Highlights
-
•
Gestational diabetes (GDM) is associated with risks for both the mother and the child.
-
•
A food composition, macro-nutrient preload, was given half an hour before each meal.
-
•
Thirty-three GDM patients were given macro-nutrient preload and 33 a control comparator.
-
•
A two-month macro-nutrient preload treatment of GDM improved post-prandial glycemia.
-
•
Macro-nutrient preload treatment is of a potential value for future management of GDM.
Keywords: Gestational diabetes, Postprandial blood glucose, Nutrition therapy, Preload
Abstract
Aim
To investigate the effect of a macro-nutrient preload (Inzone Vitality) on blood glucose levels and pregnancy outcomes of gestational diabetes. The preload method involves the ingestion of a smaller amount of a macronutrient composition half an hour before regular meals. The hypothesis was that preload treatment will reduce postprandial glycemia in gestational diabetes.
Methods
Sixty-six diagnosed cases of gestational diabetes were randomly selected from gynecology and obstetrics outpatient clinic at Xinqiao Hospital in Chongqing. The patients were divided into an intervention group (33 cases) and a control group (33 cases), according to odd–even numbers of the random cases. The intervention group was treated with a macro-nutrient preload given 0.5 h before regular meals and the control group was given a comparative treatment consisting of a milk powder with similar energy content. The two groups were studied until delivery and the measured parameters included fasting blood glucose (FBG), 2-hour postprandial blood glucose (2h-PBG), delivery mode and neonatal birth weight.
Results
The two groups showed no differences in FBG or 2h-PBG before the nutritional intervention. FBG and 2h-PBG after intervention and before delivery were significantly lower in the intervention group, treated with the macro nutrient preload compared to the control group (P < 0.01). Changes in FBG and 2h-PBG before and after the intervention were investigated and the difference in the intervention group was significantly greater than corresponding values in the control group (P < 0.05, P < 0.01). The neonatal birth weight and delivery mode was not significantly different (P > 0.05).
Conclusion
A macro-nutrient composition, used as a preload, is effective in controlling FBG and PBG of gestational diabetes.
Introduction
Gestational diabetes mellitus (GDM) refers to glucose tolerance abnormalities of varying degrees that occur or are first discovered during pregnancy. It accounts for 80–90% of diabetes in pregnancy and includes gestational impaired glucose tolerance. In 2007, the Obstetrics Branch of the Chinese Medical Association conducted a nationwide epidemiological study of patients with GDM, showing that the average prevalence of GDM in China was 6.6% with a trend of yearly increases, confirmed by a population-based study in Tianjin 2012, where the prevalence of GDM was 8.1% using the 1999 WHO criteria which was further increased to 9.3% if the 2010 IADPSG criteria were used [1]. Women with a history of GDM have an increased risk of complications during delivery and have a sevenfold increased risk of getting diabetes later in life. Likewise, their offspring have an increased risk at birth and increased risk of diabetes and obesity later in life [2]. GDM in itself can cause epigenetic “programming” of the fetal metabolism [3] and maternal hyperglycemia can therefore potentially lead to trans-generational effects with increased disease risks for the offspring. This adds to the burden many are facing in the prevention of T2DM and its micro- and macrovascular consequences. One feature of GDM is that the disease is often asymptomatic, patients rarely have any symptoms at the time of diagnosis (often at week 24–28), and an important aspect of treatment concerns life style changes [4], [5]. The screening and diagnosis of GDM are important as are interventions aiming to reduce adverse outcomes during pregnancy, immediately after delivery and in a long term perspective. A positive screening test must be linked to a safe and effective method to treat the hyperglycemia and its feared short and long term consequences.
The need for both preventive and therapeutic measures is now urgent, where life style changes, most importantly introduction of healthy diets, are crucial. A prerequisite for the introduction of this kind of healthy nutrition to the general public is that “Medical Nutrition Therapy (MNT)” is evidence based, i.e. has a strong and undisputable scientific support. Nutritional therapy can maintain blood glucose at a normal level and ensure that the mother's physiological requirements and the fetus' developmental requirements are met. Diet control is not only beneficial for controlling maternal weight and improving hyperglycemia, it can also raise the insulin sensitivity in target tissues [6].
