Highlights
-
•
Overt hypothyroidism needs to be treated with levothyroxine.
-
•
Subclinical hypothyroidism treated with thyroxine has not been proven to improve maternal outcomes.
-
•
Overt hyperthyroidism usually needs to be treated with thioamides.
-
•
Subclinical hyperthyroidism does not require treatment.
-
•
Evidence linking TPO antibodies with miscarriages and preterm labour is unequivocal.
Keywords: Pregnancy, Hyperthyroidism, Hypothyroidism, Thyroiditis, Autoimmune thyroid disease, Thioamides, Iodine
Abstract
Thyroid dysfunction is the commonest endocrine disorder in pregnancy apart from diabetes. Thyroid hormones are essential for fetal brain development in the embryonic phase. Maternal thyroid dysfunction during pregnancy may have significant adverse maternal and fetal outcomes such as preterm delivery, preeclampsia, miscarriage and low birth weight. In this review we discuss the effect of thyroid disease on pregnancy and the current evidence on the management of different thyroid conditions in pregnancy and postpartum to improve fetal and neonatal outcomes, with special reference to existing guidelines on the topic which we dissect, critique and compare with each other.
Overt hypothyroidism and hyperthyroidism should be treated appropriately in pregnancy, aiming to maintain euthyroidism. Subclinical hypothyroidism is often pragmatically treated with levothyroxine, although it has not been definitively proven whether this alters maternal or fetal outcomes. Subclinical hyperthyroidism does not usually require treatment and the possibility of non-thyroidal illness or gestational thyrotoxicosis should be considered.
Autoimmune thyroid diseases tend to improve during pregnancy but commonly flare-up or emerge in the post-partum period. Accordingly, thyroid auto-antibodies tend to decrease with pregnancy progression.
Postpartum thyroiditis should be managed based on the clinical symptoms rather than abnormal biochemical results.
Introduction
Thyroid disease, after diabetes, is the commonest endocrine disorder during pregnancy. The background prevalence of spontaneous hypothyroidism is between 1% and 2% in iodine-replete communities; it is 10 times more common in women than in men [1]; subclinical hypothyroidism, defined as a raised serum thyroid stimulating hormone (TSH) levels in presence of normal thyroid hormone levels, affects about 8% of women. Similarly, the prevalence of hyperthyroidism in women is between 0.5% and 2%, and is 10 times more common than in men in iodine-replete communities [1]; subclinical hyperthyroidism, defined as low serum TSH in the presence of normal thyroid hormone levels and in the absence of hypothalamic and pituitary disease or non-thyroidal illness or medications that inhibit TSH secretion, affects about 3% of the population. This review will explore the interplay between thyroid disease and pregnancy and the evolving evidence on the management of the different thyroid conditions in pregnancy and the postpartum period, with special emphasis on existing guidelines on this field.
Methods
We undertook a focused review of the literature and discussions with colleagues. We carried out a search of the published literature in Medline, PubMed (www.pubmed.gov) and Google Scholar (www.scholar.google.com) with a broad range of combinations of the medical subject headings (MeSH) terms, ‘pregnancy’, ‘miscarriage’, ‘breastfeeding’, ‘thyroid diseases’, ‘hypothyroidism’, ‘thyrotoxicosis’, ‘hyperthyroidism’, ‘anti-thyroid drugs’, ‘carbimazole’, ‘methimazole’, ‘propylthiouracil’, ‘thyroiditis’, ‘post-partum thyroiditis’, ‘autoimmune thyroid disease’, ‘non-thyroidal illness’, ‘thyroid function tests’, ‘congenital malformations’ and ‘neurodevelopmental defects’. Inclusion criteria were ‘English language’ and articles retrieved from 1960 to December 2015. References of articles included were read to identify any further articles that were missed from the above database searches and personal archived references were also sought. Whenever available, we gave preference to meta-analyses, systematic reviews, randomised controlled trials (RCTs) and prospective epidemiological studies. As appropriate, we included retrospective and non-randomised studies, and case reports.
Thyroid physiology in pregnancy
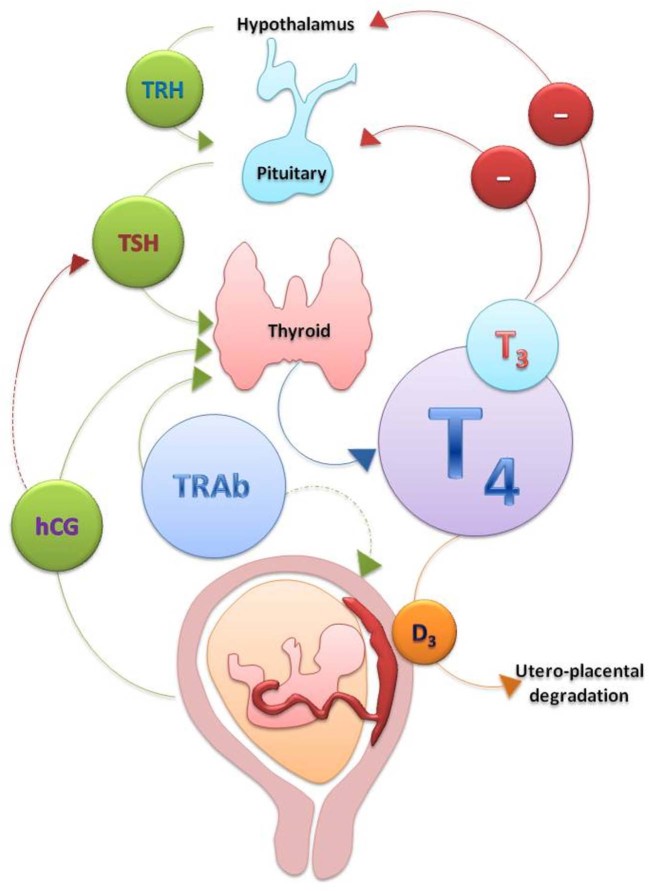
Approximately 94% of thyroid hormones are secreted by the thyroid gland as thyroxine or tetraiodothyronine (T4) and 6% as triiodothyronine (T3) (Fig. 1). T4 is catalytically converted to the more metabolically active T3 in peripheral tissues by deiodinases and a portion of peripherally-produced T3 returns to the circulation and it is because of this peripheral conversion that the plasma T4 to T3 ratio is approximately 4:1 [2], [3]. Both T4 and T3 are mostly bound to carrier proteins in the serum, chiefly thyroxine-binding globulin (TBG). However, it is the free hormones (free T4 (fT4) and free T3 (fT3)) that are available to be actively transported into cells and exert their effects.
Fig. 1.
Hypothalamic-pituitary-thyroid axis and pregnancy.
Changes in maternal thyroid function during pregnancy result from a combination of increased metabolic demands, increased serum TBG concentrations, stimulation of the TSH receptor by human chorionic gonadotropin (hCG) [4], an increased mother-to-foetus transfer of thyroxine and an increased intraplacental breakdown of T4 and T3 (resulting from the placental expression of deiodinase 3). Total T4 and T3 concentrations increase by 50% as a result of a 50% increase in circulating TBG levels by 6–8 weeks of gestation; their levels plateau at around 16 weeks of gestation [5]. Maternal TSH is usually within normal limits during pregnancy but it can be decreased in the first trimester due to the increased hCG levels and the cross-reactivity of this hormone on TSH receptors [6]; both are glycoprotein hormones with a common α subunit and a considerable homology between their β subunits. Therefore hCG has a weak thyroid stimulating activity [6]. hCG levels increase following fertilisation and peak at 10–12 weeks of gestation, leading to a rise in the total serum T4 and T3 concentrations and subsequently reduction of thyrotropin-releasing hormone (TRH) and TSH levels as a result of negative feedback. This hormonal interplay results in a biochemical picture of subclinical hyperthyroidism, which can be considered as a physiological finding. The decrease in hCG secretion later in pregnancy leads to reduction of serum fT4 and fT3 concentrations and finally the normalisation of TSH levels [4]. Thyroid hyperfunction and symptoms, if present, subside as hCG production falls, typically at 14–18 weeks of gestation. Ideally, the assay-specific TSH reference ranges for each trimester should be calculated based on the local population in iodine sufficient areas and pregnant women recruited for such calculations should be euthyroid and thyroid antibody negative. When this is not feasible, a reasonable alternative is to use the consensus ranges as per the various guidelines (Table 1) [7], [8], [9]. However, it is worth emphasizing that these guideline reference ranges are mainly drawn from Western populations; for example, the TSH in Chinese populations has been shown to be higher than these reference values [7].
Table 1.
TSH reference ranges in pregnancy.
| TSH reference ranges (mU/L) | |||
|---|---|---|---|
| First trimester | Second trimester | Third trimester | |
| American Endocrine Society | 0.1–2.5 | 0.2–3.0 | 0.3–3.0 |
| American Thyroid Association | |||
| European Thyroid Association | <2.5 | <3.0 | <3.5 |
Iodine and pregnancy
Iodine is an essential component of thyroid hormones and requirements increase during pregnancy. Iodine deficiency is associated with thyroid dysfunction and subsequently with impaired fetal development [10]. It is nowadays accepted that severe maternal iodine deficiency can have adverse implications for the mother, including hypothyroidism and goitre; for the foetus, including miscarriage and stillbirth; for the neonate, including neonatal mortality; and for the child, including impaired neurological development, faltering growth and cretinism [10], [11], [12]. Iodine supplementation is recommended as a treatment of maternal hypothyroidism in severely iodine deficient populations and there is good evidence that it improves clinical outcomes including cretinism and infant mortality rates [11], [13].
