Highlights
-
•
This is the first study in youth Hispanic with T2D born and raised in Latin America.
-
•
Insulin resistance is the main pathophysiologic characteristic in this target group.
-
•
Therapeutic target should focus on insulin resistance.
Keywords: Type 2 diabetes, Pancreatic beta cells, Insulin resistance, Obesity, Adolescent, Peru
Abstract
Objective
To characterize and compare the beta-cell function and insulin resistance among Peruvian adolescents with type 2 diabetes (T2D) and their non-diabetic, overweight and lean peers.
Methods
Cross-sectional study of 54 adolescents aged 10–19 years, distributed in three sex- and age-matched groups (n = 18): (i) adolescents with T2D; (ii) overweight adolescents without T2D; and (iii) lean adolescents without T2D, at the Diabetes, Obesity and Nutrition Research Center in Lima, Peru. Fasting glucose, insulin, C-peptide, and glycated hemoglobin were measured for all participants. In addition, a two-hour oral glucose tolerance test (OGTT, 1.75 mg of glucose/kg body weight) was performed, during which glucose and C-peptide were quantified. The homeostasis model assessment of insulin resistance (HOMA-IR) and beta-cell function (HOMA-B) were derived for all participants, and beta-cell function was further examined by the area under the curve (AUC) of C-peptide.
Results
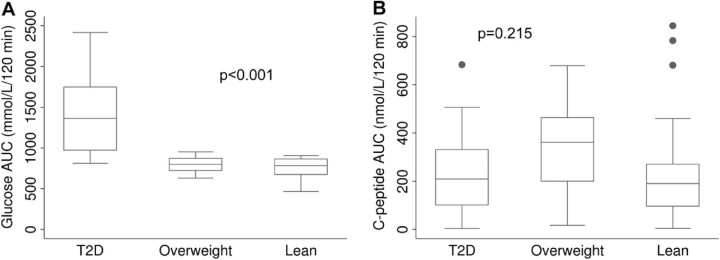
The median HOMA-IR score was higher in adolescents with T2D compared to lean adolescents (6.1 vs. 2.1; p = 0.002), but was not different from that of overweight adolescents (6.1 vs. 4.0; p = 0.322). The median HOMA-B was higher in overweight adolescents than in lean adolescents (256.9 vs. 134.2; p = 0.015), and adolescents with T2D (256.9 vs. 119.8; p = 0.011). The mean AUC of glucose in adolescents with T2D was 1.8-fold higher than that of overweight adolescents, and 1.9-fold higher than that of lean adolescents (p < 0.001). Although the median AUC of C-peptide in adolescents with T2D was lower than that of overweight and lean adolescents, this difference was not statistically significant (230.7 vs. 336.6 vs. 267.3 nmol/l120 min, respectively; p = 0.215).
Conclusion
Among Peruvian adolescents with T2D, insulin resistance is the most prominent characteristic, rather than beta-cell dysfunction.
Introduction
Type 2 diabetes (T2D) in youth is a serious public health problem that has exhibited an epidemic growth in recent decades [1], [2]. Pediatric T2D is associated with both acute and chronic complications [3], which occur more prematurely than in pediatric type 1 diabetes [4], [5]. Although initially noted in affluent settings [6], the rise in pediatric T2D has spread to low- and middle-income countries (LMICs) around the world [7]. The increased vulnerability of several of the ethnic groups living in LMICs, combined with the current epidemic of childhood obesity, will likely make the contribution of LMICs to the global T2D caseload significant. Indeed, about 80% of the 370 million of patients with diabetes will live in LMICs by 2030 [8].
Despite the importance of pediatric T2D in LMICs, the pathogenesis of T2D in non-Caucasian adolescents is still unclear [9], preventing the development of effective therapeutic strategies for these groups. In general, T2D is thought to arise from beta-cell dysfunction and increased insulin resistance [10], [11], [12], [13], usually resulting from obesity [14]. However, the relative contribution of insulin resistance and beta-cell dysfunction to the pathogenesis of T2D in adolescents is uncertain [9], partly due to the complex metabolic changes occurring in puberty [15], [16]. Furthermore, ethnic variations in glucose metabolism exist [17], [18], and add to the uncertainty about the pathophysiology of T2D in non-Caucasian adolescents.