A new method has emerged that meet these requirements, the so called macro-nutrient preload method, which has been developed to treat and prevent obesity and type 2 diabetes (T2DM). By administration of a small load of macro-nutrients at a fixed interval before a meal (30 minutes in the majority of studies) the presence of nutrients in the small intestine induces the release of gut peptides like GLP-1, which slows gastric emptying and improves the glycemic response to the subsequent meal [7], [8], [9].
In a recent study of patients with Type 2 Diabetes (T2DM) the ingestion of a macro nutrient preload before major meals, significantly reduced 2h-PBG, HbA1c, total cholesterol/LDL and CRP at the end of a 12-week treatment period [10]. The tested macro nutrient preload was a blend of three different protein sources, slow-release carbohydrates, dietary fibres, omega3/6 fatty acids and other components. The mode of action of macronutrient preloads is assumed to be increased satiety, reduced gastric emptying and attenuated postprandial blood sugar response [11]. Based on this study and previous studies of the macro-nutrient preload method, we hypothesized that the nutritional composition used in the above mentioned T2DM study (Inzone Preload – a macro-nutrient blend with low-GI and low calorie content) would reduce postprandial glycemia in GDM patients when compared to controls consisting of a regular milk powder. To test this hypothesis, we performed a controlled clinical study which aimed to explore the potential effects on blood glucose levels and pregnancy outcomes in women diagnosed with GDM for the first time.
Subjects and methods
Subjects
We collected 80 patients, 26–38 years old who received a definite diagnosis of GDM at our hospital's obstetrics and gynecology outpatient clinic from November 2013 to May 2014. Subject inclusion criteria: (1) definite diagnosis of GDM at our hospital's outpatient clinic; (2) voluntarily signed the informed consent form; (3) deemed suitable for treatment by a dietitian. Subject exclusion criteria: (1) continuously positive for urine ketone bodies; (2) fasting blood glucose and postprandial blood glucose remaining high after 1–2 weeks of nutritional intervention. In line with our clinical experience, patients with fasting blood glucose >6.1 mmol/L and two-hour postprandial blood glucose >11.1 mmol/L after 1–2 weeks of nutrition intervention will be treated with insulin to reduce blood glucose; (3) gestational hypertension, and severe hepatic or renal dysfunction; (4) refusing to follow the dietitian's instructions regarding diet. During the study all of the GDM women only used diet to control blood sugar and were not in need for any pharmacological treatment. Ethical permission was obtained from the Third Military Medical University Ethics Committee (no ChiCTR-IoR-14005522).
Diagnostic criteria
Patients who met any of the following criteria were diagnosed with GDM: (1) Fasting blood glucose ≥5.1 mmol/L; (2) blood glucose ≥10.0 mmol/L one hour after a 75-g OGTT performed after fasting for eight hours at 24–28 weeks of gestation; (3) blood glucose ≥8.5 mmol/L two hours after an OGTT. These different criteria has the following distribution: a total of 30 subjects with fasting blood glucose ≥5.1 mmol/L in line with the GDM diagnostic criteria (16 subjects in the intervention group, 18 in the control group); a total of 43 subjects in weeks 24–28 of pregnancy with blood glucose ≥10 mmol/L one hour after administration of 75 g OGTT″ (23 subjects in the intervention group, 20 in the control group); and a total of 45 subjects with blood glucose ≥8.5 mmol/L two hours after administration of OGTT″ (23 subjects in the intervention group, 22 in the control group).