There have been concerns raised recently for the adequacy of iodine intake in pregnant and child-bearing age women in the UK and other developed countries. Some of these areas, thought before as iodine-replete, were found to be mildly-to-moderately iodine deficient [10], [14], [15], [16], [17], [18], [19]. Furthermore, new emerging evidence from the large longitudinal AVON study in the UK has shown that mild-to-moderate maternal iodine deficiency was associated with lower cognition in their offspring at the age of eight and nine years in a linear manner [15], confirming the results of a smaller study in Australia [20]. Such an association may account for the previously described relationship between higher (especially oily) fish intake in pregnancy and improved cognition in the offspring [21]; indeed fish intake seems beneficial for maternal outcomes, such as gestational hypertension [22], as well as reducing thyroid autoimmunity in the antenatal and post-partum period [23]. Although such a favourable effect has been attributed to the omega-3 fatty acid content of oily fish [23], it is possible that the high iodine content of oily fish is the cause for the beneficial effects on thyroid physiology and function [15]. Nevertheless, iodine supplementation in pregnant women from areas of mild to moderate iodine deficiency is still debatable. Although the evidence has been encouraging regarding supplementation in several studies, not all of the studies had a rigorous methodology [16], [24], [25]. These studies have suggested that iodine supplementation in mildly-to-moderately iodine deficient populations have some beneficial effects on maternal and newborn serum thyroglobulin and thyroid volume (i.e. a smaller increase), while data on thyroid function is inconsistent and hard-evidence on long-term effects such as pregnancy outcomes, childhood neurodevelopment and growth are missing [10], [25], [26]. Limited evidence has shown that earlier initiation of iodine supplements is related to better outcomes [25].
Similarly, no long-term studies have examined the effect of iodine supplementation during lactation on child development. Nevertheless, an RCT in a moderate-to-severe iodine deficiency mountainous area in Morocco determined that supplementation with one dose of 400 mg iodised oil to the mother rather than 100 mg directly to the nursing infant was more effective in terms of reducing the frequency of infant thyroid hypofunction [27].
On the other hand, excessive iodine supplementation should be avoided. There is evidence that over-replacement with iodine could increase the risk of subclinical hypothyroidism, isolated hypothyroxinaemia and auto-immune hypothyroidism [13], [25]. Furthermore, kelp supplements should be avoided due to their variable, and sometimes excessive, iodine content [14].
The various guidelines seem to converge on the need for oral iodine supplementation of 150 μg daily during pregnancy and lactation. It is important to note that these guidelines were based on urinary iodine concentration which is a population assessment of iodine status, but not so useful as a tool for the assessment of the iodine status of an individual [13].
Thyroid physiology in utero
Thyroid hormones are essential for fetal brain development in the early embryonic phase; therefore, thyroid dysfunction during pregnancy may have significant adverse maternal and fetal outcomes (Table 2). That is because the fetus relies on maternal thyroid hormones during the crucial period of brain development. The fetal thyroid gland starts producing both T4 and T3 from about 10 weeks of gestation [28]; however, until approximately 20 weeks of gestation, the fetal thyroid is not fully active and therefore the fetus is very dependent on the maternal thyroxine supply [29]. TBG concentrations reach adult levels at term. T4 and fT4 concentrations reach adult levels at approximately 36 weeks of gestation, but T3 and fT3 are always below adult concentrations [30].
Table 2.
Adverse fetal and maternal outcomes associated with maternal thyroid dysfunction.
| Overt hypothyroidism | Subclinical hypothyroidism | Isolated hypothyroxinaemia | Overt hyperthyroidism | |
|---|---|---|---|---|
| Fetal outcomes | Preterm delivery | Prematurity | Low birth weight | Preterm delivery |
| Low birth weight | Fetal and neonatal death | Neuropsychological impairment | Intrauterine growth restriction | |
| Miscarriage | Neuropsychological impairment† | Fetal thyrotoxicosis | ||
| Congenital malformations | ||||
| Fetal death | ||||
| Maternal outcomes | Anaemia | Gestational diabetes | Placental abruption | Pre-eclampsia |
| Placental abruption | Pre-eclampsia | Adverse metabolic phenotype‡ | Gestational hypertension | |
| Postpartum haemorrhage | Premature rupture of membranes | Cardiac failure | ||
| Gestational hypertension | Thyroid storm |
Inconclusive results.
An adverse metabolic phenotype, such as obesity, is more likely to be the cause of isolated hypothyroxinaemia, rather than the other way around.
Hypothyroidism
Primary maternal hypothyroidism is defined as the presence of an elevated TSH concentration during gestation in the absence of rare exceptions such as TSH-secreting pituitary tumour, thyroid hormone resistance and a few cases of central hypothyroidism with biologically inactive TSH [8].
Overt hypothyroidism
Overt hypothyroidism is defined as an elevated serum TSH level (above the trimester-specific range) with fT4 below the reference range; the American Thyroid Association (ATA) is the only society that incorporated into the definition a TSH level of 10 mU/L or greater, irrespective of the fT4 level [8]. It affects 0.3–0.5% of pregnancies. It is often pre-existent although it can sometimes develop during pregnancy. Its commonest cause is chronic autoimmune (Hashimoto’s) thyroiditis. It can also result from previous surgery or radioactive iodine treatment for hyperthyroidism, goitre or thyroid cancer.
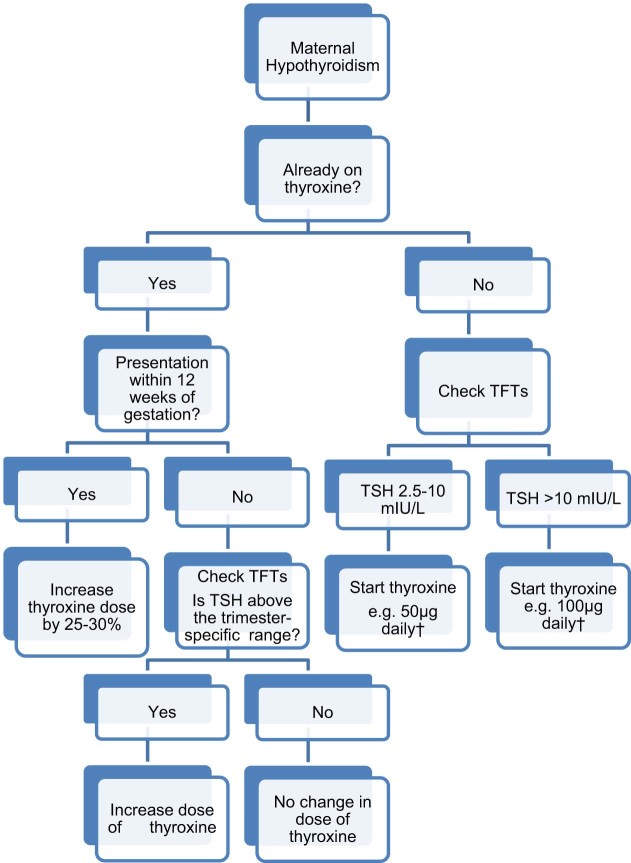
Various studies have shown an increased incidence of obstetric complications in pregnant women with untreated hypothyroidism (Table 2). These include preterm birth, low birth weight (mostly related to preterm delivery), perinatal death, pregnancy induced hypertension, pre-eclampsia, placental abruption, anaemia and postpartum haemorrhage [31]. Hypothyroidism has also been associated with adverse effects on intelligence quotient (IQ) and neuropsychological development [32]. Following the diagnosis of overt hypothyroidism, levothyroxine replacement should be commenced, aiming to achieve a TSH level within the trimester-specific pregnancy reference range [8], [9] (Fig. 2). The majority of pregnant women with pre-existing hypothyroidism will need increments of their levothyroxine dose by 25–50%, often within four to eight weeks of gestation and the dose increment tends to plateau by 16 weeks of gestation [8], [9], [33], [34]. Such a dose increment should take place immediately on confirmation of a missed cycle or a positive pregnancy test; one way of doing this is by increasing the 7 doses of levothyroxine per week to 9 doses [8], [34]. The levothyroxine requirements usually plateau from 16–20 weeks of gestation until delivery.
Fig. 2.
Treatment of maternal hypothyroidism in pregnancy TFTs, thyroid function tests. TSH, thyroid stimulating hormone. †These are conservative estimates based on our experience. Higher doses may be required and depending on the patients’ total body weight. We recommend regular TFTs and levothyroxine dose escalation until TSH drops within the trimester-specific reference range.
Subclinical hypothyroidism
Subclinical hypothyroidism is defined as an elevated TSH level (TSH 2.5–10.0 mU/L) with normal levels of free thyroxine. It can affect 0.25–2.5% of all pregnancies [35], [36], albeit in some studies the prevalence is even higher likely reflecting both differences in the definitions used and the populations studied. Subclinical hypothyroidism during pregnancy has been associated with significantly increased risk of hypertension and pre-eclampsia, placental abruption, premature rupture of membranes, early pregnancy loss, neonatal death and gestational diabetes [37], [38], [39], [40], [41]. The risk of miscarriage has been confirmed in prospective studies in iodine-replete populations and the risk seems to apply with TSH increments above, but not within, the normal range [40]. Neurodevelopmental deficits in the offspring have been reported in some studies [42], [43], but the evidence for this is inconsistent [44], [45], [46]. In the only interventional study by Lazarus et al., pregnant women were divided into a screening and a control group; the former had blood samples analysed immediately for thyroid function tests (TFTs) and subclinical hypothyroidism was treated with levothyroxine; the latter had their TFTs analysed after the end of the pregnancy [47]. There was no difference between the two groups when their children were neuropsychologically assessed at the age of three years [47]. However, this study can be criticised for the fact that the addition of thyroxine occurred at a relatively late stage in gestation (at an average of 13 weeks) and one should be aware that a lack of impact at the age of three does not necessarily translate to a lack of impact at a later stage in life. Similarly in an as yet-unpublished large multicentre double-blind RCT of levothyroxine use for subclinical hypothyroidism in pregnancy (at a mean gestational age of 17 weeks), there was no difference in intelligence quotient measurements in the offspring at the ages of three or five years [48]. There is also a scarcity of studies examining the impact of subclinical hypothyroidism in children under three years of age [49].
