] [PubMed] [Google Scholar]
- 51. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol 2011;29:3072-3077. [DOI] [PubMed] [Google Scholar]
- 52. Fisher CG, Versteeg AL, Schouten R, et al. Reliability of the spinal instability neoplastic scale among radiologists: an assessment of instability secondary to spinal metastases. AJR Am J Roentgenol 2014;203:869-874. [DOI] [PubMed] [Google Scholar]
- 53. Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol 2014;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campos M, Urrutia J, Zamora T, et al. The Spine Instability Neoplastic Score: an independent reliability and reproducibility analysis. Spine J 2014;14:1466-1469. [DOI] [PubMed] [Google Scholar]
- 55. Teixeira WGJ, de Mesquita Coutinho PR, Marchese LD, et al. Interobserver agreement for the spine instability neoplastic score varies according to the experience of the evaluator. Clinics (Sao Paulo) 2013;68:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arana E, Kovacs FM, Royuela A, et al. Spine Instability Neoplastic Score: agreement across different medical and surgical specialties. Spine J 2016;16:591-599. [DOI] [PubMed] [Google Scholar]
- 57. Lo SS, Ryu S, Chang EL, et al. ACR Appropriateness Criteria® Metastatic Epidural Spinal Cord Compression and Recurrent Spinal Metastasis. J Palliat Med 2015;18:573-584. [DOI] [PubMed] [Google Scholar]
- 58. Quinn RH, Randall RL, Benevenia J, Berven SH, Raskin KA. Contemporary management of metastatic bone disease: tips and tools of the trade for general practitioners. J Bone Joint Surg [Am] 2013;95:1887-1895. [DOI] [PubMed] [Google Scholar]
- 59. Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013;18:744-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ivanishvili Z, Fourney DR. Incorporating the Spine Instability Neoplastic Score into a treatment strategy for spinal metastasis: LMNOP. Global Spine J 2014;4:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huisman M, van der Velden JM, van Vulpen M, et al. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J 2014;14:2835-2840. [DOI] [PubMed] [Google Scholar]
- 62. Lam TC, Uno H, Krishnan M, et al. Adverse outcomes after palliative radiation therapy for uncomplicated spine metastases: role of spinal instability and single-fraction radiation therapy. Int J Radiat Oncol Biol Phys 2015;93:373-381. [DOI] [PubMed] [Google Scholar]
- 63. Lee SH, Tatsui CE, Ghia AJ, et al. Can the spinal instability neoplastic score prior to spinal radiosurgery predict compression fractures following stereotactic spinal radiosurgery for metastatic spinal tumor?: a post hoc analysis of prospective phase II single-institution trials. J Neurooncol 2016;126:509-517. [DOI] [PubMed] [Google Scholar]
- 64. Thibault I, Al-Omair A, Masucci GL, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine 2014;21:711-718. [DOI] [PubMed] [Google Scholar]
- 65. Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 2013;31:3426-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bollen L, Wibmer C, van der Linden YM, et al. Predictive value of six prognostic scoring systems for spinal bone metastases: an analysis based on 1379 patients. Spine (Phila Pa 1976) 2016;41:E155-E162. [DOI] [PubMed] [Google Scholar]
- 67. Bollen L, van der Linden YM, Pondaag W, et al. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1,043 patients. Neuro Oncol 2014;16:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Choi D, Fox Z, Albert T, et al. Prediction of quality of life and survival after surgery for symptomatic spinal metastases: a multicenter cohort study to determine suitability for surgical treatment. Neurosurgery 2015;77:698-708. [DOI] [PubMed] [Google Scholar]
- 69. Verlaan JJ, Choi D, Versteeg A, et al. Characteristics of patients who survived < 3 months or > 2 years after surgery for spinal metastases: can we avoid inappropriate patient selection? J Clin Oncol 2016;34:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976) 2016;41:S224-S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009;34:S78-S92. [DOI] [PubMed] [Google Scholar]
- 72. Chang JH, Shin JH, Yamada YJ, et al. Stereotactic body radiotherapy for spinal metastases: what are the risks and how do we minimize them? Spine (Phila Pa 1976) 2016;41:S238-S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zuckerman SL, Laufer I, Sahgal A, et al. When less is more: the indications for MIS techniques and separation surgery in metastatic spine disease. Spine (Phila Pa 1976) 2016;41:S246-S253. [DOI] [PMC free article] [PubMed] [Google Scholar]