Methods
Randomized design where one group received a macro nutrient preload (intervention) and the other group received a comparator consisting of a milk powder (control). All patients were numbered by the time of hospitalization and grouped by the random numbers, namely, intervention group (macro nutrient preload) by odd number and control group by even number, each with 40 cases. During the experiment, 7 cases from each group were canceled due to hyperglycemia or failure to follow the dietitian's instructions, thus 33 cases from each group were selected to participate in the study (Fig. 1). A total of 6 subjects were excluded due to excessive hyperglycemia, 2 subjects in the intervention group and 4 subjects in the control group. The remaining 8 subjects did not comply with the instructions of the nutritionist or moved to another part of the country. Subjects that were excluded due to excessive hyperglycemia were those who had fasting blood glucose >6.1 mmol/L and two-hour postprandial blood glucose >11.1 mmol/L after 1–2 weeks of nutrition intervention in blood glucose taken by nurses from the clinical laboratory at Xinqiao Hospital once per week after starting on nutrition intervention. These patients were treated with insulin to reduce blood glucose, in line with the clinical experience of our hospital's gynecologist, and were considered withdrawn from observation.
Figure 1.
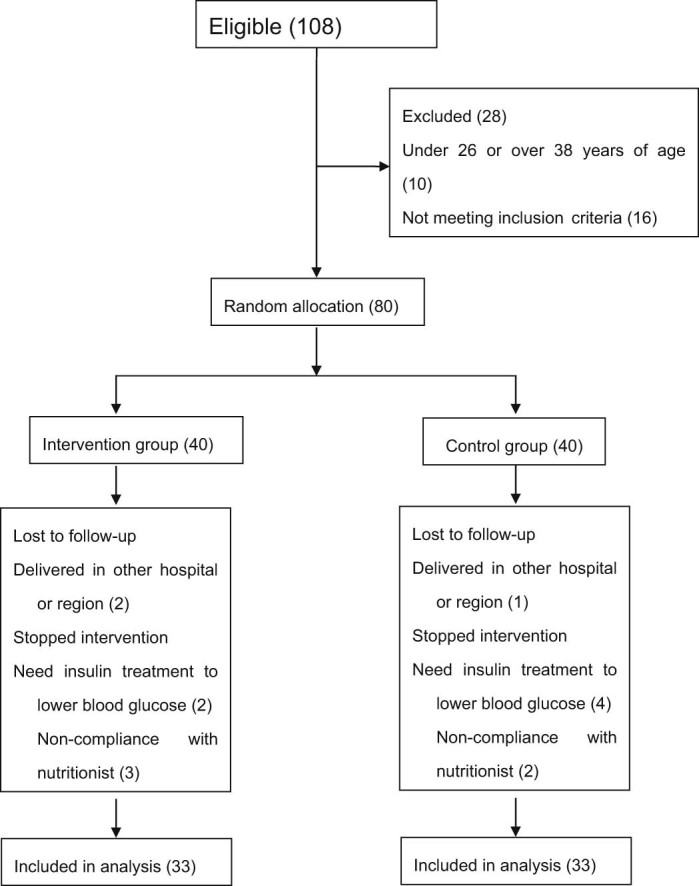
Depicts the number of patients that were enrolled to take part in the clinical testing of macronutrient preload. The diagram shows the number of patients not meeting inclusion criteria as described in the text. The selected patients, after meeting inclusion criteria, were divided into an intervention group (macro nutrient preload) and a control group where each consisted of 40 individuals. Thirty-three subjects in each group concluded the investigation. The reasons for drop-out are listed in the Figure.
The mean age of intervention group was 32.7 ± 4.9 years and the one of the control group was 30.8 ± 4.7 years, showing no significant difference (P > 0.05). In this study the patients were randomly selected to be given intervention or control treatment and the subjects were not aware if they received intervention or comparator treatment. The project leaders were aware of which treatment that was given.
Intervention measures: a record consisting of basic personal information, health status and pregnancy outcome was kept by Xinqiao Hospital where also follow-up visits were conducted. All subjects in each group were given nutritional education, and were given dietary recommendations; diets were formulated according to the daily energy demand of GDM patients up to 30–38 kcal/kg ideal body weight and was adapted to the actual weight of patients, weight increment in pregnancy, age, physical activities, family history, etc. These mainly consisted of six meals per day, consisting of three regular meals and three snacks. For the intervention group, a macronutrient preload nutrition powder (Inzone Vitality; imported from Sweden, original packing, provided by Tianjin Health Promotion Technology Co., Ltd.) was given as three pre-meals 30 minutes before ordinary meals (meal times were: 7:30, 11:30, and 17:30). The Inzone Vitality product consists only of natural food ingredients (pea-protein, whey protein, egg albumin, Ω 3/6 fatty acids, whole eggs, apple, rosehip and sugar beet fiber). Each serving of Inzone Vitality (18 gram) contains 7.6 g protein, 1.8 g fat (saturated and unsaturated fat are 0.6 g and 1.2 g respectively), 1.6 g fiber and 5.2 g carbohydrates, which provided 71 kcal energy (Indevex Biotech, Sweden) and a low Glycemic Index, GI. Each sachet containing 18 g was dissolved in water at ambient temperature before use. The patient directly drank in a single draft the entire preparation made by mixing the powder with 150 mL water. After drinking the preparation, the patient drank another 100 mL water and then started a regular meal half an hour later.
