Abstract
A Salmonella Typhimurium ghost vaccine was constructed with the use of a recombinant fusion protein consisting of lysozyme and porcine myeloid antimicrobial peptide 36 expressed by the Escherichia coli overexpression system. After confirmation of its effectiveness by transmission electron microscopy the vaccine was evaluated in a murine model. Of the 60 BALB/c mice equally divided into 4 groups, group A mice were intramuscularly inoculated with 100 μL of sterile phosphate-buffered saline, and the mice in groups B, C, and D were intramuscularly inoculated with approximately 1.0 × 104, 1.0 × 105, or 1.0 × 106 cells of the S. Typhimurium ghost vaccine, respectively, in 100-μL amounts. The serum IgG titers against S. Typhimurium outer membrane proteins were significantly higher in groups B to D than in group A, as were the concentrations of interleukin-10 and interferon gamma in supernatants of harvested splenocytes. After challenge with wild-type S. Typhimurium, all the vaccinated groups showed significant protection compared with group A, notably perfect protection in groups C and D. Overall, these results show that intramuscular vaccination with 1.0 × 105 cells of this ghost vaccine candidate provided efficient protection against systemic infection with virulent S. Typhimurium.
Résumé
Un vaccin fantôme dirigé contre Salmonella Typhimurium a été construit en utilisant une protéine de fusion recombinante composée de lysozyme et du peptide myéloïde antimicrobien 36 d’origine porcine exprimée par le système de surexpression d’Escherichia coli. Après confirmation de son efficacité par microscopie électronique à transmission, le vaccin a été évalué dans un modèle murin. Soixante souris BALB/c ont été séparées en quatre groupes. Les souris du groupe A ont été inoculées par voie intramusculaire (IM) avec 100 μL de saline tamponnée stérile, alors que les souris des groupes B, C, et D ont été inoculées IM avec approximativement 1,0 × 104, 1,0 × 105, ou 1,0 × 106 cellules du vaccin fantôme S. Typhimurium, respectivement, dans des volumes de 100 μL. Les titres d’IgG sériques contre les protéines de la membrane externe de S. Typhimurium étaient significativement plus élevés dans les groupes B à D que dans le groupe A, de même que les concentrations d’interleukine-10 et d’interféron gamma dans les surnageants de splénocytes récoltés. Suite à une infection défi avec une souche sauvage de S. Typhimurium, les animaux de tous les groupes vaccinés étaient protégés de manière significative comparativement à ceux du groupe A, notamment une protection parfaite pour les groupes C et D. De manière générale, ces résultats montrent que la vaccination IM avec 1,0 × 105 de ce vaccin fantôme candidat fourni une protection efficace contre une infection systémique par une souche virulente de S. Typhimurium.
(Traduit par Docteur Serge Messier)
Introduction
Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium) mainly causes gastroenteritis in domestic animals and humans (1,2). In addition, in mice it can cause enteric fever with symptoms similar to those observed in humans after S. Typhi infection (1). An effective means of preventing salmonellosis is vaccination against S. Typhimurium infection (2–4). Cell-mediated immunity (CMI) is crucial (5,6), and a humoral immune response, such as the production of serum IgG and secretory IgA, is also known to contribute to the clearing of Salmonella under some circumstances (3,6). Protection against virulent bacterial infection can be induced through vaccination with killed or attenuated Salmonella or Salmonella ghost cells (5–14).
Use of a vaccine with live, attenuated Salmonella is a general protocol for protection against Salmonella infections, but it is risky given the potential for reversion to a virulent strain. Many live, attenuated Salmonella vaccine strains have been generated by mutating or deleting metabolism- or virulence-associated genes (4,9,10,15–17). Hence, many different approaches, including killed vaccine, subunit vaccines, and vector vaccines, have been tried for protection against Salmonella infection, with varying success (6,14,18,19).
Recently, bacterial ghost cells have emerged as an effective inactivated vaccine candidate for protection against various Gram-negative bacterial infections. Antimicrobial peptide (AMP), or host defence peptide, is a part of the innate immune system (20,21). Peptides work by disrupting the barrier function of the cell membrane, forming pores or inducing membrane permeability without disturbing the integrity of the membrane (22–24). Porcine myeloid antimicrobial peptide 36 (PMAP36) has the highest reported positive charge among porcine AMPs (25). Some bacteriophage endolysins, such as the lysozyme of Salmonella phage P22, have been known to act as antimicrobials by disrupting the activity of the bacterial cell wall (26). The enzymes attack the cell walls of Gram-negative bacteria, eventually resulting in cell wall lysis (27).