Delineating the specific metabolic defects underlying T2D in non-Caucasian adolescents is critical for the development of effective therapeutic strategies targeting the specific metabolic defects in each ethnic group. This is apparent in adults, with African-Americans exhibiting a greater response to insulin sensitizers (e.g. thiazolidinediones), given their diminished insulin sensitivity [19].
Hispanic adolescents have a high prevalence of obesity [20], [21], type 2 diabetes [22], [23], and its complications [24]. This increased vulnerability has been linked to both environmental and genetic factors, expressed as a low disposition index [25], and higher insulin resistance than their Caucasian peers [26]. Despite these observations, evidence on ethnic variations in glucose metabolism in Hispanics is very limited [26], particularly for Latin American adolescents. Latin Americans share a common genetic ancestry, which may affect their risk of T2D and the pathogenesis of the disease. Nevertheless, the extent to which this genetic background affects the role of insulin resistance and impaired beta-cell function in the pathogenesis of T2D in Latin American adolescents is currently unknown.
In this context, we aimed to assess the beta-cell function and insulin resistance in three groups of adolescents aged 10–19 years in Peru: adolescents with T2D, overweight adolescents without T2D, and lean adolescents without T2D. This evidence will improve our understanding of the pathogenesis of diabetes in Latin American adolescents and, more importantly, it will contribute to tailor effective and safe therapeutic strategies targeting the main metabolic defects underlying diabetes in this population.
Materials and methods
Study design and setting
We conducted a cross-sectional study to assess the metabolic defects in Peruvian adolescents with type 2 diabetes mellitus, by comparing the metabolic parameters of sex- and age-matched patients with T2D, overweight and lean adolescents aged 10–19 years at the Diabetes, Obesity and Nutrition Research Center (CIDON), in Lima, Peru.
Participants
This study included three groups of adolescents between 10 and 19 years, matched by sex and age (within one year), in a 1:1:1 scheme: (i) adolescents with T2D, (ii) overweight or obese adolescents without T2D, and (iii) lean adolescents without T2D. Adolescents with T2D were selected randomly from a list of patients aged 10–19 years, and with T2D attending CIDON. The diagnosis of T2D was based on the diagnostic criteria of the International Society for Pediatric and Adolescent Diabetes, and included: symptoms of diabetes plus casual plasma glucose ≥200 mg/dl, fasting plasma glucose ≥126 mg/dl or two-hour plasma glucose ≥200 mg/dl during an oral glucose tolerance test (OGTT) [27]. For the comparison groups, lean and overweight adolescents fulfilling the eligibility and matching criteria were systematically selected from adolescents consecutively attending CIDON. Overweight was defined as a Body Mass Index (BMI) for age and sex at or above the 85th percentile of the 2000 clinical growth charts of the Center for Disease Control and Prevention [28]. Normal weight was defined as a BMI for age and sex within the 5th and 84th percentiles of the same standard. Adolescents with a diagnosis of with type 1 diabetes and the presence of markers of beta-cell autoimmunity (antibodies against glutamic acid decarboxylase, islet antigen 2, and insulin; and islet cell autoantibodies) were excluded from the study. Participants with active concurrent illnesses were excluded from the study.
Data collection and variables
Metabolic testing took place between January 8th and March 15, 2014 at CIDON. As instructed, participants arrived at CIDON at 08:00 h on their scheduled date, after a 12-hour overnight fast. Insulin treatment was discontinued 48 hours prior to metabolic testing, but oral antidiabetic therapy was not modified, because close follow-up was not possible.
A short survey was conducted to collect basic demographic data (age, sex) and relevant clinical antecedents (family history of diabetes, age at diagnosis and duration of diabetes). Weight and height were measured by experienced health professionals, using a previously-calibrated scale and stadiometer, respectively. Vital signs were checked prior to initiating metabolic testing. A peripheral catheter was placed in the forearm and 4 ml of fasting venous blood was collected for measuring glucose, insulin, HbA1C and C-peptide. Fifteen minutes later, a two-hour oral glucose tolerance test (OGTT) using 1.75 g of glucose per kilogram of body weight (up to a maximum of 75 g) was performed, and C-peptide and glucose were measured at baseline, and 15, 30, 60 and 120 minutes after the glucose load. Glucose, fasting insulin and HbA1C were measured using kinetic methods (ByoSystems, Barcelona, Spain), and C-peptide was measured by radioimmunoassay (C-PEP II-RIA-CT, DIAsource ImmunoAssays S.A., Louvain-la-Neuve, Belgium).