The control group was given a comparator consisting of a milk powder for pregnant women (Nestle Ange milk powder (https://www.nestle.com.cn/)). The product was produced in Heilongjiang, China (QS serial number 2300 0510109) and had the following composition: 18 g per serving, energy 73 kcal, protein 3.5 g, fat 1.1 g carbohydrates 11.2 g. Treatment of the control group was given in accordance with the principles described above for the Inzone Vitality (preload 30 minutes before major meals).
NGC preload was developed to provide a high protein/low carbohydrate composition. As a control composition (comparator) we selected a milk based composition characterized by less protein and a higher content of carbohydrates. A comparison between preload and control is shown in Table 1.
Table 1.
Comparison between macronutrient preload and control comparator
| NCG preload | Control | |
|---|---|---|
| Protein | 7.6 g (42%) | 3.5 g(19%) |
| Fat | 1.8 g (10%) | 1.1 g(6.1%) |
| Carbohydrates | 5.2 g (28%) | 11.2 g(62%) |
| Energy | 71 kcal | 73 kcal |
Table 1 shows a comparison between intervention treatment i.e. macro-nutrient preload (Inzone Vitality) and Control (Nestle Ange milk) in terms of protein, fat and carbohydrate contents. The table lists the amount (g) in each 18 g serving. Numbers in parenthesis shows the percent content per serving.
In addition to nutritional treatment, the remaining treatment for the two groups was subjected to the same or similar modality. Intervention for each patient was concluded after discharge following delivery. The duration of treatments was for the intervention group 65.3 ± 8.2 days and for the control group 65.9 ± 8.6 days, i.e. no significant difference between the two groups.
Methods of evaluation: primary outcome measurement was two-hour postprandial blood glucose levels following a standardized meal. Secondary outcome comparisons were made with fasting blood glucose measurements. Additional data were collected and included delivery method, neonatal birth weight and Apgar score.
Instruments and tools
Adult weight and height were measured using the RGZ120 body scale (weight calibrated to ±0.2 kg; height calibrated to ±0.5 cm) manufactured by the Auxin Scales Factory in Wuxi City, China. Neonatal birth weight was measured using the Life Sense neonatal scale (calibrated to ±0.05 kg). Blood glucose was tested using the Roche Accu-Chek Active II. Diets were formulated using Nutrition System of Chinese Traditional Medicine Combining Western Medicine (NCCW) MX1 (version: V11.0) software. Patients had their blood glucose tested once every week after nutritional intervention, by nurses from the clinical laboratory at Xinqiao Hospital. In line with clinical experience, the gynecologist requires patients with fasting blood glucose >6.1 mmol/L and two-hour postprandial blood glucose >11.1 mmol/L after 1–2 weeks of nutrition intervention be treated with insulin to reduce blood glucose.
Fasting blood glucose is blood glucose taken before the first meal of the day after rising at 7:00 in the morning, and having taken the final meal of the previous day at 21:00.
Statistical methods
A power calculation (0.8) was carried out using a significance level of 0.025, SD of 1.29 and a difference in means of 1 mmol/L, for the 2 h post prandial glucose level between the intervention group (macro-nutrient preload) and the control group. This resulted in that minimally 56 patients should enter this two treatment (intervention and control) parallel-design study. Data from the subjects in this investigation (n = 66) were entered and saved in an Excel software system. SPSS18.0 software was used for statistical analysis and charting. Quantitative data were analyzed using a two-tailed t-test after normality testing. Qualitative data were analyzed using a χ2 test. The level of statistical significance was P < 0.05.
