Highlights
-
•
CKD-MBD can lead to bone fragility, either with high bone turnover or low bone turnover.
-
•
Adynamic bone disease is characterized by a low-bone-turnover state.
-
•
Bone biopsy remains the diagnostic gold standard for adynamic bone disease.
-
•
Aggressive PTH suppression can increase the risk of this disease process.
-
•
Novel, anabolic agents may play a therapeutic role in ABD management.
Keywords: Chronic kidney disease-mineral and bone disorders, Adynamic bone disease, Anabolic osteoporosis therapy
Abstract
The Kidney Disease: Improving Global Outcomes (KDIGO) work group released recommendations in 2006 to define the bone-related pathology associated with chronic kidney disease as renal osteodystrophy. In 2009, KDIGO released revised clinical practice guidelines which redefined systemic disorders of bone and mineral metabolism due to chronic kidney disease as chronic kidney disease-mineral and bone disorders. Conditions under this overarching term include osteitis fibrosa cystica, osteomalacia, and adynamic bone disease. We aim to provide a brief review of the histopathology, pathophysiology, epidemiology, and diagnostic features of adynamic bone disease, focusing on current trends in the management of this complex bone disorder.
Introduction
Patients with end-stage renal disease (ESRD) requiring hemodialysis have an increased fracture risk, where the incidence of hip fractures in one study was fourfold greater in ESRD patients on dialysis when compared to non-dialysis patients [1]. Chronic kidney disease (CKD) is associated with various bone-related pathologies, each with unique histopathological findings. In spite of their varied underlying processes, the clinical presentations of the diseases which comprise chronic kidney disease-mineral and bone disorders (CKD-MBD) are oftentimes similar [2]. Patients with adynamic bone disease (ABD), a condition of low-bone turnover, and those with osteitis fibrosa cystica (OFC), a condition of high-bone turnover, are typically asymptomatic at the time of their diagnosis, though either can present with fragility fractures.
Histopathology
ABD is characterized by markedly low-bone turnover resulting from a reduced number of osteoclasts and osteoblasts without osteoid accumulation [3]. This distinguishes ABD from OFC, which in contrast, is a disorder characterized by increased bone turnover with a resulting increase in bone formation and resorption [4] [Fig. 1].
Figure 1.
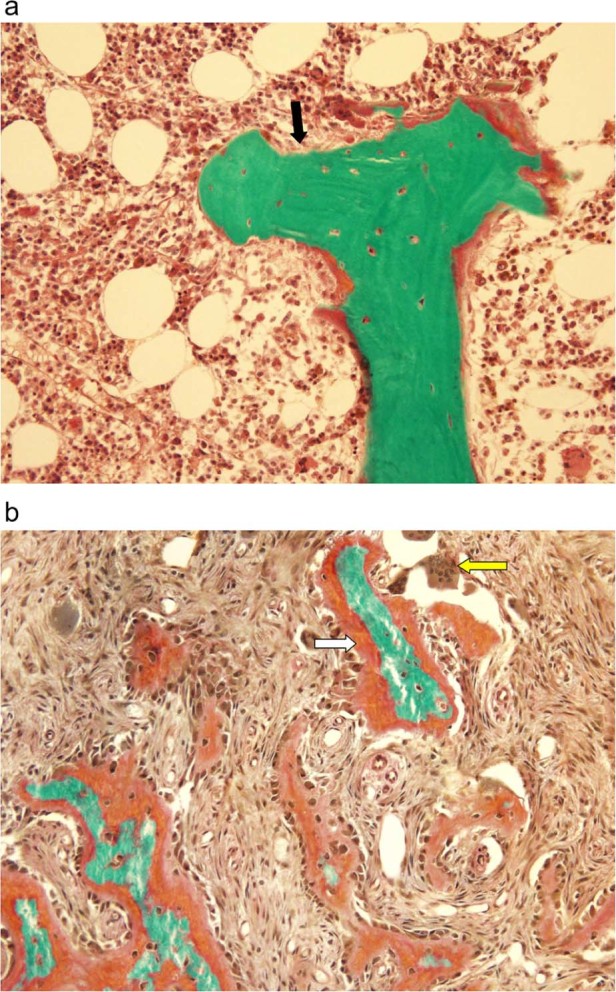
a: Adynamic bone disease. Depicts a classic bone biopsy featuring minimal osteoid (black arrow) and nearly no activity of osteoblasts or osteoclasts.