Beta-cell function was assessed by calculating the area under the curve (AUC) of C-peptide levels during an OGTT, using the trapezoidal method [29]. The post-stimulation C-peptide concentrations constitute a widely-accepted, accurate marker of beta-cell function among children and adolescents with T2D [30], [31]. In addition, we analyzed the 15-minute C-peptide concentration during OGTT as a measure of beta-cell function [31]. Finally, we also calculated the Homeostasis Model Assessment of Beta-cell function (HOMA-B) index, which is based on the fasting glucose and insulin levels [32], [33]. This index has been shown to characterize beta-cell function accurately in adolescents [34].
Insulin resistance was measured using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index [32], [33]. The HOMA-IR index has been shown to correlate closely with well-established methods for assessing insulin resistance, including the hyperinsulinemic-euglycemic clamp and minimal models, in patients with T2D and lean and obese children and adolescents without T2D [34], [35], [36], [37].
Statistical analysis
Initially, we evaluated the distribution of variables using numeric and graphic methods, and checked for the presence of outliers. Non-normally distributed, numeric variables were transformed using simple power functions (logarithmic, inverse, square-root, square, cubic, etc.), and their distribution was re-checked after transformation. Variables that were not normalized after transformation were analyzed in their original form, using non-parametric tests. Normally-distributed variables were summarized using the mean and standard deviation (the geometric mean and geometric standard deviation were used for log-transformed numeric variables), and the median and interquartile range was used for non-normally distributed variables. In the bivariate analysis, we compared the distribution of age, anthropometric and metabolic parameters across the three study groups using Analysis of Variance (ANOVA) or the Kruskal–Wallis test, as appropriate. The association between the AUC of C-peptide and the duration of T2D among adolescents with T2D was evaluated using Spearman's correlation coefficient and its corresponding p-value. The assumptions underlying each test were validated prior to the implementation of the tests. Adjustment for multiple comparisons was performed using Hommel's procedure, a modification of Bonferroni's adjustment that is considerably more powerful, and maintains adequate protection against type I errors [38]. Statistical tests were two-sided, and a significance level of 5% was considered relevant. All statistical analyses were conducted using Stata 13.1 for Windows (StataCorp LP, College Station, Texas, United States).
Ethical aspects
Patients and their parents or legal guardians signed a written informed consent prior the onset of the study. This study was approved by the Institutional Review Board of Universidad Peruana Cayetano Heredia, in Lima, Peru.
Results
A total of 54 adolescents aged 10–19 years participated in the study, equally distributed in the three study groups (n = 18). Slightly over half of the adolescents were male (55.5%), and had a mean age (±standard deviation) of 16.2 ± 2.5 years. As expected by the study's matched design, the sex distribution was identical across the three study groups, and there were minimal variations in the age distribution across the groups (owing to caliper matching). The mean BMI of overweight adolescents was higher than that of the lean adolescents (p = 0.002), but was not significantly higher than that of adolescents with T2D (p = 0.081). Adolescents with T2D had a mean age at diagnosis (±standard deviation) of 14.8 ± 3.0 years, and a median disease length (interquartile range) of 9.7 (25.3) months. Half of the adolescents with T2D (n = 9) was obese (BMI for age and sex at or above the 95th percentile), while 27.7% (n = 5) was overweight, but not obese (BMI for age and sex between 85th and 94th percentile). A family history of T2D was reported in 72.2% of adolescents with T2D (n = 13). The characteristics of the three study groups are detailed in Table 1.
Table 1.
Characteristics of the study population
| Characteristic | T2D (n = 18) | OW (n = 18) | LN (n = 18) |
|---|---|---|---|
| Age (years)a | 16.2 ± 2.5 | 16.1 ± 2.8 | 16.4 ± 2.4 |
| Sex | |||
| Male | 10(55.5) | 10(55.5) | 10(55.5) |
| Female | 8(44.5) | 8(44.5) | 8(44.5) |
| Weight(kg)a | 74.9 ± 22.1 | 86.7 ± 24.0 | 62.9 ± 12.9 |
| Height(m)a | 1.6 ± 0.9 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| BMI(kg/m2)a | 28.8 ± 6.5 | 32.3 ± 5.3 | 22.5 ± 2.1 |
| Age at diagnosis(years)a | 14.9 ± 3.1 | N/A | N/A |
| Disease duration(months)b | 9.7(6.5–31.8) | N/A | N/A |
T2D, adolescents with type 2 diabetes; OW, overweight/obese adolescents; LN, lean adolescents; BMI, body mass index; N/A, not applicable.