Results
The results in Fig. 2 show no significant difference between the groups with respect to fasting blood glucose and postprandial blood glucose before intervention. One group of GDM patients were subsequently treated with the macro-nutrient preload for an average of 65 days and this group was compared to a similar sized control group treated with a comparator for a similar time period. As shown in Fig. 2, there was a significant difference between the groups with respect to fasting blood glucose and postprandial blood glucose after the intervention and before delivery. The preload treated group (intervention group) had a significantly reduced (P < 0.01) blood glucose level compared to controls and this was also the case with postprandial glucose levels. This demonstrates that an Inzone preload is beneficial for the control of postprandial blood glucose in patients with gestational diabetes.
Figure 2.
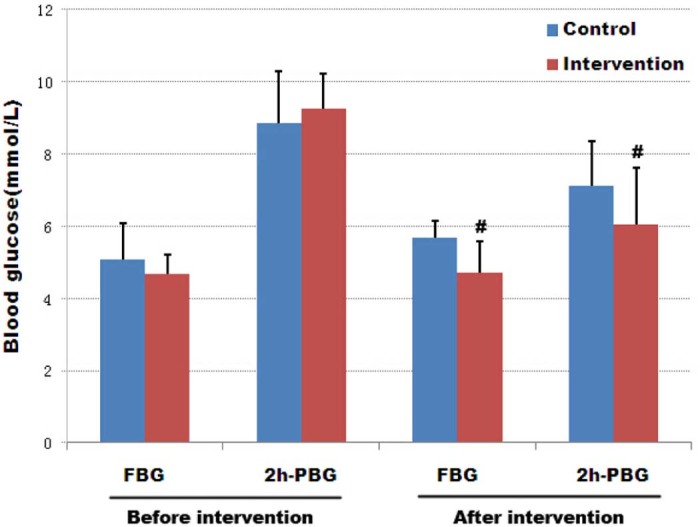
Effect of a macro-nutrient preload on blood glucose before and after intervention in GDM patients. The graph depicts the mean fasting blood glucose (FBG) and the mean 2 h post-prandial blood glucose levels (mmol/L) before and after macro-nutrient preload treatment. Both control and intervention groups include 33 cases; error bars indicate one SEM, #P < 0.01.
Table 2 shows that mean change in fasting blood glucose was more pronounced in the control group compared to the macro-nutrient preload treated group (P < 0.05). This demonstrates that Inzone Vitality is beneficial for controlling fasting blood glucose increments. The change in two-hour postprandial blood glucose, measured before and after nutritional intervention, was decreased in both groups, but the decrease was more pronounced in the intervention (macro-nutrient preload) group (P < 0.01). This demonstrates that Inzone preload treatment significantly improves postprandial glucose levels.
Table 2.
Effect of a macronutrient preload on blood glucose changes in GDM before and after intervention ( ± SEM, n = 33)
| Group | Fasting blood glucose change (mmol/L) | 2-hour postprandial blood glucose change (mmol/L) |
|---|---|---|
| Intervention group (n = 33) | 0.04 ± 1.19* | −3.19 ± 1.48** |
| Control group (n = 33) | 0.62 ± 0.91 | −1.74 ± 1.87 |
P < 0.05.
P < 0.01.
Neonatal birth weights were as shown in Table 3. There was a trend toward a reduced birth weight in macro-nutrient preload treated GDM patients, but this difference was not statistically significant (P > 0.05). Delivery was carried out by Cesarean section in a majority of cases (60%) in both groups. Table 3 shows that there were no significant differences between the two groups with respect to the number of Cesarian sections or in the neonatal Apgar score.
Table 3.