The objective of this study was to express and purify a recombinant lysozyme–PMAP36 fusion protein by means of the Escherichia coli overexpression system with a pET expression vector, use the recombinant protein along with S. Typhimurium to construct an S. Typhimurium ghost vaccine candidate, and investigate the efficacy of the ghost vaccine’s protection in a mouse model.
Materials and methods
Bacterial strains and growth conditions
Salmonella Typhimurium isolate HJL456, from a broiler chicken in Korea, was used for vaccine construction with the recombinant lysozyme–PMAP36 fusion protein and was also used as the challenge strain. Escherichia coli isolates HJL505 and BL21(DE3) (Invitrogen, Carlsbad, California, USA) with the pET30a plasmid (an expression vector inducible by isopropyl β-D-1-thiogalactopyranoside) (Novagen, Temecula, California, USA) containing the genes for the recombinant fusion protein were used in overexpression of the fusion protein (Table I). These strains were grown in Luria–Bertani (LB) broth and on LB agar (Becton Dickinson, Sparks, Maryland, USA) at 37°C.
Table I.
Bacterial strains and plasmids used in this study.
| Strain/plasmid | Description | Source |
|---|---|---|
| Escherichia coli | ||
| BL21(DE3) | F−, ompT, hsdSB(rB−,mB−), dcm, gal, λ (DE3) | Invitrogen |
| HJL505 | BL21(DE3) with pET30a containing gene for lysozyme–PMAP36 fusion protein | Lab stock |
| Salmonella Typhimurium | ||
| HJL456 | Isolate from broiler chicken in Korea | Lab stock |
| Plasmid | ||
| pET30a | Expression vector inducible by IPTG; Kmr | Novagen |
PMAP36 — porcine myeloid antimicrobial peptide 36; IPTG — isopropyl β-D-1-thiogalactopyranoside; Kmr — kanamycin resistance.
Preparation of the recombinant fusion protein
The modified fusion gene for the lysozyme (containing restriction enzyme and HIS-tag) — PMAP36 (including restriction enzyme) fusion protein was synthesized at Bioneer, Daejeon, Republic of Korea (Table II) (28,29). The gene was inserted into restriction sites NdeI and Not I of the pET30a plasmid (Novagen) and the plasmid introduced into E. coli BL21(DE3) (Invitrogen) and designated as HJL505. The recombinant fusion protein was expressed in HJL505 and purified according to a previously reported method (11). All purified antigens were mixed with 50% glycerol and stored at −70°C until further used.
Table II.
| Gene product | Nucleotide sequencea | Size (number of base pairs) | Restriction enzyme | Gene coordinates | Accession number |
|---|---|---|---|---|---|
| Lysozyme | CATATGcaccatcatcaccatcacATGCAAATCAGCAGTAACGGAATCACCAGATTAAAACGTGAAGAAGGTGAGAGACTAAAAGCCTATTCAGATAGCAGGGGGATACCAACCATTGGGGTTGGGCATACCGGAAAAGTGGATGGTAATTCTGTCGCATCAGGGATGACAATCACCGCCGAAAAATCTTCTGAACTGCTTAAAGAGGATTTGCAGTGGGTTGAAGATGCGATAAGTAGTCTTGTTCGCGTCCCGCTAAATCAGAACCAGTATGATGCGCTATGTAGCCTGATATTCAACATAGGTAAATCAGCATTTGCCGGCTCTACCGTTCTTCGCCAGTTGAATTTAAAGAATTACCAGGCAGCAGCAGATGCTTTCCTGTTATGGAAAAAAGCTGGTAAAGACCCTGATATTCTCCTTCCACGGAGGCGGCGAGAAAGAGCGCTGTTCTTATCG | 435 | NdeI | 366–800 | M10997.1 |
| ATGCAAATCAGCAGTAACGGAATCACCAGATTAAAACGTGAAGAAGGTGAGAGACTAAAAGCCTATTCAGATAGCAGGGGGATACCAACCATTGGGGTTGGGCATACCGGAAAAGTGGATGGTAATTCTGTCGCATCAGGGATGACAATCACCGCCGAAAAATCTTCTGAACTGCTTAAAGAGGATTTGCAGTGGGTTGAAGATGCGATAAGTAGTCTTGTTCGCGTCCCGCTAAATCAGAACCAGTATGATGCGCTATGTAGCCTGATATTCAACATAGGTAAATCAGCATTTGCCGGCTCTACCGTTCTTCGCCAGTTGAATTTAAAGAATTACCAGGCAGCAGCAGATGCTTTCCTGTTATGGAAAAAAGCTGGTAAAGACCCTGATATTCTCCTTCCACGGAGGCGGCGAGAAAGAGCGCTGTTCTTATCG | |||||
| Thrombin | CTGGTTCCGCGTGGATCC | 18 | CTGGTGCCACGCGGTTCT | ||