The ATA recommended that women with subclinical hypothyroidism who have elevated titres of thyroid peroxidase antibody (TPOAb) should be treated with levothyroxine based on one study [8]; however, they commented that there is limited data to either recommend or discourage levothyroxine treatment for TPOAb negative women with subclinical hypothyroidism. The RCT in question by Negro et al. has indeed shown a statistically important reduction in miscarriages and pre-term deliveries in euthyroid TPOAb-positive women who received levothyroxine and a reduction of the trend towards declining fT4 and increasing TSH longitudinally in gestation [50]. Nevertheless, levothyroxine was commenced on average at around 10 weeks of gestation and all but one miscarriage took place prior to the 12 weeks so it is questionable whether indeed the thyroxine therapy influenced this outcome. Furthermore, this study took place in Southern Italy which has some iodine deficiency and hence the findings cannot be easily extrapolated to other populations. The European Thyroid Association (ETA) and The Endocrine Society recommend levothyroxine replacement in all women with subclinical hypothyroidism regardless of TPOAb status, as they consider the advantages of therapy to far outweigh the potential disadvantages [9], [49]. Indeed, the general consensus among endocrinologists has been to treat all such women with levothyroxine to achieve a TSH level within the trimester-specific range [49]. However, a recent large, population-based prospective Dutch study has cast doubt on such practices because it revealed an association between early pregnancy maternal TFTs and child IQ (at six years) and brain morphology on MRI (at eight years of age); both low and high fT4 levels were associated with reduced IQ and reduced grey matter and cortical volume [51]. Hence, the authors recommended caution in prescribing levothyroxine for subclinical hypothyroidism given that this often results in high-normal fT4 levels [51], albeit this study did not specifically look at the effect of levothyroxine therapy for subclinical hypothyroidism. Nevertheless, based on this study and the general dearth of evidence on the ability of levothyroxine to counter the complications observed with subclinical hypothyroidism, a position statement has been published by some investigators challenging the existing orthodoxy on the topic and essentially stating that at present we are not in a good position to recommend for or against treatment of this pathology in pregnancy and that further RCTs are necessary to guide practice [39].
For both overt and subclinical hypothyroidism, it is advisable that TFTs should be performed regularly, particularly in the first half of pregnancy, and every time there is a change in treatment. There is usually no requirement for additional fetal surveillance in mothers with well controlled hypothyroidism. Following childbirth, it is common practice to reduce the levothyroxine dose to pre-pregnancy levels immediately post-delivery, although some authors advocate that the dose should be reduced to the pre-pregnancy levels two weeks post-partum, followed by repeated TFTs [52].
Isolated hypothyroxinaemia
Maternal isolated hypothyroxinaemia (IH) is defined by some authors as TSH concentrations within normal range and fT4 levels below the reference range [52], whereas other common definitions include a normal TSH with fT4 in the lowest 2.5th or 5th or even 10th percentile of the normal pregnant population [49], [53]. The prevalence varies between studies, and given the aforementioned differences in definition, from 1.3% to 10% of pregnant women in iodine sufficient regions [44], [53], [54], [55]. The diagnosis of IH can indicate the early stages of thyroid insufficiency and rarely secondary hypothyroidism, which should always be considered [52]. Some authors have reported increased rates of IH in areas of mild to moderate iodine deficiency and with advancing gestational age, reaching 25% in the third trimester [56], [57]. Moreover, a recent study of a large population of iodine-sufficient pregnant women from China has shown markedly increased risk of IH with iron deficiency [58].
Studies have shown that IH is associated with obstetric risks (Table 2), including placental abruption, low birth weight, neurodevelopmental impairment and pregnancy loss [52]. Two large prospective studies have given conflicting results regarding the significance of IH. Both measured TFTs in the first 20 weeks of gestation and compared them to pregnancy outcomes [54], [55]. The first study found similar pregnancy outcomes and TPOAb levels between women with IH and those who were euthyroid [54]. The second study identified an increased risk of fetal distress, small-for-gestational age and musculoskeletal malformations in the offspring [55]. It is not clear why there is such a discrepancy; admittedly, the former study had a higher population (about 18,000 vs. 1000 women, respectively), but the classification of complications was better overall in the latter study. It is also conceivable that the different populations (US vs. Chinese, respectively) have influenced these findings. Another recent longitudinal cohort study assessed the effect of hypothyroxinaemia during early pregnancy on school performance and concluded that there is an association with subnormal arithmetic (but not language) performance tests in their offspring at the age of five [59]; as with many studies of this kind there was a large number of cases lost to follow-up, which can introduce attrition bias. Another study has reported that IH, but not subclinical hypothyroidism, is associated with an adverse metabolic phenotype in pregnancy, as is decreasing maternal fT4 and increasing fT3:fT4 ratio [60]. IH was traditionally left untreated as it was thought to be a normal variant but nowadays some centres tend to treat it. Although the ATA guidelines do not recommend treatment [8], and The Endocrine Society have refrained from giving clear guidance [9], the ETA guidelines are the only ones which suggested that levothyroxine supplementation should be considered in the first trimester [49], because of the aforementioned association with neuropsychological impairment in the offspring. However, they do not recommend treatment after the first trimester (Table 3).
Table 3.
Comparison of the recommendations of the current guidelines on the management of thyroid disease in pregnancy.
| Guideline aspect | American Thyroid Association [8], [70]* | The Endocrine Society [9] | European Thyroid Association [7] |
|---|---|---|---|
| Hypothyroidism: levothyroxine therapy | Treatment is recommended | Treatment is recommended | Treatment is recommended |
| Subclinical hypothyroidism: levothyroxine therapy | Treatment is recommended if TPOAb-positive;Treatment is discretionary if TPOAb-negative | Treatment is recommended | Treatmet is recommended |
| Lack of RCT evidence documenting benefit of levothyroxine therapy in women with subclinical hypothyroidism and no antibodies | Potential benefits are considered more than potential risks | ‘Levothyroxine therapy would appear to have the potential benefits which outweigh the potential risks’† | |
| Isolated hypothyroxinaemia: levothyroxine therapy | Treatment is discouraged | Treatment is discretionary | Treatment is recommended if diagnosed in the 1st trimester Treatment is discouraged if diagnosed in the 2nd and 3rd trimester |
| ‘Management…is controversial and requires further study’ ‘…partial replacement therapy may be initiated at the discretion of the caregiver, with continued monitoring’ |
|||
| Hyperthyroidism: anti-thyroid drugs | PTU in the 1st trimester MMI in the 2nd and 3rd trimester |
PTU in the 1st trimester MMI in the 2nd and 3rd trimester |
N/A |
| PTU in 1st trimester; MMI in the 2nd and 3rd trimester, or, alternatively, continue with PTU (ATA 2016 [70]) | |||
| Subclinical hyperthyroidism: | Treatment is discouraged | Treatment is discouraged | N/A |
| Treatment is discouraged [70] | |||
| Autoimmune Thyroid Disease (TPOAb positive) | Treatment is discretionary | Treatment is discouraged | N/A |
| ‘Insufficient evidence to recommend for or against screening for TPO Abs or for treatment with LT4 of TPOAb + euthyroid women…’ | ‘Universal screening for thyroid antibodies, and possible treatment, cannot be recommended…’ ‡ | N/A | |
| Iodine supplementation§ | 150 μg in the form of potassium iodide. Avoid kelp supplements. | 150–200 μg in the form of potassium iodide or iodate. Commence ideally before conception. Avoid excessive intake above 500mcg | 150 μg. Commence ideally before conception. |
| Avoid excessive intake that is 500–1100mcg | Avoid excessive intake above 500mcg |
TPOAb: thyroid peroxidase antibodies; RCT: randomised control trial; PTU: propylthiouracil; MMI: methimazole or carbimazole; N/A: not applicable.
All these recommendations are derived from the 2011 ATA guidelines [8] for the diagnosis and management of thyroid disease during pregnancy and postpartum, apart for the recommendations for hyperthyroidism where the 2016 ATA guidelines on thyrotoxicosis were also utilized [70].
Pre-conception it is reasonable to maintain TSH <2.5 mU/L, especially in those with TPO positivity.
If euthyroid with TPOAb positivity, then TSH should be measured before pregnancy and during the 1st and 2nd trimester.
These recommendations are also valid for lactation.
Hyperthyroidism
Overt hyperthyroidism, defined as suppressed TSH with elevated fT4 and/or fT3 levels, is relatively uncommon during pregnancy, occurring in 0.1–0.4% of all pregnancies [61]. A suppressed or undetectable TSH level during pregnancy and especially in the first trimester is usually due to the direct stimulation of the thyroid gland by hCG which can result in transient gestational thyrotoxicosis. Hyperemesis gravidarum, hydatidiform mole, choriocarcinoma and multiple pregnancies can produce the same effect [62].
Graves’ disease
Graves’ disease (autoimmune hyperthyroidism) is the commonest cause (in 85% of cases) of overt hyperthyroidism [9], [63]; solitary or multi-nodular toxic nodules or factitious thyrotoxicosis are less common causes. The disease usually exacerbates within the first trimester and improves thereafter, and recurs following delivery [61], [64]. This follows the same pattern as other autoimmune conditions during pregnancy and the puerperium, therefore the dose of thioamides is often reduced following the first trimester.
Differentiation of Graves’ disease from gestational thyrotoxicosis is supported by the presence of clinical stigmata of Graves’ disease, such as a typical goitre, ophthalmopathy and pretibial myxoedema, and the presence of TSH receptor antibodies (TRAbs). TPOAb may be present in either case. Thyroid ultrasound may show enlarged gland dimensions and typical increased blood flow in Graves’ disease, but there are no absolute differentiating features from gestational thyrotoxicosis [65]; hence it is not usually essential. Radioisotope scanning is contraindicated in pregnancy.