Image courtesy of Avudai Maran, PhD and Bart Clarke, MD – Biomaterials and Histomorphometry Core Lab, Mayo Clinic, Rochester, MN. b: Osteitis fibrosa cystica. Characterized by thick osteoid production (white arrow) with increased osteoclast activity (yellow arrow). Image courtesy of Avudai Maran, PhD and Bart Clarke, MD – Biomaterials and Histomorphometry Core Lab, Mayo Clinic, Rochester, MN. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Pathophysiology
The underlying pathophysiology of ABD is both intricate and multi-factorial. Fundamentally, ABD is due to either the resistance of parathyroid hormone (PTH) on bone metabolism or the oversuppression of PTH release, though several events precede this outcome. One of the earliest biomarkers of CKD appears to be a decreased expression in α-Klotho, which is a co-receptor of the phosphatonin Fibroblast growth factor 23 (FGF-23) [5]. This peptide is secreted by osteocytes and osteoblasts in response to elevated phosphate and calcitriol levels thus allowing for increased excretion of phosphate [6]. The resistance to FGF-23 by the decreased expression of α-Klotho leads to an increase in FGF-23 production, a decrease in calcitriol, relative hypocalcemia, and secondary hyperparathyroidism [5], [7]. This cascade eventually results in PTH receptor down-regulation with subsequent skeletal resistance to PTH action [8].
Oversuppression of PTH is an additional hallmark of ABD. While very high levels of PTH are associated with an increase in both fracture risk and mortality, normalizing the secondary hyperparathyroidism associated with early and late-stage CKD can also lead to similar increases in morbidity [9]. Patients with CKD and intact parathyroid hormone (iPTH) levels <195 pg/mL have been found to have a 22% increased risk of fractures as studied retrospectively by Atsumi et al. [10]. Hence, dietary phosphate restriction, the use of calcium-based phosphate binders, activated vitamin D analogs, and calcimimetics can all trigger PTH suppression thus creating a low-bone-turnover state [8], [11].
Epidemiology
Of the disorders which comprise CKD-MBD, ABD is by far the most prevalent and should therefore be at the forefront of metabolic bone disease management. In patients with CKD stages 3–5, there is an 18% prevalence of ABD [2]. This low-bone-turnover disease is seen in 50% of patients with CKD-MBD who receive peritoneal dialysis and in 19% of CKD-MBD patients receiving hemodialysis [2], [8], [12]. Several direct and indirect risk factors for ABD exist and include the use of calcium-based phosphate binders, activated vitamin D analogs, calcimimetics, peritoneal dialysis, high-calcium dialysate, glucocorticoid-induced osteoporosis, bisphosphonate use, diabetes, post-menopausal state, hypogonadism, increased age, malnutrition, parathyroidectomy, and systemic inflammation [2], [3], [4], [5], [6], [7], [8], [12]. Of the aforementioned risk factors, the increased global prevalence of diabetes and ESRD, as well as the aggressive treatment of secondary hyperparathyroidism have greatly contributed to the growing prevalence of ABD over the past 20 years [3].