| PMAP36 | 108 to 111 | NotI | 404 to 514 | NM_0011 | |
| GGACGATTTAGACGTTTACGTAAAAAAACCCGGAAACGTCTGAAAAAGATTGGGAAAGTGTTGAAATGGATTCCTCCTATTGTCGGTTCAATACCCTTAGGTTGTGGATAAGCGGCCGC | 29965.1 | ||||
| GGACGATTTAGACGGTTGCGTAAGAAGACCCGAAAACGTTTGAAGAAGATCGGGAAGGTTTTGAAGTGGATTCCTCCCATTGTCGGCTCAATACCCTTGGGTTGTGGGTAA | |||||
Underlining indicates the sites at which the restriction enzymes acted.
Lower-case letters indicate His-tag.
Construction of the ghost vaccine candidate
A single colony of S. Typhimurium HJL456 was inoculated into 200 mL of LB broth and incubated at 37°C with slow agitation to an optical density of 0.3 at 600 nm. The fusion protein, 40 μg/mL, was added into the cultured broth and the mixture incubated at 37°C to induce the ghost isolates (30). After 16 h, induction of the ghost isolates against all cells was confirmed by counting the number of viable bacteria after incubation on LB agar for 72 h at 37°C.
Transmission electron microscopy (TEM)
The ghost samples underwent TEM with a transmission electron microscope (H-7600; Hitachi High-Technologies Corporation, Tokyo, Japan) according to a previously described method (25) for observation of intracellular alteration of the S. Typhimurium vaccine candidate before and after addition of the recombinant fusion protein. The samples had been prepared in the same manner as for construction of the S. Typhimurium ghost vaccine candidate.
Vaccination and sample collection
Four groups of BALB/c female mice, each group containing 15 mice, were inoculated intramuscularly (IM) at 6 wk of age (0 wk after primary vaccination) and given a booster IM at 8 wk of age (2 wk after primary vaccination). All 15 mice forming group A were injected with sterile phosphate-buffered saline (PBS) and acted as the controls. The mice in groups B, C, and D were inoculated with approximately 1.0 × 104 cells, 1.0 × 105 cells, and 1.0 × 106 cells, respectively, of the Salmonella ghost vaccine strain in 100-μL amounts. Blood and fecal samples were collected at 0, 2, and 4 wk after primary vaccination to evaluate the immune response. All the animal experiments were conducted with ethics approval (CBU 2012–0017) of the Animal Ethics Committee of Chonbuk National University, Iksan, Republic of Korea, in accordance with the guidelines of the Korean Council on Animal Care.
Evaluation of immune response
The serum and fecal titers, respectively, of IgG and IgA specific for S. Typhimurium outer membrane proteins (OMPs) were determined according to previously described methods (12) of enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well, flat-bottom ELISA plates (Microlon; Greiner Bio-One, Frickenhausen, Germany) were coated with the OMPs (500 ng/well) and incubated overnight at 4°C. Serum was diluted 1:200 in PBS, and feces were diluted 1:3. The plates were treated with goat IgG or IgA against mouse antigen conjugated with horseradish peroxidase (Southern Biotechnology Associates, Birmingham, Alabama, USA). Enzymatic reactions were produced through the addition of substrate containing o-phenylenediamine (Sigma-Aldrich, St. Louis, Missouri, USA) and were measured with an automated ELISA spectrophotometer (TECAN, Salzburg, Austria) at 492 nm. In addition, ELISA was used to measure the concentration of interleukin (IL)-10 and interferon gamma (IFN-γ) in the supernatants with the mouse cytokine ELISA Ready-SET-Go! reagent kit (eBioscience, San Diego, California, USA). The ELISA results were expressed as the mean concentration ± standard deviation.