Inadequately treated hyperthyroidism is associated with an increased risk of preterm labour, intrauterine growth restriction, pre-eclampsia and fetal death [66]; fetal malformations have been reported in most [55], [67], [68], but not all studies [69]. The explanation for this discrepancy may be that in the latter study by Yoshihara et al. patients that received anti-thyroid drugs (ATD) were excluded from the analysis [69], hence it is conceivable that the milder spectrum of Graves’ disease is not associated with congenital malformations. In view of adverse fetal outcomes (Table 2), growth scans should be offered in women with hyperthyroidism; and absence of fetal tachycardia (<160 beats per minute) must also be documented at each antenatal visit [66]. The treatment of overt hyperthyroidism aims to maintain maternal fT4 levels at or slightly above the upper limit of the trimester- and assay-specific reference range or, alternatively, maintain total T4 and T3 levels at or slightly above the normal reference range in pregnancy or at 1.5 fold the upper limit of the non-pregnant reference range in the second and third trimester [70]. TSH below the reference range for pregnancy would be acceptable as TSH levels can be misleadingly ‘low’ and consequently lead to overtreatment.
Treatment of Graves’ disease in pregnancy
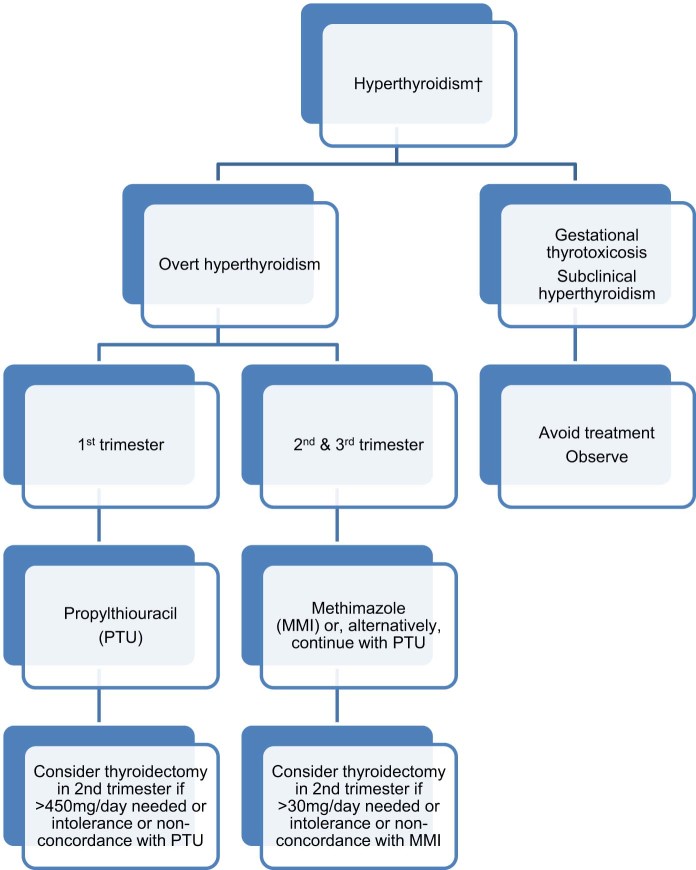
The use of ATD is recommended for the treatment of Graves’ disease or toxic nodular disease causing overt hyperthyroidism (Fig. 3). Radioiodine therapy is contraindicated in pregnancy. Therapeutic administration of radioiodine to the mother after the fetal gland is formed (i.e. after about 10 weeks into gestation) can result in fetal hypothyroidism and may be associated with attention deficit disorders and impairment of figurative memory in the offspring [71]. Incidentally, when radioiodine therapy is applied in non-pregnant women, it is recommended to avoid conception for at least six months.
Fig. 3.
Treatment of maternal hyperthyroidism in pregnancy. †In most patients hyperthyroidism appears outside of pregnancy and in such cases definitive treatments with thyroidectomy or radioidine ablation should be considered if pregnancy is contemplated in the near-future (see main text).
The first line ATD has traditionally been propylthiouracil (PTU). This is because methimazole (MMI) and carbimazole (CBZ, which is a prodrug to MMI; the term MMI shall be used to cover both these medications for simplicity) have been traditionally associated with a higher risk of congenital malformations, whereas PTU was not thought to increase such risk [69]. However, in other studies both MMI and PTU use in early pregnancy were associated with birth defects [72], [73], but the risk remained higher with MMI comparative to PTU (Odds Ratio 1.66 with MMI vs. 1.41 with PTU) [73]. Moreover, there was differential expression of complications between the two medications, with MMI causing ‘MMI-induced embryopathy’ (choanal atresia, oesophageal atresia, omphalocele, omphalomesenteric tract anomalies and/or aplasia cutis), eye and urinary tract anomalies, whereas PTU was associated with malformations in the face and neck region and urinary tract anomalies [72], [73]. Situs inversus, cardiac outflow abnormalities and unilateral kidney a/dysgenesis were commoner with PTU use in another case-affected control study [74]. Data from the UK Yellow Card Scheme (for reporting medication adverse effects) documented only six cases with 11 birth anomalies related to PTU exposure in the past 50 years [75]; the corresponding number for MMI was 57 cases with 97 anomalies, hence also suggestive of a MMI embryopathy.
Nevertheless, MMI is the agent most widely used outside of pregnancy and its administration is more convenient (once daily vs. split dosing). During the second and third trimester, MMI is recommended in preference to PTU [8], [9], [70], in view of rare reports of severe hepatotoxicity with the latter (about 1 in 10,000) [2]. However, in daily practice switching of ATD can be somewhat ‘messy’ as it may lead to variations in TFTs in the short-term. Moreover, the case for such a switch has never been proven [76] and indeed a shift between PTU and MMI in early pregnancy has recently been linked with an increased risk of congenital malformations by 82–88% [73], [76]. Moreover, a recent large population-based cohort study that assessed the complications of ATD has indicated that the incidence of liver dysfunction and agranulocytosis are exceedingly rare in pregnancy, whereas ATD-induced congenital defects are comparatively more frequent (less than one case of agranulocytosis or liver dysfunction per 5 million population per 10 year period versus 44 live births with birth defects in same time period) [77].
In conclusion, there isn’t much evidence that the switch from PTU to MMI after the first trimester reduces the risk of complications. Fulminant hepatic failure associated with PTU is vanishingly rare, but when it occurs is a devastating complication and has been encountered in both pregnant women and their foetuses. Furthermore, although both PTU and MMI seem to be linked with congenital malformations, the incidence of such defects is higher with MMI and overall the defects encountered are of a more serious nature. There is a wide variation in practice for newly diagnosed Graves’ disease during pregnancy; a survey of ETA members reported that 53% would treat with PTU, 12% with MMI, and only 34% with PTU initially and switch to MMI after the first trimester as per guidelines [78]. The very recent ATA guidelines on thyrotoxicosis have incorporated these gaps in evidence and mention that for females taking PTU in the first trimester they may either switch to MMI or, alternatively, remain on PTU for the second and third trimester. Whichever ATD is used the patients should be provided with oral and written information that if a pruritic rash, jaundice, acolic stools or dark urine, arthralgias, abdominal pain, nausea, fatigue or fever were to occur then they have to stop the medications immediately and inform their physicians [70].
We recommend a discussion with the patient regarding the pros and cons of these therapies, preferably at pre-conception counselling. Furthermore, for women who are contemplating pregnancy in the near-future we encourage them to consider a definitive therapy, such as radioidine therapy or surgery, especially if requiring high doses of ATD as these will abolish the need to for ATD antenatally [70], [79] Radioiodine would be acceptable when pregnancy is contemplated after at least six months, and thyroidectomy if conception is envisaged within a six month interval and/or if there is a large goitre. It is worth noting that TRAb titres increase for at least a year post-radioiodine therapy (vs. a common, but not universal [80], reduction with surgery) [81] and this could theoretically raise the risk for fetal or neonatal thyrotoxicosis. Hence, surgery may be a more attractive option for those women with persistently raised TRAb, especially if planning to conceive within one year [70]. In any case, regardless of the definitive therapy employed to treat Graves’ disease, the patient should be rendered euthyroid through appropriate and prompt adjustments of levothyroxine, before conception is attempted. Pregnant women should have their TFTs checked regularly about every 3–4 weeks whilst on PTU treatment, and two weeks after switching from PTU to CBZ, followed by 2–4 week intervals until thyroid function is within normal limits [8].
A surgical approach should be considered as a last resort in pregnant women with overt hyperthyroidism who are unable to take ATD because of either a severe adverse reaction or non-concordance. Moreover, if high doses of ATD (e.g. >30 mg/d of CBZ or >450 mg/d of PTU) are required to achieve optimal treatment, this may be another indication for surgical intervention. The optimal timing for surgery is the second trimester given the risks of teratogenicity and miscarriage with first trimester and preterm delivery with third trimester surgery.
Breastfeeding and use of anti-thyroid drugs
For many years breastfeeding was strongly discouraged in women treated with ATD. However, studies have shown that only limited quantities of PTU and MMI are secreted in milk, therefore the neonatal exposure to ATD is minimal and clinically insignificant [63], [82]. Furthermore, few studies, albeit small-scale, have shown normal TFTs and no increased risk of malformations in neonates whose mothers received MMI in pregnancy [83], [84], [85]. Hence, women with hyperthyroidism using low-to-moderate doses of CBZ (<20 mg) or PTU (<300 mg) should be encouraged to breastfeed, as the benefits of breastfeeding largely outweigh the theoretical risks from ATD treatment [86]. The ATD of choice in such circumstances is MMI f [8], [70].