Effect of a macronutrient preload on birth weight, Cesarian section and Apgar score
| Group | n | Birth weight, kg | Cesarian section [n (%)] | Apgar score 10 [n (%)] |
|---|---|---|---|---|
| Intervention | 33 | 3.33 ± 0.45 | 20 (60.6) | 33 (100) |
| Control | 33 | 3.44 ± 0.50 | 21 (63.4) | 33 (100) |
Discussion
The incidence of GDM is increasing year by year and has become the most common pregnancy/delivery complication, with health risks for the mother and the child [12]. Our study shows that ingestion of a specified blend of nutrients before major meals, the macro nutrient preload method, improves gestational hyperglycemia. This method thereby has the potential to decrease the risks associated with GDM for the mother and the fetus/newborn child. To our knowledge this is the first study of this method in gestational diabetes, and also the first controlled study using a specific diet regimen to GDM patients in general.
Normal pregnancy is associated with a progressive increase of maternal insulin resistance and hyper-insulinemia from the increased insulin secretion from the mother. This is vital for delivery of nutrients to the fetus and fetal growth. Insulin sensitivity is either normal or slightly increased during early gestation (<20 weeks), whereas it decreases during mid to late pregnancy, with compensatory increases in maternal insulin secretion. It is for this reason that the body's fasting blood glucose and postprandial blood glucose exhibit a tendency to be increased as pregnancy progresses [13].
Glucose intolerance in pregnancy increases risks for both mother and child, such as higher rates of preeclampsia, operative deliveries, macrosomia and birth injury. High maternal glycemia results in increased fetal insulin production, which is considered to be the main driver of large for gestational age, LGA, and macrosomia. Hyperglycemia in pregnancy and neonatal adiposity has also been linked to increased subsequent childhood obesity and early adulthood T2DM. How the intrauterine environment confers higher risk is not fully understood, but data from animal studies suggest that epigenetic processes modulate gene transcription in utero.
Diet control is fundamental in both preventing and treating GDM [14], [15], [16], [17]. In China, insulin therapy is only considered for those patients in whom blood glucose control is inadequate after 1–2 weeks of diet control [18]. Healthy nutrition ensures normal development of the fetus and controls the mother's postprandial blood glucose and weight gain during pregnancy within an ideal range. In addition, performing suitable exercise can increase the body's sensitivity to insulin, which helps lower blood glucose in GDM patients. Thus, life style measures lower the risks for developing GDM and its consequences like hyperglycemia, hypoglycemia, ketosis, and other symptoms that adversely affect the mother and child [19]. In the present study, GDM patients underwent nutritional education and were informed of suitable food choices. A suitable diet was also formulated for each mother. These diets provided the daily intakes and compositional ratios of energy, protein, fat, carbohydrate, and other nutrients. In addition, to prevent patients from consuming only single foods, simple food substitutions were provided. The foods and drinks that were permitted and those that were not were also explained to the patients in detail.
In contrast to traditional nutritional therapy, which focuses on the amount of total energy, macro-nutrients and the amount of carbohydrates, the macro-nutrient preload method focus on the therapeutic potential, with a defined mode of action. Several studies suggest that the macronutrient preload can have a vital role in the care of diabetic patients [7], [11], [20]. These studies show that a preload of macronutrients significantly increases the release of gut hormones which in turn slow gastric emptying, increases the release of insulin and attenuates the blood glucose response after a meal – the postprandial blood glucose (PBG). When nutrients interact with the small intestine, they generate feedback that slows further gastric emptying and suppresses appetite, via both neural and hormonal mechanisms. The latter include GLP-1, cholecystokinin (CCK) and peptide YY (PYY). The small and large intestines also secrete peptides that account for the “incretin” effect – the phenomenon by which insulin secretion is at least doubled when glucose is given by the enteral route when compared to an isoglycemic intravenous glucose infusion [21], [22]. Several studies have established that the peak changes in hormone concentration are occurring within 30–60 minutes of preload ingestion, which also is the basis for the “30 minute rule” in the preload method [7], [20].