Mean ± standard deviation.
Median(1st quartile–3rd quartile); Otherwise, n(%).
Mean fasting glucose levels were higher in adolescents with T2D than in overweight adolescents (p = 0.005) or lean adolescents (p = 0.001). Mean fasting insulin levels were higher in adolescents with T2D than in lean adolescents (p = 0.026), but were similar to those in overweight adolescents (p = 0.993). The HbA1C mean was higher in adolescents with T2D than in overweight adolescents (p = 0.001) or lean adolescents (p = 0.001) (Table 2).
Table 2.
Fasting serum glucose, insulin, glycated hemoglobin (HbA1C), 15-minute C-peptide (15-min C-peptide), and Homeostasis Model Assessment for Insulin Resistance Index (HOMA-IR) and β-cell function (HOMA-B)
| Characteristic | T2D (n = 18) | OW (n = 18) | LN (n = 18) | Global p-value |
|---|---|---|---|---|
| Fasting glucose (mmol/l)a | 7.3 ± 2.8 | 5.90 ± 0.4 | 4.6 ± 0.5 | <0.001 |
| Fasting insulin(pmol/l)b | 119.2 ± 2.3 | 121.4 ± 1.8 | 58.2 ± 2.3 | 0.007 |
| HbA1C(%)a | 8.4 ± 2.9 | 5.4 ± 0.4 | 5.4 ± 0.4 | <0.001 |
| 15-min C-peptide(ng/ml)c | 4.5(1.3–6.4) | 6.8(3.3–11.0) | 3.2(0.9–7.8) | 0.388 |
| HOMA-B indexc | 119.8(57.8–347.6) | 256.9(162.1–389.8) | 134.2(98.5–238.0) | 0.015 |
| HOMA-IR indexc | 6.1(3-3-8.1) | 4.0(2.7–6.0) | 2.1(1.4–2.8) | <0.001 |
T2D, adolescents with type 2 diabetes; OW, overweight/obese adolescents; LN, lean adolescents; BMI, body mass index; N/A, not applicable.
Arithmetic mean ± standard deviation.
Geometric mean ± geometric standard deviation.
Median(1st quartile-3rd quartile).
The median C-peptide concentrations at 15 minutes during the OGTT were not statistically different between overweight adolescents (6.8 ng/ml), lean adolescents (3.2 ng/ml) and adolescents with T2D (4.5 ng/ml) (p = 0.388). However, the median (1st quartile–3rd quartile) of the HOMA-B index was higher in overweight adolescents than in lean adolescents [256.9 (162.1–389.8) vs. 134.2 (98.5–238.0); p = 0.015], and adolescents with T2D [256.9 (162.1–389.8) vs. 119.8 (57.8–347.6); p = 0.011]. No difference in the HOMA-B index was found between lean adolescents and adolescents with T2D (134.2 vs. 119.8, p = 0.439) (Table 2).
The median (1st quartile–3rd quartile) of the HOMA-IR scores was higher in adolescents with T2D than in lean adolescents [6.1 (3.3–8.1) vs. 2.1 (1.4–2.8); p = 0.001], but were similar to those in overweight adolescents [6.1 (3.3–8.1) vs. 4.0 (2.7–6.0); p = 0.174] (Table 2 and Fig. 1).
Figure 1.
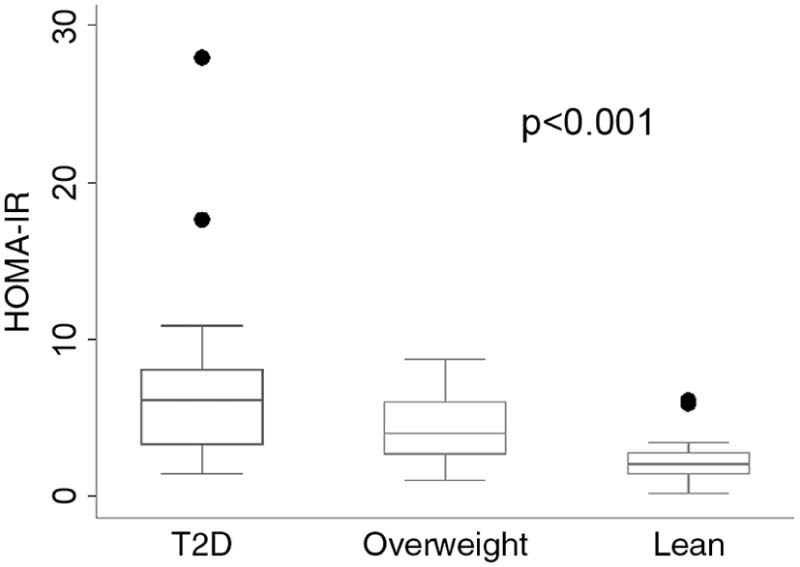
Homeostatic model assessment for insulin resistance (HOMA-IR) in patients with T2D, overweight, and lean (●) adolescents.