Treatment of subclinical hyperthyroidism in pregnancy
In subclinical hyperthyroidism (reduced TSH, normal fT4 and fT3), treatment should be avoided and consideration should be given whether this is a normal variant (due to high hCG levels) or, occasionally, due to non-thyroidal illness. In one large study of 25,765 women who underwent thyroid screening and delivered singleton infants, subclinical hyperthyroidism (1.7%) was not associated with adverse pregnancy or neonatal outcomes, and indeed there was a significantly reduced risk of gestational hypertension (but not severe pre-eclampsia) [54], although it is unclear why this should be, or indeed if this represents a chance finding (Table 3).
Gestational hyperthyroidism
Gestational hyperthyroidism is defined as transient hyperthyroidism limited to the first half of pregnancy, associated with a high fT4 and a suppressed TSH, but no clinical nor any antibody evidence of thyroid autoimmunity. fT3 is elevated less frequently. It complicates 0.5–10 per 1000 pregnancies [87].
Regarding hyperemesis gravidarum (characterized by 5% weight loss, dehydration and ketonuria), various hormonal, mechanical and psychological factors have been implicated in its pathophysiology. The current literature suggests three pathophysiological mechanisms: placental growth and function as reflected by free hCG, maternal endocrine function, and pre-existing gastro-intestinal (GI) disease. The temporal relationship between the level of hCG (peaking between 6 and 12 weeks) and severity of vomiting suggest hCG per se may have a causative role [88]. The exact mechanism, however, for how elevated levels of hCG leads to hyperemesis is unclear. High levels of estradiol may be implicated, whereas another proposed mechanism involves the stimulatory effect of hCG on the secretory pathways in the upper GI tract [89], albeit a decreased gastric tone and motility due to elevations in progesterone in pregnancy may also be involved.
The vast majority of patients with hyperemesis gravidarum and hyperthyroidism do not require ATD. Instead, they should be managed with supportive measures and their illness should resolve spontaneously. Clinical judgment should be followed in women who are clinically thyrotoxic or in addition have a serum T4 level above the reference range for pregnancy. Beta-blockers (such as propranolol or metoprolol) may be used cautiously if very symptomatic, and always after liaison with the obstetric team.
On rare occasions when gestational hyperthyroidism persists well into the second trimester with prominent symptomatology and a biochemical picture of thyrotoxicosis and despite hCG levels that are relatively low or reducing, in such cases liaising with relevant research institutions could be considered for sequencing the TSH receptor gene to look for mutant TSH receptor with enhanced hCG sensitivity [90], [91].
In women with hyperemesis gravidarum and clinical features of hyperthyroidism, TFTs and TRAbs should be measured. TRAbs have high sensitivity and specificity for the diagnosis of Graves’ disease [72], [92].
Thyroid storm
Thyroid storm is a rare medical emergency, characterized by an extreme hypermetabolic state precipitated by high levels of endogenous thyroid hormones. It occurs in 1% of women with untreated or inadequately treated hyperthyroidism during pregnancy and has a high risk of maternal heart failure [93], shock, stupor and coma, as well as a maternal mortality of up to 25% [70], [94], [95]. Thyroid storm should be suspected in every pregnant woman with pre-existing hyperthyroidism who presents with unexplained fever, altered mental status, cardiac arrhythmias, confusion, and seizures [94]. Often there is an identified precipitating factor, such as infection, surgery, labour or delivery.
Its treatment does not differ from non-pregnant women and it should be managed by a multidisciplinary team consisting of endocrinologists and fetal specialists in the intensive care unit with continuous fetal monitoring if the fetus has reached 26 weeks of gestational age [94], [95]. Prompt recognition and management is paramount.
The pharmacological treatment is directed against thyroid hormone synthesis and secretion as well as the peripheral action of thyroid hormone at the tissue level. A multimodality treatment approach should be employed, including beta-adrenergic blockade (propranolol), high doses of PTU, inorganic iodide and corticosteroid therapy (dexamethasone). In addition to the pharmacological management, general supportive care should be undertaken such as oxygen administration, antipyretics, cooling blankets, volume resuscitation, nutritional support, respiratory care and monitoring in an intensive care [70]. In general, it is recommended to avoid delivery in the presence of thyroid storm unless fetal indications for delivery outweigh the risks to the woman [94], [95].
Autoimmune thyroid disease
Maternal TSH does not cross the placenta; however, maternal T4 and T3 do cross the placenta in small quantities and are important for early fetal growth. TRAbs may cross the placenta and directly stimulate the fetal thyroid when present in high concentrations. TPO antibodies per se are not known to directly damage the foetus, but are considered indicative of an immune dysfunction and increase the risk of maternal thyroid dysfunction.
Maternal TSH receptor antibodies
TRAb titre decreases as the pregnancy progresses. Fetal hyperthyroidism due to maternal TRAb stimulation is rare with a reported rate of 1 in 4000–40,000 pregnancies [96].
It is recommended that TRAb should be checked at 20–26 weeks of gestation (Table 4), albeit fetal Graves’ disease has been reported as early as 18 weeks gestation [97]. Women with TRAb titres two-to-three-fold above the normal level and women treated with ATD should have TFTs performed and the fetal thyroid appearances checked during the fetal anatomy ultrasound at 20 weeks of gestation; these should be repeated every 4–6 weeks thereafter [66]. Features suggestive of fetal thyroid dysfunction include goitre, growth restriction, hydrops, tachycardia or heart failure [98]. Umbilical cord sampling should be used as a last resort and only be considered if the diagnosis of fetal thyroid disease remains uncertain with the available clinical and sonographic data, and only if such an invasive test could potentially change the management given that this procedure itself carries a non-trivial risk of fetal loss. If fetal hyperthyroidism is present and is thought to endanger the pregnancy, ATD should be given to the mother to suppress the fetal thyroid. This represents the only potential indication for concomitant use of ATD and levothyroxine (‘block and replace’ regime) in pregnancy.
Table 4.
Guidelines & recommendations on the use of Thyroid Receptor Antibodies (TRAb) during pregnancy.
| Guidelines & recommendations on the use of Thyroid Receptor Antibodies (TRAb) during pregnancy | |
|---|---|
| American Thyroid Association (ATA) 2011 [8] | 22–24/40 if previous medical history of Graves’ disease |
| The Endocrine Society (TES) [9] | 22/40 if previous history or active Graves’ disease, if prior radioactive iodine (RAI) therapy or thyroidectomy, previous neonate with Graves’ disease or previously raised TRAb |
| If TRAb negative and not on anti-thyroid drugs, then there is very low risk of fetal or neonatal thyroid dysfunction | |
| Italian Thyroid Association (AIT) & Italian Association of Clinical Endocrinologists (AME) [113] | Early pregnancy and 22–26/40 if on anti-thyroid drugs for active Graves’ disease and if recent radioidine therapy |
| 22–26/40 only if previous Graves’ disease (and no relapse in pregnancy) and if surgically treated >1 year before pregnancy onset | |
| American Thyroid Association (ATA) 2016 [70] | First trimester and if raised re-check again at 18–22/40 if prior radioactive iodine therapy or thyroidectomy |
| Initial pregnancy visit or at diagnosis and if raised again at 18–22/40 for active Graves’ disease requiring ATD or new Graves’ disease in pregnancy. If TRAb raised at 18–22/40 then re-check at 30–34/40 |
Beware of the specific assay used locally and also about the fact that TRAb assays do not routinely measure antibody activity. However sensitive bioassays do exist that measure antibody activity and these can be used in exceptional circumstances e.g. in a pregnant woman with no thyroid gland in situ but positive TRAb, in order to know whether these are stimulating or inhibiting antibodies.
Maternal thyroid peroxidase antibodies
Women of reproductive age have a prevalence of TPOAb of 6–20% [99]; these are strongly associated with polyhydramnios [100], premature rupture of membranes, low birth weight [49], and preterm delivery. Thyroid autoimmunity is also associated with increased risk of miscarriage even in euthyroid, iodine sufficient populations [40]. Nevertheless, thyroid autoimmunity and subclinical hypothyroidism seem to act synergistically to multiply the risk of miscarriage, which overall takes place at an earlier stage in gestation compared to unaffected women [40]. In a meta-analysis of case-control and cohort studies euthyroid women with elevated TPO autoantibodies were reported to have a two-to-four-fold higher risk of spontaneous miscarriage, three-fold higher risk of subfertility and a two-fold higher risk of preterm birth than those without [101]. One study reported that levothyroxine treatment in the first trimester reduces miscarriages in euthyroid TPOAb-positive women [50]. However, it had a small sample size and as previously mentioned levothyroxine initiation was late. At present there is insufficient evidence to recommend for or against screening for thyroid antibodies, or treatment with levothyroxine during the first trimester of pregnancy in euthyroid women, or those with sporadic or recurrent miscarriages, or in women undergoing in vitro fertilisation [8]. The Endocrine Society made a somewhat more stern statement that it cannot recommend screening or treatment with thyroxine in such circumstances [9] (Table 3).
Nevertheless, women identified as TPOAb positive should have TFTs checked before pregnancy and during the first and second trimester, as they are at increased risk of developing subclinical or overt hypothyroidism; these women can be monitored as per overt/subclinical hypothyroidism recommendations for simplicity [8].
Recurrent pregnancy loss has been correlated with reduced selenium levels and selenium supplementation may reduce thyroid antibody levels in euthyroid patients [102], [103]. Indeed, when selenomethionine was given to euthyroid pregnant women with TPOAb positivity, TPOAb titers reduced more steeply as the gestation progressed and the incidence of post-partum thyroid dysfunction and hypothyroidism diminished [102]. However, the sample size was small (85 women in the intervention group) and it involved an Italian population alone.