Diagnosis
Bone biopsy remains the diagnostic gold standard in distinguishing a disease of low-bone turnover such as ABD from other bone-related pathologies such as OFC which is characterized by a marked increase in bone turnover and remodeling [4]. Guidelines set forth by the KDIGO work group as well as the National Kidney Foundation: Kidney Disease Outcomes Quality Initiative (NKF KDOQI) recommend performing a bone biopsy in patients with CKD who also possess one or more of the following conditions [2], [3], [13]:
-
•
Patients with iPTH levels between 100 and 500 pg/mL with unexplained hypercalcemia, bone pain, or increased bone-specific alkaline phosphatase (BSAP)
-
•
Fragility fractures not explained by additional etiologies such as malignancy
-
•
Prior to initiating bisphosphonate therapy
-
•
Unexplained hypercalcemia
-
•
Suspected aluminum toxicity
-
•
Severe vascular calcification
Serological markers, though unable to solely confirm the diagnosis of ABD, can be helpful in guiding clinicians who are unsure of whether or not to proceed with a bone biopsy. This especially holds true in the case of an asymptomatic patient, as persistent bone pain and fragility fractures are seen in only a minority of patients at the time of their initial diagnosis [8]. Patients with PTH levels <150 pg/mL have a 97% positive predictive value for ABD, whereas PTH levels >450 pg/mL have a 100% positive predictive value for OF [14]. One serum marker directly implicated in assessing bone formation is BSAP [8]. BSAP of >20 ng/mL virtually excludes the diagnosis of ABD particularly when patients have a concurrent PTH >200 pg/mL. Patients with BSAP <12.9 ng/mL can further bolster the diagnosis of ABD as this is 100% sensitive and 93.7% specific [8], [15], [16].
Management
In spite of its underlying complexity, the traditional therapies geared toward managing ABD are firmly rooted in the prevention of CKD progression, management of ABD risk factors such as diabetes, and decreasing calcium and vitamin D load so as to relax PTH suppression in order to re-establish its activity [3], [8].
Relaxing PTH suppression
As discussed earlier, several factors are involved in the suppression of PTH which can ultimately lead to ABD in patients with CKD. Though the most favorable PTH level to maintain in patients with CKD stages 3–5 is currently not known, the 2009 KDIGO Guidelines suggest maintaining iPTH levels for patients with CKD stage 5D between 2 and 9 times the upper limit of normal for the assay utilized [2].
The administration of activated vitamin D analogs can cause decreased bone turnover in patients with CKD. In a 1994 study by Goodman et al., 6 out of 14 study participants with end-stage renal disease developed biopsy-proven ABD following the administration of high dose calcitriol [17]. Therefore, limiting the use of calcitriol can assist in alleviating PTH oversuppression which is a driving force for the development of ABD. Similarly, the use of calcimimetics should be avoided in patients who have developed this low-bone-turnover disease seeing that these medications also serve to suppress PTH activity [8]. Consideration should also be given toward switching patients with ESRD receiving hemodialysis from a calcium-containing phosphate binder to a non-calcium-containing phosphate binder, as this could assist in relaxing PTH suppression and increase the rate of bone formation. In a study of 68 patients with ESRD on hemodialysis, Ferreira et al. showed that patients receiving sevelamer experienced an increase in bone formation rate per bone surface from their baseline when compared to patients receiving calcium carbonate [18]. Also pertinent for patients undergoing dialysis is the use of low-calcium dialysate. A 2006 prospective study by Haris et al. revealed that the use of a low-calcium dialysate in 51 patients with biopsy-proven ABD receiving peritoneal dialysis not only lowered levels of serum ionized calcium, but led to an increase in PTH and a significant improvement in bone turnover [19].
Treatment with teriparatide
Teriparatide (PTH 1–34), which has been FDA approved to treat post-menopausal osteoporosis, may have a growing role in the treatment of ABD. Seeing that ABD is a disease process caused by both PTH resistance and relative PTH deficiency, it would seem that the use of this PTH analog could be beneficial in improving bone-related outcomes.
An English-language literature search for teriparatide and ABD revealed 4 individual studies where a total of 17 patients with biopsy-proven ABD received teriparatide therapy [20], [21], [22], [23]. The results from these studies are promising in that patients receiving teriparatide not only sustained increases to their PTH levels, but saw improvement in their bone mineral density (BMD) as measured on Dual-energy X-ray Absorptiometry (DXA) in all 4 studies [20], [21], [22], [23]. Though these studies speculate that increased BMD may also result in a decreased fracture risk, no supportive data are currently available.
Pipeline anabolic therapies
The future of ABD treatment is a promising area for research, as several anabolic therapies currently in development for the treatment of post-menopausal osteoporosis could also potentially offer novel therapies for ABD.