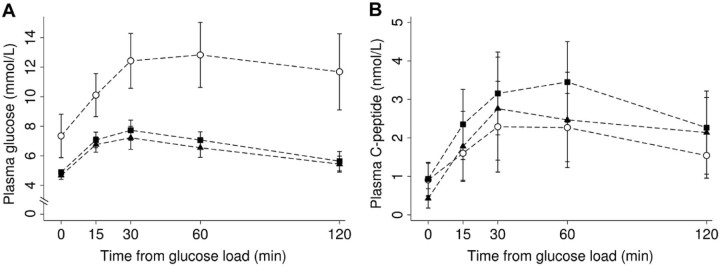
The AUC of glucose during OGTT was higher in adolescents with T2D compared to overweight adolescents and lean adolescents (p < 0.001), but there was no difference between the glucose AUC of lean and overweight adolescents (p = 0.318) (Fig. 2). Regarding the AUC of C-peptide, there was no significant difference between the three study groups (p = 0.215) (Fig. 3). There was no correlation between disease length and AUC of C-peptide (Spearman's correlation coefficient = −0.21; p = 0.415).
Figure 2.
(A) Plasmatic glucose and (B) C-peptide concentrations during the oral glucose tolerance test in patients with T2D (○), overweight (▲), and lean (■) adolescents.
Figure 3.
(A) Box-and-whisker plots of the area under the curve (AUC)* of plasmatic glucose and (B) C-peptide during the oral glucose tolerance test in patient with T2D, overweight, and lean adolescents.
Discussion
Main findings
The present study shows that insulin resistance is the most prominent characteristic, rather than beta-cell dysfunction, among Peruvian adolescents with T2D. The median HOMA-IR score was higher in adolescents with T2D compared to lean adolescents (6.1 vs. 2.1; p = 0.002), but it was not different from overweight adolescents (6.1 vs. 4.0; p = 0.322); and although the median AUC of C-peptide in adolescents with T2D was lower than overweight and lean adolescents, this difference was not statistically significant (230.7 vs. 336.6 vs. 267.3 nmol/l/120 min, respectively; p = 0.215).
It is not feasible to assess beta-cell mass, but it is possible to assess beta-cell function by glucose tolerance test. In our study, the most outstanding findings were that the AUC of glucose during OGTT was significantly higher in adolescents with T2D than in lean adolescents without T2D, while the AUC of C-peptide were not significant among the three study groups, that beta cell dysfunction was not significant different in these three groups.
The mean of HbA1c of the participants in the study was 8.4% ± 2.9, which is lower compared to a study of the International Diabetes Federation (8.9 ± 0.6) [27], while it was higher a recent one done in Caucasians and African Americans (6.6 ± 1.0) [9]. The possible factors that might explain these differences in Hba1c, as a metabolic control marker, may be related to the genetic predisposition for insulin resistance in the pathogenesis of T2D in different population groups.
Role of race on insulin resistant and beta-cell dysfunction
Race and genetics are important factors to consider as potential determinants of the pathophysiology of T2D in youth. The genetic contribution to the pathophysiology of T2D are based on several identified genetic polymorphisms linked to T2D in youth, such as CC polymorphism of Apolipoprotein A-II (APOA-II) [39]. Also, a recent publication has identified SLC16A11, as a novel candidate gene for developing T2D, in Mexican people which is also possible may have a role in triacylglycerol metabolism; findings that confirm the important role of genetics in the development of T2D predisposition [40].