Therefore, further RCTs are needed to investigate the effects on the maternal and neonatal outcomes when levothyroxine or selenium or other treatments are given to pregnant euthyroid women with positive thyroid antibodies and two such RCTs are currently underway: a randomised controlled trial of the efficacy and mechanism of levothyroxine treatment on pregnancy and neonatal outcomes in women with thyroid antibodies (TABLET: Thyroid AntiBodies and LEvoThyroxine study; https://www.clinicaltrialsregister.eu/ctr-search/trial/2011–000719-19/GB), and a randomised controlled trial of levothyroxine for euthyroid women with recurrent miscarriage and thyroid autoimmunity (T4Life trial; https://www.clinicaltrialsregister.eu/ctr-search/trial/2011–001820-39/DK).
Postpartum thyroiditis
The prevalence of postpartum thyroiditis varies from 1% to 17% and is more common in women with type 1 diabetes, a family history of hypothyroidism and in euthyroid pregnant women with TPOAb positivity [50], [100]. Given that autoimmune thyroid disease mainly revolves around cell-mediated autoimmunity and the fact that pregnancy diminishes cellular immunity, it frequently improves during pregnancy. During the post-partum period relative hypocortisolism seems critical for the development of autoimmune thyroid disease via a disinhibition of specific cytokine production (e.g. TNFα, IL-12 and IFNγ); the relative reduction in sex steroids is also a likely contributing factor [104].
The classical pattern of postpartum thyroiditis is characterized by transient thyrotoxicosis followed by transient or, in 5% of cases permanent, hypothyroidism, before a return into a euthyroid state, usually within the 12-month post-partum period. Two thirds of patients with TPOAb positivity can be expected to present with the classical form of the disease, whereas two thirds of those with absent TPOAb will present with isolated thyrotoxicosis [100]; presentation with isolated hypothyroidism is also common [70]. Post-partum thyroiditis usually presents clinically between three and four months postpartum but may be delayed to as long as six months, [66] which is when the TPOAb titers are expected to be at their peak. The diagnosis is made on clinical and biochemical grounds. A distinction should be made from Graves’ disease, for example the latter may have a bigger goitre, a bruit on auscultation, Graves’ ophthalmopathy and high TRAb levels. Radioactive iodine or technetium uptake tests can help distinguish between postpartum thyroiditis (low uptake) and Graves’ disease (high uptake); they need to be used with caution if women are breast-feeding; they should be instructed to pump and discard breast milk for 10 half-lives i.e. for three and five days with 99 m-Tc-pertechnetate and 123-Iodine,respectively [70]. Thyroid ultrasound scanning can provide further evidence of its presence; classic appearances include focal or diffuse non-specific hypoechogenicity which resolves on serial scanning and reduced flow on power Doppler compared with less hypoechogenicity and an increased blood flow (including ‘thyroid inferno’) often observed with Graves’ disease. The sonographer also needs to be aware that an inflamed thyroid gland and associated reactive lymph nodes can give appearances that mimic neoplastic disease [105]. Finally, it is worth mentioning that the spectrum of disease in association with thyroid autoimmunity has expanded in recent years, for example post-partum depression and alexithymia and even psychosis have been directly linked with thyroid autoimmunity [106], [107], [108]. However, no association was noted in a large Norwegian population-based study between anxiety and depression and thyroid autoimmunity [109]. Moreover, there is RCT evidence that the use of levothyroxine in TPO-Ab positive women from 6 weeks to 6 months post-partum did not reduce the risk of occurrence of post-natal depression [110].
Treatment of post-partum thyroiditis
The management of postpartum thyroiditis should be determined by the degree of clinical symptoms rather than the severity of the abnormal biochemistry. The thyrotoxic phase may be treated with beta-blockers (propranolol or metoprolol) for symptom control; ATD are ineffective given that post-partum thyroiditis does not represent a state of increased thyroid hormone synthesis and secretion, hence there is no role for drugs that block thyroid hormone synthesis. Close monitoring is the key to its management. If hypothyroidism develops then treatment is only indicated if this is persistent and/or symptomatic. The TFTs trend is helpful in guiding treatment decisions. For example, if the fT4 is low but slowly rising and the patient is asymptomatic then no treatment is required. It is recommended that women with postpartum thyroiditis should be monitored with annual TSH and fT4 measurements [111] and in the post-partum period of future pregnancies given the high risk of recurrence (up to 70% [112]).
Conclusions
Early diagnosis and management of thyroid dysfunction in pregnancy is essential to avoid adverse maternal and fetal outcomes. Overt hypothyroidism and hyperthyroidism should be treated appropriately. Subclinical hypothyroidism is often treated with levothyroxine, although it has not been proven beyond doubt that it improves maternal or fetal outcomes. Subclinical hyperthyroidism does not usually require treatment and the possibility of non-thyroidal illness or gestational thyrotoxicosis should be considered. Despite the demonstrated adverse maternal and fetal outcomes in observational studies with euthyroid women having positive thyroid autoantibodies, to date there is insufficient data to prove that such outcomes improve with thyroxine supplementation. Further RCTs are required to investigate the effects of treating pregnant euthyroid women with autoimmune thyroid disease and, indeed, women with subclinical hypothyroidism and isolated hypothyroxinaemia. Current practice guidelines provide a safe framework for practice and have many similarities among them, as well as differences which generally indicate gaps in our current knowledge of thyroid disorders in pregnancy.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work did not receive funding for any aspect of compilation or publication.
References
- 1.Vanderpump M.P.J. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 2.Kyriacou A., McLaughlin J., Syed A.A. Thyroid disorders and gastrointestinal and liver dysfunction: A state of the art review. Eur J Intern Med. 2015;26:563–571. doi: 10.1016/j.ejim.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N., Braunstein G.D., Butts C.L., Martin K.A., McDermott M., Pinkerton J.V. Compounded bioidentical hormones in endocrinology practice: an endocrine society scientific statement. J Clin Endocrinol Metab. 2016;101:1318–1343. doi: 10.1210/jc.2016-1271. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 5.Weeke J., Dybkjaer L., Granlie K., Eskjaer Jensen S., Kjaerulff E., Laurberg P. A longitudinal study of serum TSH, and total and free iodothyronines during normal pregnancy. Acta Endocrinol (Copenh) 1982;101:531–537. doi: 10.1530/acta.0.1010531. [DOI] [PubMed] [Google Scholar]
- 6.Ballabio M., Poshychinda M., Ekins R.P. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73:824–831. doi: 10.1210/jcem-73-4-824. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus J., Brown R.S., Daumerie C., Hubalewska-Dydejczyk A., Negro R., Vaidya B. European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagnaro-Green A., Abalovich M., Alexander E., Azizi F., Mestman J., Negro R. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Groot L., Abalovich M., Alexander E.K., Amino N., Barbour L., Cobin R.H. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M.B. The adverse effects of mild-to-moderate iodine deficiency during pregnancy and childhood: a review. Thyroid. 2007;17:829–835. doi: 10.1089/thy.2007.0108. [DOI] [PubMed] [Google Scholar]
- 11.Qian M., Wang D., Watkins W.E., Gebski V., Yan Y.Q., Li M. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr. 2005;14:32–42. [PubMed] [Google Scholar]
- 12.WHO, Assessment of iodine deficiency disorders and monitoring their elimination, 2007. Available from: http://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en/.