The most important result of the preload intervention in our study is the impact on the postprandial blood glucose 2 hours after the meals (2h-PBG). Given that nutrients emptying from the stomach are in the range of 1–4 kcal/min [23] and that people nowadays generally consume three main meals per day, often with snacks in between, it is clear that most of the day is spent in the postprandial state, with only a few hours of true fasting before breakfast [24]. The traditional focus on controlling “fasting” blood glucose in the management of type 2 diabetes is, therefore, often inappropriate. For the majority with type 2 diabetes, who have relatively good overall glycemic control like the GDM subjects, postprandial glycemia predominates over fasting blood glucose in contributing to HbA1c, and a deterioration in postprandial glycemic control precedes any substantial elevation of fasting blood glucose [25]. Therapies that specifically target postprandial glycemic excursions are therefore of fundamental importance in preventing the progression of hyperglycemic conditions like GDM and T2DM and the emergence of micro- and macro-vascular complications, whose incidence is related closely to the HbA1c [26]. In the recently updated ADA/EASD guidelines for managing type 2 diabetes, nutritional strategies to reduce postprandial glycemia are recommended as a first line treatment, and represent the greatest opportunity for optimizing glycemic control at affordable costs as the healthcare demands of our society escalate [27].
The strength of this study is the design where a macro-nutrient preload was compared to a control milk powder with a higher GI. It is a challenge to find a suitable placebo treatment for macro nutrient treatments and in the present study we choose regular milk powder as a comparator but this treatment is likely to exert some biological effects. A recent, not placebo controlled, study shows that a 12 week macro-nutrient preload treatment had positive effects on T2DM patients [10]. Ma et al. demonstrate sustained effects on glycemia and gastric emptying in T2DM patients following a four week trial using a protein based preload [20]. The present study suggest GDM as an indication for macro-nutrient preload treatment and possibly this can be a first line therapy to women with impaired glucose control during pregnancy. Ideally the treatment should be commenced as early as possible, to prevent the development of impaired glucose intolerance to GDM or T2DM, but further research is necessary for clarification. There are limitation of the present study and follow up studies are needed that could e.g. include a larger number of patients, a comparison with standard care, more specific classification of GDM, more advanced monitoring of treatment effects, dynamic effects on several metabolic parameters and effects on GDM patients from other countries.
In summary our study found that macro-nutrient preload treatment has positive effects on GDM. We found that postprandial blood glucose was significantly lower in the intervention group compared to the control group but also that there was a tendency in control group for a reduced postprandial glucose (Fig. 2). This indicates that nutritional education and nutritional intervention per se are beneficial in controlling GDM patients' postprandial blood glucose level. With respect to neonatal birth weight and macrosomia in our study, there was a trend of decreased birth weight after macro-nutritional preload intervention, although not significant. The short and long term health effects of the macro-nutrient preload method for the mother and newborn child need to be further investigated, including the impact on the risk of developing T2DM for both mother and child, recurrence of GDM during next pregnancy and other metabolic consequences.
Conflict of interest
The authors declare they have no conflicts of interest.
Acknowledgment
The authors are grateful for financial support for this study from Xinqiao Hospital Clinical Scientific Research Project (yclkt-201436).
References
- 1.Pan L., Leng J., Liu G., Zhang C., Liu H., Li M. Pregnancy outcomes of Chinese women with gestational diabetes mellitus defined by the IADPSG's but not by the 1999 WHO's criteria. Clin Endocrinol (Oxf) 2015;83:684–693. doi: 10.1111/cen.12801. [DOI] [PubMed] [Google Scholar]