Different studies have aimed to identify the main processes in the T2D pathophysiology in youth, but most of these studies have included children and adolescents in North-Americas. They showed that a rapid deterioration of beta cell function over time is the principal characteristic in T2D in youth, with no significant change in insulin sensitivity [9], [41], [42]. Expanding on these findings, the TODAY trial [43] showed that metformin and lifestyle modification are insufficient to maintain an adequate glucose balance in adolescents with T2D, probably because insulin resistance is not the predominant characteristic at the onset of diabetes.
Opposite to our findings, a multi-ethnic study [44] showed that obese adolescents with normal glucose tolerance who progressed to Impaired Glucose tolerance (IGT) had a normal beta-cell function at the beginning of the study, proposing insulin deficiency as the main mechanism of action. Despite of its prospective nature, the study is limited in that it evaluates a heterogeneous, multi-ethnic group, and did not analyze the progression to IGT in Hispanics.
Regarding studies of T2D in Hispanic youth, a community-based study in Mexico found a high prevalence of pre-diabetes in children, and a higher prevalence of T2D among the obese [45]. However, the majority of the studies about T2D in Hispanic children and adolescents have only included children and adolescents of Hispanic descent who were raised (and even born) in developed countries. As stated above, this is important because the Latino adolescents born and raised in a South American setting may possess unique genetic and environmentally- determined characteristics that could influence the pathogenesis of T2D. Nevertheless, previous studies have reported that Hispanic children have greater insulin resistance than Caucasian, and, that the second phase insulin secretion is greater in Hispanic than in African-American youth [18]. In addition, a high prevalence of obesity and metabolic syndrome reflected by a high HOMA-IR was found in Latin American youth living in Spain (compared to Caucasian Spanish youth) [46]. Also, it has been found a negative correlation between adiponectin and insulin resistance among Mexican youth, findings that could be linked to their genetic background [47]. A possible reason of these findings can be related to the fact that Hispanics are genetically linked to the Pima Indians of Arizona, in whom the natural history of T2D starts with insulin resistance, which is later followed by an impairment in insulin secretion [48], [49].
This study expands the existing literature by focusing in adolescents born and raised in a South American country. Evidence on the pathophysiology of T2D is limited in this group, despite the potential influence exerted by genetic and environmental factors unique to this population. We believe it is important to understand the pathophysiology of T2D in youth because several metabolic changes occurring in puberty render its treatment challenging, including the reduction in insulin sensitivity secondary to an increase secretion of growth hormone [50], testosterone, estrogens and an increase of fat mass [51]. This challenge is even greater in LMICs, such as Peru, as adolescents with T2D may exhibit a poor metabolic control and are overweight at the time of the diagnosis [52].
Limitations and strengths
The cross-sectional nature of the study may constitute a limitation, as many cases will have established disease, and some associations may be confounded by disease duration; however, the study results are important (albeit preliminary) because this is a novel study done with patients born and raised in a Latin American country. Second, selection bias could have resulted from the selection of participants in a private research center, instead of primary care facilities or the population. The strength of this study was based on the standardized assessment conducted by one healthcare staff in order achieves an accurate diagnosis of the three groups studied, as well as the use of one of the main diabetes research centers in Lima to perform the study. Another strength is that we have considered lean adolescents as an additional group, who were matched with adolescents with T2D and overweight adolescents. This addition allows a better assessment of the role of both overweight and diabetes on glucose metabolism. In addition to that, we determined that all T2D adolescents did not have T1D, did not have clinical suspicion of maturity onset diabetes of the young (MODY) as well as other hybrid diabetes states.
Conclusion
Insulin resistance is the main characteristic in adolescents diagnosed with T2D born and raised in a Latino country, such as Peru. Improving insulin resistance, through diet and pharmacologic treatment in this target group, may contribute to have an adequate control of T2D.
Funding
Funding awarded by School of Medicine of the Universidad Peruana Cayetano Heredia.
Conflicts of interest
The authors declare they have no conflicts of interest.