- 13.Taylor P.N., Vaidya B. Iodine supplementation in pregnancy - is it time? Clin Endocrinol (Oxf) 2016 doi: 10.1111/cen.13065. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann M., Delange F. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004;58:979–984. doi: 10.1038/sj.ejcn.1601933. [DOI] [PubMed] [Google Scholar]
- 15.Bath S.C., Steer C.D., Golding J., Emmett P., Rayman M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC) Lancet. 2013;382:331–337. doi: 10.1016/S0140-6736(13)60436-5. [DOI] [PubMed] [Google Scholar]
- 16.Bath S.C., Rayman M.P. Iodine deficiency in the U.K.: an overlooked cause of impaired neurodevelopment? Proc Nutr Soc. 2013;72:226–235. doi: 10.1017/S0029665113001006. [DOI] [PubMed] [Google Scholar]
- 17.Bath S.C., Furmidge-Owen V.L., Redman C.W., Rayman M.P. Gestational changes in iodine status in a cohort study of pregnant women from the United Kingdom: season as an effect modifier. Am J Clin Nutr. 2015;101:1180–1187. doi: 10.3945/ajcn.114.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caldwell K.L., Pan Y., Mortensen M.E., Makhmudov A., Merrill L., Moye J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid. 2013;23:927–937. doi: 10.1089/thy.2013.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce E.N., Andersson M., Zimmermann M.B. Global iodine nutrition: Where do we stand in 2013? Thyroid. 2013;23:523–528. doi: 10.1089/thy.2013.0128. [DOI] [PubMed] [Google Scholar]
- 20.Hynes K.L., Otahal P., Hay I., Burgess J.R. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab. 2013;98:1954–1962. doi: 10.1210/jc.2012-4249. [DOI] [PubMed] [Google Scholar]
- 21.Oken E., Wright R.O., Kleinman K.P., Bellinger D., Amarasiriwardena C.J., Hu H. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Donne M., Alibrandi A., Vita R., Zanghì D., Triolo O., Benvenga S. Does eating oily fish improve gestational and neonatal outcomes? Findings from a Sicilian study. Women Birth. 2016;29:e50–7. doi: 10.1016/j.wombi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Benvenga S., Vigo M.T., Metro D., Granese R., Vita R., Le Donne M. Type of fish consumed and thyroid autoimmunity in pregnancy and postpartum. Endocrine. 2016;52:120–129. doi: 10.1007/s12020-015-0698-3. [DOI] [PubMed] [Google Scholar]
- 24.Melse-Boonstra A., Gowachirapant S., Jaiswal N., Winichagoon P., Srinivasan K., Zimmermann M.B. Iodine supplementation in pregnancy and its effect on child cognition. J Trace Elem Med Biol. 2012;26:134–136. doi: 10.1016/j.jtemb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Taylor P.N., Okosieme O.E., Dayan C.M., Lazarus J.H. Therapy of endocrine disease: Impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis. Eur J Endocrinol. 2014;170 doi: 10.1530/EJE-13-0651. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S.J., Anderson A.J., Gibson R.a., Makrides M. Effect of iodine supplementation in pregnancy on child development and other clinical outcomes: a systematic review of randomized controlled trials. Am J Clin Nutr. 2013;98:1241–1254. doi: 10.3945/ajcn.113.065854. [DOI] [PubMed] [Google Scholar]
- 27.Bouhouch R.R., Bouhouch S., Cherkaoui M., Aboussad A., Stinca S., Haldimann M. Direct iodine supplementation of infants versus supplementation of their breastfeeding mothers: a double-blind randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:197–209. doi: 10.1016/S2213-8587(13)70155-4. [DOI] [PubMed] [Google Scholar]
- 28.Fisher D.A. Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol. 1997;40:16–31. doi: 10.1097/00003081-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Puig-Domingo M., Vila L. The implications of iodine and its supplementation during pregnancy in fetal brain development. Curr Clin Pharmacol. 2013;8:97–109. doi: 10.2174/1574884711308020002. [DOI] [PubMed] [Google Scholar]
- 30.Thorpe-Beeston J.G., Nicolaides K.H., McGregor A.M. Fetal thyroid function. Thyroid. 1992;2:207–217. doi: 10.1089/thy.1992.2.207. [DOI] [PubMed] [Google Scholar]
- 31.Saki F., Dabbaghmanesh M.H., Ghaemi S.Z., Forouhari S., Ranjbar Omrani G., Bakhshayeshkaram M. Thyroid function in pregnancy and its influences on maternal and fetal outcomes. Int J Endocrinol Metab. 2014;12:e19378. doi: 10.5812/ijem.19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haddow J.E., Palomaki G.E., Allan W.C., Williams J.R., Knight G.J., Gagnon J. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 33.Alexander E.K., Marqusee E., Lawrence J., Jarolim P., Fischer G.A., Larsen P.R. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 34.Yassa L., Marqusee E., Fawcett R., Alexander E.K. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab. 2010;95:3234–3241. doi: 10.1210/jc.2010-0013. [DOI] [PubMed] [Google Scholar]
- 35.Huang M.J., Li K.L., Wei J.S., Wu S.S., Fan K.D., Liaw Y.F. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol. 1994;89:1071–1076. [PubMed] [Google Scholar]
- 36.Chang D.L.F., Pearce E.N. Screening for maternal thyroid dysfunction in pregnancy: a review of the clinical evidence and current guidelines. J Thyroid Res. 2013;2013:851326. doi: 10.1155/2013/851326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajmani S.N., Aggarwal D., Bhatia P., Sharma M., Sarabhai V., Paul M. Prevalence of overt and subclinical thyroid dysfunction among pregnant women and its effect on maternal and fetal outcome. J Obstet Gynaecol India. 2014;64:105–110. doi: 10.1007/s13224-013-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maraka S., Singh Ospina N.M., O’Keeffe D.T., Espinosa De Ycaza A.E., Gionfriddo M.R., Erwin P.J. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580–590. doi: 10.1089/thy.2015.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brabant G., Peeters R.P., Chan S.Y., Bernal J., Bouchard P., Salvatore D. Management of subclinical hypothyroidism in pregnancy: are we too simplistic? Eur J Endocrinol. 2015;173:P1–11. doi: 10.1530/EJE-14-1005. [DOI] [PubMed] [Google Scholar]
- 40.Liu H., Shan Z., Li C., Mao J., Xie X., Wang W. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014;24:1642–1649. doi: 10.1089/thy.2014.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toulis K.A., Stagnaro-Green A., Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract. 2014;20:703–714. doi: 10.4158/EP13440.RA. [DOI] [PubMed] [Google Scholar]
- 42.Ghassabian A., Bongers-Schokking J.J., Henrichs J., Jaddoe V.W.V., Visser T.J., Visser W. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69:454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Shan Z., Teng W., Yu X., Li Y., Fan C. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf) 2010;72:825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 44.Henrichs J., Bongers-Schokking J.J., Schenk J.J., Ghassabian A., Schmidt H.G., Visser T.J. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227–4234. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 45.Julvez J., Alvarez-Pedrerol M., Rebagliato M., Murcia M., Forns J., Garcia-Esteban R. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology. 2013;24:150–157. doi: 10.1097/EDE.0b013e318276ccd3. [DOI] [PubMed] [Google Scholar]
- 46.Behrooz H.G., Tohidi M., Mehrabi Y., Behrooz E.G., Tehranidoost M., Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid. 2011;21:1143–1147. doi: 10.1089/thy.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazarus J.H., Bestwick J.P., Channon S., Paradice R., Maina A., Rees R. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493–501. doi: 10.1056/NEJMoa1106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casey B. Effect of treatment of maternal subclinical hypothyroidism or hypothyroxinemia on IQ in offspring. Am J Obs Gynaecol. 2015 Available from: http://www.ajog.org/article/S0002-9378(15)01319-8/pdf. [Google Scholar]
- 49.Lazarus J., Brown R.S., Daumerie C., Hubalewska-Dydejczyk A., Negro R., Vaidya B. European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76–94. doi: 10.1159/000362597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Negro R., Formoso G., Mangieri T., Pezzarossa A., Dazzi D., Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587–2591. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 51.Korevaar T.I.M., Muetzel R., Medici M., Chaker L., Jaddoe V.W.V., de Rijke Y.B. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:35–43. doi: 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- 52.Chan S., Boelaert K. Optimal management of hypothyroidism, hypothyroxinaemia and euthyroid TPO antibody positivity preconception and in pregnancy. Clin Endocrinol (Oxf) 2015;82:313–326. doi: 10.1111/cen.12605. [DOI] [PubMed] [Google Scholar]
- 53.Moleti M., Trimarchi F., Vermiglio F., Moleti M., Trimarchi F., Vermiglio F. Doubts and concerns about isolated maternal hypothyroxinemia. J Thyroid Res. 2011;2011:463029. doi: 10.4061/2011/463029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casey B.M., Dashe J.S., Wells C.E., McIntire D.D., Leveno K.J., Cunningham F.G. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–341. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 55.Su P.-Y., Huang K., Hao J.-H., Xu Y.-Q., Yan S.-Q., Li T. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011;96:3234–3241. doi: 10.1210/jc.2011-0274. [DOI] [PubMed] [Google Scholar]
- 56.Warner M.H., Beckett G.J. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010;205:1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 57.Moleti M., Lo Presti V.P., Mattina F., Mancuso A., De Vivo A., Giorgianni G. Gestational thyroid function abnormalities in conditions of mild iodine deficiency: early screening versus continuous monitoring of maternal thyroid status. Eur J Endocrinol. 2009;160:611–617. doi: 10.1530/EJE-08-0709. [DOI] [PubMed] [Google Scholar]
- 58.Yu X., Shan Z., Li C., Mao J., Wang W., Xie X. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab. 2015;100:1594–1601. doi: 10.1210/jc.2014-3887. [DOI] [PubMed] [Google Scholar]
- 59.Noten A.M.E., Loomans E.M., Vrijkotte T.G.M., van de Ven P.M., van Trotsenburg A.S.P., Rotteveel J. Maternal hypothyroxinaemia in early pregnancy and school performance in 5-year-old offspring. Eur J Endocrinol. 2015;173:563–571. doi: 10.1530/EJE-15-0397. [DOI] [PubMed] [Google Scholar]
- 60.Knight B.A., Shields B.M., Hattersley A.T., Vaidya B. Maternal hypothyroxinaemia in pregnancy is associated with obesity and adverse maternal metabolic parameters. Eur J Endocrinol. 2016;174:51–57. doi: 10.1530/EJE-15-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krassas G.E., Poppe K., Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 62.Tan J.Y.L., Loh K.C., Yeo G.S.H., Chee Y.C. Transient hyperthyroidism of hyperemesis gravidarum. BJOG. 2002;109:683–688. doi: 10.1111/j.1471-0528.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- 63.Marx H., Amin P., Lazarus J.H. Hyperthyroidism and pregnancy. BMJ. 2008;336:663–667. doi: 10.1136/bmj.39462.709005.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patil-Sisodia K., Mestman J.H. Graves hyperthyroidism and pregnancy: a clinical update. Endocr Pract. 2010;16:118–129. doi: 10.4158/EP09233.RA. [DOI] [PubMed] [Google Scholar]