- 2.Ma R.C., Tutino G.E., Lillycrop K.A., Hanson M.A., Tam W.H. Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol. 2015;118(1–2):55–68. doi: 10.1016/j.pbiomolbio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey K.M., Sheppard A., Gluckman P.D., Lillycrop K.A., Burdge G.C., McLean C. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes. 2011;60(5):1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez T.L., Anderson M.A., Chartier-Logan C., Friedman J.E., Barbour L.A. Strategies in the nutritional management of gestational diabetes. Clin Obstet Gynecol. 2013;56(4):803–815. doi: 10.1097/GRF.0b013e3182a8e0e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halperin I.J., Feig D.S. The role of lifestyle interventions in the prevention of gestational diabetes. Curr Diab Rep. 2014;14(1):452. doi: 10.1007/s11892-013-0452-2. [DOI] [PubMed] [Google Scholar]
- 6.Noctor E., Crowe C., Carmody L.A., Kirwan B., O'Dea A., Glynn L.G. ATLANTIC-DIP: prevalence of metabolic syndrome and insulin resistance in women with previous gestational diabetes mellitus by International Association of Diabetes in Pregnancy Study Groups criteria. Acta Diabetol. 2015;52(1):153–160. doi: 10.1007/s00592-014-0621-z. [DOI] [PubMed] [Google Scholar]
- 7.Ma J., Stevens J.E., Cukier K., Maddox A.F., Wishart J.M., Jones K.L. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care. 2009;32(9):1600–1602. doi: 10.2337/dc09-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu T., Bound M.J., Zhao B.R., Standfield S.D., Bellon M., Jones K.L. Effects of a D-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care. 2013;36(7):1913–1918. doi: 10.2337/dc12-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Samra R., Keersmaekers L., Brienza D., Mukherjee R., Macé K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr J. 2011;10:139. doi: 10.1186/1475-2891-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C.J., Norstedt G., Hu Z.G., Yu P., Li D.Q., Li J. Effects of a macro-nutrient preload on type 2 diabetic patients. Front Endocrinol (Lausanne) 2015;6:139. doi: 10.3389/fendo.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowicz D., Froy O., Ahrén B., Boaz M., Landau Z., Bar-Dayan Y. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57(9):1807–1811. doi: 10.1007/s00125-014-3305-x. [DOI] [PubMed] [Google Scholar]
- 12.Triunfo S., Lanzone A. Impact of overweight and obesity on obstetric outcomes. J Endocrinol Invest. 2014;37(4):323–329. doi: 10.1007/s40618-014-0058-9. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre H.D., Chang A.M., Callaway L.K., Cowley D.M., Dyer A.R., Radaelli T. Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care. 2010;33(2):356–360. doi: 10.2337/dc09-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 15.He J.R., Yuan M.Y., Chen N.N., Lu J.H., Hu C.Y., Mai W.B. Maternal dietary patterns and gestational diabetes mellitus: a large prospective cohort study in China. Br J Nutr. 2015;113(8):1292–1300. doi: 10.1017/S0007114515000707. [DOI] [PubMed] [Google Scholar]
- 16.Rogozinska E., Chamillard M., Hitman G.A., Khan K.S., Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS ONE. 2015;10(2):e0115526. doi: 10.1371/journal.pone.0115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobias D.K., Zhang C., Chavarro J., Bowers K., Rich-Edwards J., Rosner B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96(2):289–295. doi: 10.3945/ajcn.111.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bain E., Crane M., Tieu J., Han S., Crowther C.A., Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;(4) doi: 10.1002/14651858.CD010443.pub2. CD010443. [DOI] [PubMed] [Google Scholar]
- 19.Morisset A.S., Côté J.A., Michaud A., Robitaille J., Tchernof A., Dubé M.C. Dietary intakes in the nutritional management of gestational diabetes mellitus. Can J Diet Pract Res. 2014;75(2):64–71. doi: 10.3148/75.2.2014.64. [DOI] [PubMed] [Google Scholar]
- 20.Ma J., Jesudason D.R., Stevens J.E., Keogh J.B., Jones K.L., Clifton P.M. Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2015;108(2):e31–4. doi: 10.1016/j.diabres.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre N., Holdsworth C.D., Turner D.S. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 22.Elrick H., Stimmler L., Hlad C.J., Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 23.Brener W., Hendrix T.R., McHugh P.R. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85(1):76–82. [PubMed] [Google Scholar]
- 24.Monnier L., Colette C., Dunseath G.J., Owens D.R. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30(2):263–269. doi: 10.2337/dc06-1612. [DOI] [PubMed] [Google Scholar]
- 25.Monnier L. Is postprandial glucose a neglected cardiovascular risk factor in type 2 diabetes? Eur J Clin Invest. 2000;30(Suppl. 2):3–11. [PubMed] [Google Scholar]
- 26.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 27.Inzucchi S.E., Matthews D.R., Management of Hyperglycemia in Type 2 Diabetes American Diabetes Association and European Association for the Study of Diabetes Position Statement Writing Group Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]