References
- 1.Cali A.M., Caprio S. Prediabetes and type 2 diabetes in youth: an emerging epidemic disease? Curr Opin Endocrinol Diabetes Obes. 2008;15(2):123–127. doi: 10.1097/MED.0b013e3282f57251. [DOI] [PubMed] [Google Scholar]
- 2.Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23(3):381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O., Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369(9575):1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 4.Dart A.B., Martens P.J., Rigatto C., Brownell M.D., Dean H.J., Sellers E.A. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37(2):436–443. doi: 10.2337/dc13-0954. [DOI] [PubMed] [Google Scholar]
- 5.Eppens M.C., Craig M.E., Cusumano J., Hing S., Chan A.K., Howard N.J. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 6.Fagot-Campagna A., Pettitt D.J., Engelgau M.M., Burrows N.R., Geiss L.S., Valdez R. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 7.Pinhas-Hamiel O., Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 8.The diabetes pandemic. Lancet. 2011;378(9786):99. doi: 10.1016/S0140-6736(11)61068-4. [DOI] [PubMed] [Google Scholar]
- 9.Bacha F., Gungor N., Lee S., Arslanian S.A. Progressive deterioration of beta-cell function in obese youth with type 2 diabetes. Pediatr Diabetes. 2013;14(2):106–111. doi: 10.1111/j.1399-5448.2012.00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Adamo E., Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34(Suppl. 2):S161–5. doi: 10.2337/dc11-s212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss R., Taksali S.E., Caprio S. Development of type 2 diabetes in children and adolescents. Curr Diab Rep. 2006;6(3):182–187. doi: 10.1007/s11892-006-0032-9. [DOI] [PubMed] [Google Scholar]
- 12.Weiss R., Gillis D. Patho-physiology and dynamics of altered glucose metabolism in obese children and adolescents. Int J Pediatr Obes. 2008;3(Suppl. 1):15–20. doi: 10.1080/17477160801896499. [DOI] [PubMed] [Google Scholar]
- 13.Gungor N., Arslanian S. Pathophysiology of type 2 diabetes mellitus in children and adolescents: treatment implications. Treat Endocrinol. 2002;1(6):359–371. doi: 10.2165/00024677-200201060-00002. [DOI] [PubMed] [Google Scholar]
- 14.Caprio S. Insulin resistance in childhood obesity. J Pediatr Endocrinol Metab. 2002;15(Suppl. 1):487–492. [PubMed] [Google Scholar]
- 15.Goran M.I., Gower B.A. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 16.Kelly L.A., Lane C.J., Weigensberg M.J., Toledo-Corral C.M., Goran M.I. Pubertal changes of insulin sensitivity, acute insulin response, and beta-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442–446. doi: 10.1016/j.jpeds.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss R., Dziura J.D., Burgert T.S., Taksali S.E., Tamborlane W.V., Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49(3):571–579. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 18.Goran M.I., Bergman R.N., Cruz M.L., Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25(12):2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 19.Umpierrez G., Dagogo-Jack S. Role of thiazolidinediones in the management of type 2 diabetes: focus on ethnic minority populations. Ethn Dis. 2006;16(1):51–57. [PubMed] [Google Scholar]
- 20.Dwyer J.T., Stone E.J., Yang M., Webber L.S., Must A., Feldman H.A. Prevalence of marked overweight and obesity in a multiethnic pediatric population: findings from the Child and Adolescent Trial for Cardiovascular Health (CATCH) study. J Am Diet Assoc. 2000;100(10):1149–1156. doi: 10.1016/s0002-8223(00)00337-0. [DOI] [PubMed] [Google Scholar]
- 21.Rivera J.A., de Cossio T.G., Pedraza L.S., Aburto T.C., Sanchez T.G., Martorell R. Childhood and adolescent overweight and obesity in Latin America: a systematic review. Lancet Diabetes Endocrinol. 2014;2(4):321–332. doi: 10.1016/S2213-8587(13)70173-6. [DOI] [PubMed] [Google Scholar]
- 22.Narayan K.M., Boyle J.P., Thompson T.J., Sorensen S.W., Williamson D.F. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 23.Search Study Group SEARCH for diabetes in youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25(5):458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence J.M., Mayer-Davis E.J., Reynolds K., Beyer J., Pettitt D.J., D'Agostino R.B., Jr Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl. 2):S123–32. doi: 10.2337/dc09-S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goran M.I., Bergman R.N., Avila Q., Watkins M., Ball G.D., Shaibi G.Q. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89(1):207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 26.Cruz M.L., Shaibi G.Q., Weigensberg M.J., Spruijt-Metz D., Ball G.D., Goran M.I. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr. 2005;25:435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- 27.International Diabetes Federation . International Diabetes Federation; Brussels: 2011. Global IDF/ISPAD guideline for diabetes in childhood and adolescence. [Google Scholar]