- 65.Labadzhyan A., Brent G.A., Hershman J.M., Leung A.M., Kersten I., Lange A.E. Thyrotoxicosis of pregnancy. J Clin Transl Endocrinol. 2014;1:140–144. doi: 10.1016/j.jcte.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson-Piercy C. 4th ed. Medicine & Health Science Books @ Amazon.com; 2010. Handbook of obstetric medicine; pp. 95–107. [Google Scholar]
- 67.Momotani N., Ito K., Hamada N., Ban Y., Nishikawa Y., Mimura T. Maternal hyperthyroidism and congenital malformation in the offspring. Clin Endocrinol (Oxf) 1984;20:695–700. doi: 10.1111/j.1365-2265.1984.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 68.Galofre J.C., Davies T.F. Autoimmune thyroid disease in pregnancy: a review. J Womens Health (Larchmt) 2009;18:1847–1856. doi: 10.1089/jwh.2008.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshihara A., Noh J., Yamaguchi T., Ohye H., Sato S., Sekiya K. Treatment of graves’ disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab. 2012;97:2396–2403. doi: 10.1210/jc.2011-2860. [DOI] [PubMed] [Google Scholar]
- 70.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L. American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 71.Gorman C.A. Radioiodine and pregnancy. Thyroid. 1999;9:721–726. doi: 10.1089/thy.1999.9.721. [DOI] [PubMed] [Google Scholar]
- 72.Vos X.G., Smit N., Endert E., Tijssen J.G.P., Wiersinga W.M. Frequency and characteristics of TBII-seronegative patients in a population with untreated Graves’ hyperthyroidism: a prospective study. Clin Endocrinol (Oxf) 2008;69:311–317. doi: 10.1111/j.1365-2265.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 73.Andersen S.L., Olsen J., Wu C.S., Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J Clin Endocrinol Metab. 2013;98:4373–4381. doi: 10.1210/jc.2013-2831. [DOI] [PubMed] [Google Scholar]
- 74.Clementi M., Di Gianantonio E., Cassina M., Leoncini E., Botto L.D., Mastroiacovo P. Treatment of hyperthyroidism in pregnancy and birth defects. J Clin Endocrinol Metab. 2010;95:E337–41. doi: 10.1210/jc.2010-0652. [DOI] [PubMed] [Google Scholar]
- 75.Bowman P., Vaidya B. Suspected spontaneous reports of birth defects in the UK associated with the use of carbimazole and propylthiouracil in pregnancy. J Thyroid Res. 2011;2011:235130. doi: 10.4061/2011/235130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., Liu G.-Y., Ma J.-L., Zhou L. Risk of congenital anomalies associated with antithyroid treatment during pregnancy: a meta-analysis. Clinics (Sao Paulo) 2015;70:453–459. doi: 10.6061/clinics/2015(06)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andersen S.L., Olsen J., Laurberg P. Antithyroid drug side effects in the population and in pregnancy. J Clin Endocrinol Metab. 2016;101:1606–1614. doi: 10.1210/jc.2015-4274. [DOI] [PubMed] [Google Scholar]
- 78.Poppe K., Hubalewska-Dydejczyk A., Laurberg P., Negro R., Vermiglio F., Vaidya B. Management of hyperthyroidism in pregnancy: results of a survey among members of the European thyroid association. Eur Thyroid J. 2012;1:34–40. doi: 10.1159/000336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burch H.B., Cooper D.S. Management of graves disease: a review. JAMA. 2015;314:2544–2554. doi: 10.1001/jama.2015.16535. [DOI] [PubMed] [Google Scholar]
- 80.Syed A.A., Jones N.A.G., Perros P. Medical image. Acute pretibial myxoedema following thyroidectomy for Graves’ disease. N Zngl Med J. 2008;121:108–109. [PubMed] [Google Scholar]
- 81.Laurberg P., Wallin G., Tallstedt L., Abraham-Nordling M., Lundell G., Tørring O. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158:69–75. doi: 10.1530/EJE-07-0450. [DOI] [PubMed] [Google Scholar]
- 82.Cooper D.S., Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1:238–249. doi: 10.1016/S2213-8587(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 83.Azizi F. Effect of methimazole treatment of maternal thyrotoxicosis on thyroid function in breast-feeding infants. J Pediatr. 1996;128:855–858. doi: 10.1016/s0022-3476(96)70342-6. [DOI] [PubMed] [Google Scholar]
- 84.Azizi F. Thyroid function in breast-fed infants is not affected by methimazole-induced maternal hypothyroidism: results of a retrospective study. J Endocrinol Invest. 2003;26:301–304. doi: 10.1007/BF03345176. [DOI] [PubMed] [Google Scholar]
- 85.Lamberg B., Ikonen E., Osterlund K., Teramo K., Pekonen F., Peltola J. Antithyroid treatment of maternal hyperthyroidism during lactation. Clin Endocrinol (Oxf) 1984:81–87. doi: 10.1111/j.1365-2265.1984.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 86.Glatstein M.M., Garcia-Bournissen F., Giglio N., Finkelstein Y., Koren G. Pharmacologic treatment of hyperthyroidism during lactation. Can Fam Physician. 2009;55:797–798. [PMC free article] [PubMed] [Google Scholar]
- 87.Garber J., Cobin R., Gharib H., Hennessey J., Klein I., Mechanick J. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of clinical endocrinologists and the American thyroid association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 88.Neill A.-M.C., Nelson-Piercy C. Hyperemesis gravidarum. Obstet Gynaecol. 2003;5:204–207. doi: 10.1111/j.1471-0528.1994.tb13053.x. [DOI] [PubMed] [Google Scholar]
- 89.Body C., Christie J.A. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016:267–283. doi: 10.1016/j.gtc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 90.Coulon A.L., Savagner F., Briet C., Vernin M., Munier M., Chabre O. Prolonged and severe gestational thyrotoxicosis due to enhanced hCG sensitivity of a mutant thyrotropin receptor. J Clin Endocrinol Metab. 2016;101:10–11. doi: 10.1210/jc.2015-3670. [DOI] [PubMed] [Google Scholar]
- 91.Rodien P., Brémont C., Sanson M.L., Parma J., Van Sande J., Costagliola S. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823–1826. doi: 10.1056/NEJM199812173392505. [DOI] [PubMed] [Google Scholar]
- 92.Matthews D.C., Syed A.A. The role of TSH receptor antibodies in the management of Graves’ disease. Eur J Intern Med. 2011;22:213–216. doi: 10.1016/j.ejim.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Davis L.E., Lucas M.J., Hankins G.D., Roark M.L., Cunningham F.G. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63–70. doi: 10.1016/0002-9378(89)90088-4. [DOI] [PubMed] [Google Scholar]
- 94.Khoo C.M., Lee K.O. Endocrine emergencies in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:885–891. doi: 10.1016/j.bpobgyn.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Sullivan S.A., Goodier C. Endocrine emergencies. Obstet Gynecol Clin North Am. 2013;40:121–135. doi: 10.1016/j.ogc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 96.Radetti G., Zavallone A., Gentili L., Beck-Peccoz P., Bona G. Foetal and neonatal thyroid disorders. Minerva Pediatr. 2002;54:383–400. [PubMed] [Google Scholar]
- 97.Donnelly M.A., Wood C., Casey B., Hobbins J., Barbour L.A. Early severe fetal Graves disease in a mother after thyroid ablation and thyroidectomy. Obstet Gynecol. 2015;125:1059–1062. doi: 10.1097/AOG.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 98.Alamdari S., Azizi F., Delshad H., Sarvghadi F., Amouzegar A., Mehran L. Management of hyperthyroidism in pregnancy: comparison of recommendations of american thyroid association and endocrine society. J Thyroid Res. 2013;2013:878467. doi: 10.1155/2013/878467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poppe K., Velkeniers B., Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab. 2008;4:394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 100.Chen X., Jin B., Xia J., Tao X., Huang X., Sun L. Effects of thyroid peroxidase antibody on maternal and neonatal outcomes in pregnant women in an iodine-sufficient area in China. Int J Endocrinol. 2016;2016:6461380. doi: 10.1155/2016/6461380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thangaratinam S., Tan A., Knox E., Kilby M.D., Franklyn J., Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011;342 doi: 10.1136/bmj.d2616. d2616–d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Negro R., Greco G., Mangieri T., Pezzarossa A., Dazzi D., Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007;92:1263–1268. doi: 10.1210/jc.2006-1821. [DOI] [PubMed] [Google Scholar]
- 103.Fan Y., Xu S., Zhang H., Cao W., Wang K., Chen G. Selenium supplementation for autoimmune thyroiditis: a systematic review and meta-analysis. Int J Endocrinol. 2014;2014:904573. doi: 10.1155/2014/904573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilder R.L. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 105.Kyriacou A., Salazar L.V., Ghattamaneni S. Lymph nodes close to the thyroid isthmus can masquerade as malignant thyroid nodules in chronic lymphocytic thyroiditis. Int J Clin Endocrinol Metab. 2012;1:37–39. [Google Scholar]
- 106.Le Donne M., Settineri S., Benvenga S. Early pospartum alexithymia and risk for depression: relationship with serum thyrotropin, free thyroid hormones and thyroid autoantibodies. Psychoneuroendocrinology. 2012;37:519–533. doi: 10.1016/j.psyneuen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Harris B., Othman S., Davies J.A., Weppner G.J., Richards C.J., Newcombe R.G. Association between postpartum thyroid dysfunction and thyroid antibodies and depression. BMJ. 1992;305:152–156. doi: 10.1136/bmj.305.6846.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergink V., Kushner S.A., Pop V., Kuijpens H., Lambregtse-van den Berg M.P., Drexhage R.C. Prevalence of autoimmune thyroid dysfunction in postpartum psychosis. Br J Psychiatry. 2011;198 doi: 10.1192/bjp.bp.110.082990. [DOI] [PubMed] [Google Scholar]
- 109.Engum A., Bjøro T., Mykletun A., Dahl A.A. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J Psychosom Res. 2005;59:263–268. doi: 10.1016/j.jpsychores.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Harris B., Oretti R., Lazarus J., Parkes A., John R., Richards C. Randomised trial of thyroxine to prevent postnatal depression in thyroid-antibody-positive women. Br J Psychiatry. 2002;180 doi: 10.1192/bjp.180.4.327. [DOI] [PubMed] [Google Scholar]
- 111.Nicholson W.K., Robinson K.A., Smallridge R.C., Ladenson P.W., Powe N.R. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573–582. doi: 10.1089/thy.2006.16.573. [DOI] [PubMed] [Google Scholar]
- 112.Lazarus J.H., Ammari F., Oretti R., Parkes A.B., Richards C.J., Harris B. Clinical aspects of recurrent postpartum thyroiditis. Br J Gen Pract. 1997;47:305–308. [PMC free article] [PubMed] [Google Scholar]
- 113.Negro R., Beck-Peccoz P., Chiovato L., Garofalo P., Guglielmi R., Papini E. Hyperthyroidism and pregnancy. An Italian Thyroid Association (AIT) and Italian Association of Clinical Endocrinologists (AME) joint statement for clinical practice. J Endocrinol Invest. 2011;34:225–231. doi: 10.1007/BF03347071. [DOI] [PubMed] [Google Scholar]