Cytokine quantitation in splenocytes
Five mice from each group were euthanized and their spleens removed aseptically at 4 wk after primary vaccination. Splenocytes were prepared according to methods described previously (30–32) and seeded in 24-well tissue culture plates, 2 × 106/well. The splenocytes were stimulated in vitro with the Salmonella vaccine candidate (108 cells/well), concanavalin A (0.5 μg/well) as a positive control, or the medium as an unstimulated control, and incubated at 37°C in 5% CO2 at 95% humidity. Culture supernatants were collected after 48 h and stored at −70°C until used for cytokine quantification.
Challenge experiments
Challenge strain HJL456 was prepared according to a previously described method (9). The remaining 40 mice were orally administered 2 × 108 colony-forming units of HJL456 in 20 μL of sterile PBS at 4 wk after primary vaccination and monitored for up to 14 d.
Statistical analysis
To test for differences in absorbance between the various vaccinated groups, data from the ELISA results were compared by analysis of variance with a post hoc Tukey’s test for pairwise comparisons and calculated with SPSS version 16.0 (SPSS, Chicago, Illinois, USA). Statistical significance was set at P < 0.01.
Results
No colony of ghost cells was observed on LB agar after incubation. The ghost cells were harvested by centrifugation at 4000 × g for 30 min, washed thrice, and resuspended in sterile PBS to a concentration of approximately 1 × 107 cells/mL. This suspension was used as the S. Typhimurium ghost vaccine candidate.
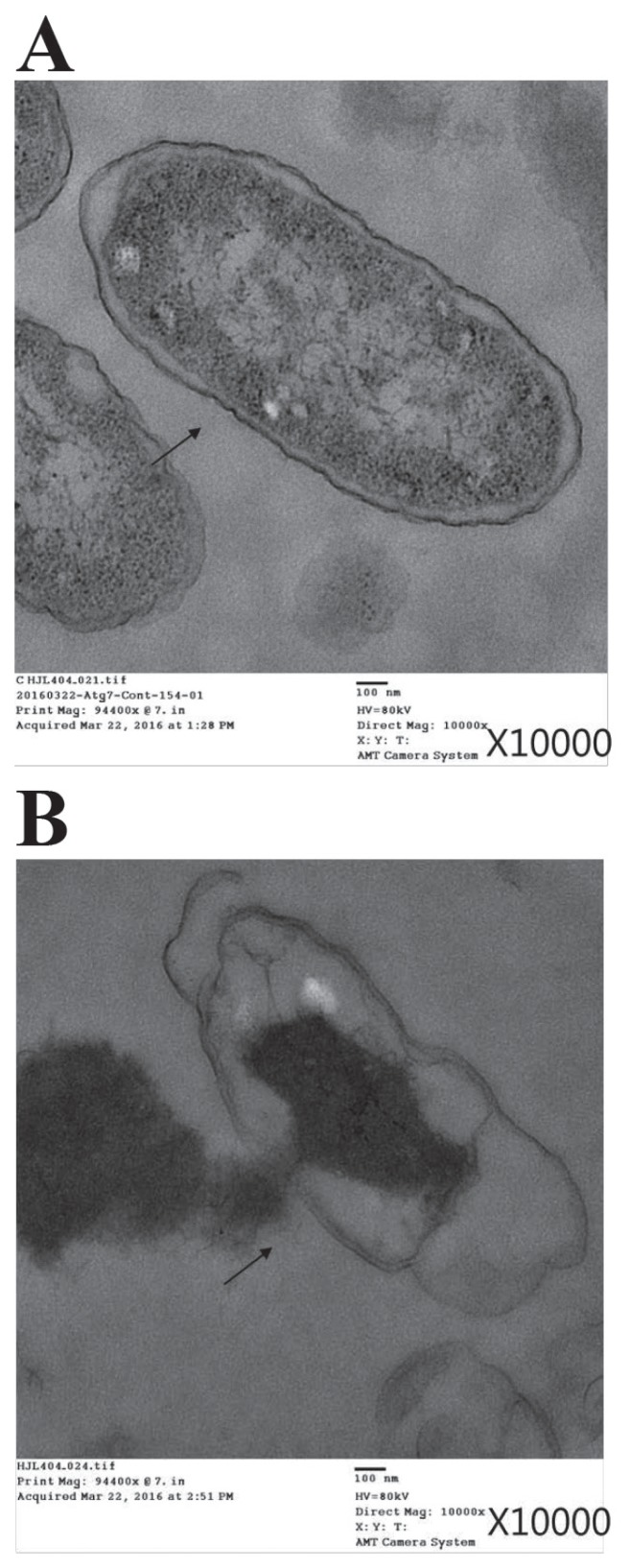
A complete cell membrane and full intracellular contents were observed by TEM in the untreated S. Typhimurium cells (Figure 1A), whereas after treatment with the fusion protein the cells exhibited obvious cytoplasmic clear zones and disruption of the cell membrane, with visible pores (Figure 1B).