Abaloparatide
Parathyroid hormone-related peptide (PTHrP) activates the PTH type 1 receptor, which when done intermittently, can lead to bone formation and increased osteoblast activity. Abaloparatide is a fragment of PTHrP (1–34) which has been shown to exert these aforementioned effects in both Phase II and Phase III trials in post-menopausal women with osteoporosis [24]. In a 2015 multi-center, multi-national, double-blind, placebo-controlled trial by Leder et al., post-menopausal women with osteoporosis who received 80 mcg of abaloparatide daily for 24 weeks experienced a 6.7% increase in the BMD of their lumbar spine and a 2.6% increase in the BMD of their total hip [25]. Furthermore, their risk of sustaining a major osteoporotic fracture was reduced by 67% when compared to teriparatide [26]. Given these findings, one could speculate that patients with ABD could sustain similar increases in bone formation with improvements to their BMD, as well as a reduction to their fracture risk with abaloparatide use.
Romosozumab
The wingless in Drosophila and integrated in vertebrate (Wnt) signaling pathway is a crucial regulator of osteoblast recruitment. Sclerostin is an endogenous inhibitor of this pathway, thereby inhibiting osteoblast recruitment and decreasing bone formation [27]. The use of romosozumab, a sclerostin antibody which possesses anabolic properties, is currently in Phase III trials for the treatment of post-menopausal osteoporosis. Based on pooled clinical trial data, the use of a monthly, 210-mg, subcutaneous dose of romosozumab resulted in an 11.3% increase in the BMD of the lumbar spine and a 4.1% increase in the BMD of the total hip [24]. Based on the marked improvements noted in BMD for the treatment of post-menopausal osteoporosis, similar success in using romosozumab to improve bone density in patients with ABD may be promising.
Ronacaleret
Endogenous PTH release can be stimulated when calcium-sensing receptor (CASR) activity is inhibited. Ronacaleret is a calcilytic which allows for bone formation by serving as a CASR antagonist, thus increasing endogenous production of PTH [28]. In a 2012 randomized, placebo-controlled, dose-ranging trial by Fitzpatrick et al., the effects of ronacaleret on volumetric BMD (vBMD) as measured by quantitative computed tomography, was studied in women with post-menopausal osteoporosis, as compared to both teriparatide and alendronate [28]. Patients treated with teriparatide saw an improvement in vBMD of both the lumbar and trabecular spine, with increases of 14.8% and 24.4%, respectively. Ronacaleret, administered at a 400-mg daily dose for 12 months, was associated with a 3.9% increase in vBMD in the lumbar spine and a 13.3% increase in the trabecular spine, though, a 1.79% decrease in the cortical femur vBMD was observed at this dose. This, however, was not seen with teriparatide. The decrease at cortical sites could be attributed to the duration of the increase in PTH production. This could prove to be a limiting factor in the development of these agents. Regardless, it is interesting to consider the potential benefits of such a therapy on ABD.
Conclusion
ABD is a complex disease process which, given its increasing prevalence and associated morbidity, requires careful diagnosis and management. This disorder is characterized by histopathological findings of low bone turnover and pathophysiological features of both PTH resistance and oversuppression. The management of underlying ABD risk factors is paramount, particularly with the increased incidence of diabetes, ESRD, and aggressive treatment of secondary hyperparathyroidism. In addition to controlling risk factors and balancing the risks and benefits of PTH suppression, the use of novel anabolic bone therapies could provide clinicians with new therapeutic options to potentially improve bone-related outcomes in patients with this unique disease process.