- 28.Kuczmarski R.J., Ogden C.L., Guo S.S., Grummer-Strawn L.M., Flegal K.M., Mei Z. Department of Health and Human Services; Washington, DC: 2002. 2000 CDC growth charts for the United States: methods and development. Contract No.: 246. [PubMed] [Google Scholar]
- 29.Tai M.M. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17(2):152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- 30.Palmer J.P., Fleming G.A., Greenbaum C.J., Herold K.C., Jansa L.D., Kolb H. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53(1):250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 31.Bacha F., Gungor N., Arslanian S.A. Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr. 2008;152(5):618–621. doi: 10.1016/j.jpeds.2007.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 34.Conwell L.S., Trost S.G., Brown W.J., Batch J.A. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27(2):314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 35.Yeckel C.W., Weiss R., Dziura J., Taksali S.E., Dufour S., Burgert T.S. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 36.Sinha R., Fisch G., Teague B., Tamborlane W.V., Banyas B., Allen K. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 37.Keskin M., Kurtoglu S., Kendirci M., Atabek M.E., Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 38.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- 39.Zaki M.E., Amr K.S., Abdel-Hamid M. Evaluating the association of APOA2 polymorphism with insulin resistance in adolescents. Meta Gene. 2014;2:366–373. doi: 10.1016/j.mgene.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SIGMA Type 2 Diabetes Consortium Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature. 2014;506(7486):97–101. doi: 10.1038/nature12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elder D.A., Woo J.G., D'Alessio D.A. Impaired beta-cell sensitivity to glucose and maximal insulin secretory capacity in adolescents with type 2 diabetes. Pediatr Diabetes. 2010;11:314–321. doi: 10.1111/j.1399-5448.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elder D.A., Herbers P.M., Weis T., Standiford D., Woo J.G., D'Alessio D.A. β-cell dysfunction in adolescents and adults with newly diagnosed type 2 diabetes mellitus. J Pediatr. 2012;160(6):904–910. doi: 10.1016/j.jpeds.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cali A.M., Man C.D., Cobelli C., Dziura J., Seyal A., Shaw M. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care. 2009;32:456–461. doi: 10.2337/dc08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerrero-Romero F., Violante R., Rodríguez-Morán M. Distribution of fasting plasma glucose and prevalence of impaired fasting glucose, impaired glucose tolerance and type 2 diabetes in the Mexican paediatric population. Paediatr Perinat Epidemiol. 2009;23(4):363–369. doi: 10.1111/j.1365-3016.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 46.Enes Romero P., Cano Gutiérrez B., Alvarez Gil N., Martín-Frías M., Alonso Blanco M., Barrio Castellanos R. Influencia étnica en la prevalencia de síndrome metabólico en población pediátrica obesa. An Pediatr (Barc) 2013;78(2):75–80. doi: 10.1016/j.anpedi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Cruz M., García-Macedo R., García-Valerio Y., Gutiérrez M., Medina-Navarro R., Duran G. Low adiponectin levels predict type 2 diabetes in Mexican children. Diabetes Care. 2004;27(6):1451–1453. doi: 10.2337/diacare.27.6.1451. [DOI] [PubMed] [Google Scholar]
- 48.Villarreal-Molina M.T., Aguilar-Salinas C.A., Rodriguez-Cruz M., Riaño D., Villalobos-Comparan M., Coral-Vazquez R. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes. 2007;56:1881–1887. doi: 10.2337/db06-0905. [DOI] [PubMed] [Google Scholar]
- 49.Villarreal-Molina M.T., Flores-Dorantes M.T., Arellano-Campos O., Villalobos-Comparan M., Rodríguez-Cruz M., Miliar-García A. Association of the ATP-binding cassette transporter A1 R230C variant with early-onset type 2 diabetes in a Mexican population. Diabetes. 2008;57:509–513. doi: 10.2337/db07-0484. [DOI] [PubMed] [Google Scholar]
- 50.Hannon T.S., Rao G., Arslanian S.A. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- 51.Roemmich J.N., Clark P.A., Lusk M., Friel A., Weltman A., Epstein L.H. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26(5):701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 52.Manrique-Hurtado H., Aro-Guardia P., Pinto-Valdivia M. Diabetes tipo 2 en niños: serie de casos. Rev Med Hered. 2015;26(1):5–9. [Google Scholar]