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Maravic M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;1:159–165. doi: 10.1007/s00198-013-2435-1. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Chapter 4.3. Kidney Int. 2009;76(Suppl. 113):90–99. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 3.Brandenburg V., Floege J. Adynamic bone disease – bone and beyond. NDT Plus. 2008;3:135–137. doi: 10.1093/ndtplus/sfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sprague S. The role of the bone biopsy in the diagnosis of renal osteodystrophy. Semin Dial. 2000;13(3):152–155. doi: 10.1046/j.1525-139x.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuro-o M. Phosphate and klotho. Kidney Int. 2011;79(121):S20–3. doi: 10.1038/ki.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juppner H. Phosphate and FGF-23. Kidney Int. 2011;79(121):524–527. doi: 10.1038/ki.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drueke T. Endotext online endocrinology book. 2015. Hyperparathyroidism in chronic kidney disease. [Google Scholar]
- 8.Bover J., Ureña P., Brandenburg V. Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin Nephrol. 2014;34(6):626–640. doi: 10.1016/j.semnephrol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Block G., Hulbert-Shearon T.E., Levin N.W. Association of serum phosphorous and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 10.Atsumi K., Kushida K., Yamazaki K. Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis. 1999;33:287–293. doi: 10.1016/s0272-6386(99)70302-1. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E., Shoback D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab. 2013;27:373–384. doi: 10.1016/j.beem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Ng A., Omelon S., Variola F. Adynamic bone decreases bone toughness during aging by affecting mineral and matrix. J Back Musculoskelet Rehabil. 2016;31(2):369–379. doi: 10.1002/jbmr.2702. [DOI] [PubMed] [Google Scholar]
- 13.Noordzij M., Korevaar J.C., Boeschoten E.W. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46(5):925–932. doi: 10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez M.C., Bajo A., Selgas R. Parathormone secretion in peritoneal dialysis patients with adynamic bone disease. Am J Kidney Dis. 2000;36:953–961. doi: 10.1053/ajkd.2000.19093. [DOI] [PubMed] [Google Scholar]
- 15.Ureña P., Hruby M., Ferreira A. Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol. 1996;7:506–512. doi: 10.1681/ASN.V73506. [DOI] [PubMed] [Google Scholar]
- 16.Coen G., Ballanti P., Bonucci E. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant. 1998;13(9):2294–2302. doi: 10.1093/ndt/13.9.2294. [DOI] [PubMed] [Google Scholar]
- 17.Goodman W.G., Ramirez J.A., Belin T.R. Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int. 1994;46(4):1160–1166. doi: 10.1038/ki.1994.380. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira A., Frazão J.M., Monier-Faugere M.C. Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol. 2008;19(2):405–412. doi: 10.1681/ASN.2006101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haris A., Sherrard D., Hercz G. Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int. 2006;70:931–937. doi: 10.1038/sj.ki.5001666. [DOI] [PubMed] [Google Scholar]
- 20.Cejka D., Kodras K., Bader T., Haas M. Treatment of hemodialysis-associated adynamic bone disease with teriparatide (PTH1-34): a pilot study. Kidney Blood Press Res. 2010;33:221–226. doi: 10.1159/000316708. [DOI] [PubMed] [Google Scholar]
- 21.Palcu P., Dion N., St-Marie L.G. Teriparatide and bone turnover and formation in a hemodialysis patient with low-turnover bone disease: a case report. Am J Kidney Dis. 2015;65(6):933–936. doi: 10.1053/j.ajkd.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Mitsopoulos E., Ginikopoulou E., Economidou D. Impact of long-term cinacalcet, ibandronate or teriparatide therapy on bone mineral density of hemodialysis patients: a pilot study. Am J Nephrol. 2012;36:238–244. doi: 10.1159/000341864. [DOI] [PubMed] [Google Scholar]
- 23.Giamalis P., Economidou D., Dimitriadis C. Treatment of adynamic bone disease in hemodialysis patient with teriparatide. Clin Kidney J. 2015;8:188–190. doi: 10.1093/ckj/sfv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harslof T., Langdahl B.L. New horizons in osteoporosis therapies. Curr Opin Pharmacol. 2016;28:38–42. doi: 10.1016/j.coph.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Leder B.Z., O'Dea L.S., Zanchetta J.R. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 26.Mallarkey G., Reid D. Osteoporosis therapeutics: recent developments at ASBMR. Ther Adv Musculoskelet Dis. 2016;8(1):3–7. doi: 10.1177/1759720X15623489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLung M., Frauer A., Boonen S. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick L.A., Dabrowski C.E., Cicconetti G. Ronacaleret, a calcium-sensing receptor antagonist increases trabecular but not cortical bone in postmenopausal women. J Bone Miner Res. 2012;27(2):255–262. doi: 10.1002/jbmr.554. [DOI] [PubMed] [Google Scholar]