Figure 1.
Transmission electron micrographs of Salmonella Typhimurium before (A) and after (B) treatment with a recombinant fusion protein consisting of lysozyme and porcine myeloid antimicrobial peptide 36. The bacterial cells were incubated with 40 μg/mL of the fusion protein for 16 h at 37°C. The arrows indicate a complete cell membrane (A) or a disrupted membrane with pores (B).
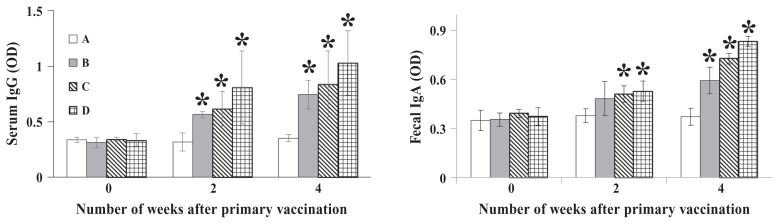
Serum titers of IgG against the OMPs of S. Typhimurium in mouse groups B, C, and D increased gradually from 2 wk after primary vaccination and were 2.1, 2.4, and 2.9 times higher, respectively, than those in group A at 4 wk (P < 0.05). In addition, the fecal titers of IgA against the OMPs in groups B, C, and D were 1.6, 2.0, and 2.2 times higher, respectively, than those in group A at 4 wk (P < 0.01) (Figure 2).
Figure 2.
Titers in mice of serum IgG and fecal IgA against outer membrane proteins of S. Typhimurium at various times after primary vaccination with sterile phosphate-buffered saline (group A) or the S. Typhimurium ghost vaccine, at doses of approximately 1.0 × 104, 1.0 × 105, and 1.0 × 106 cells in groups B, C, and D, respectively. Shown are the means for the 15 mice in each group and the standard deviations (SDs). Asterisks indicate a significant difference (P < 0.05) between the values for the vaccinated groups compared with the control group. OD — optical density.
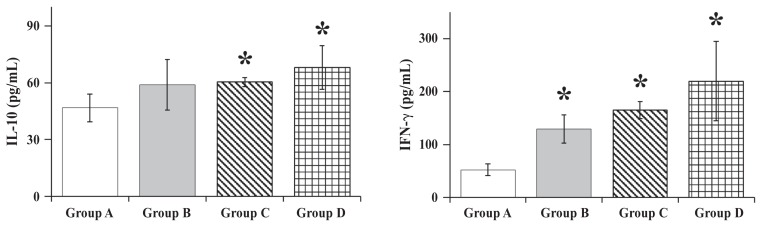
As shown in Figure 3, the mean concentrations of IL-10 against the S. Typhimurium ghost cells in the splenocytes harvested from mice in groups B, C, and D, respectively, at 4 wk after primary vaccination were 1.26, 1.29, and 1.46 times the mean for group A (P < 0.05). The mean concentrations of IFN-γ in the harvested splenocytes of groups B, C, and D, respectively, were 2.5, 3.1, and 4.2 times the mean for group A (P < 0.05).
Figure 3.
Concentrations of interleukin (IL)-10 and interferon gamma (IFN-γ) in the supernatants of splenocytes harvested 4 wk after primary vaccination from 5 mice in each group and stimulated with the ghost vaccine. Means, SDs, and asterisks as for Figure 2.
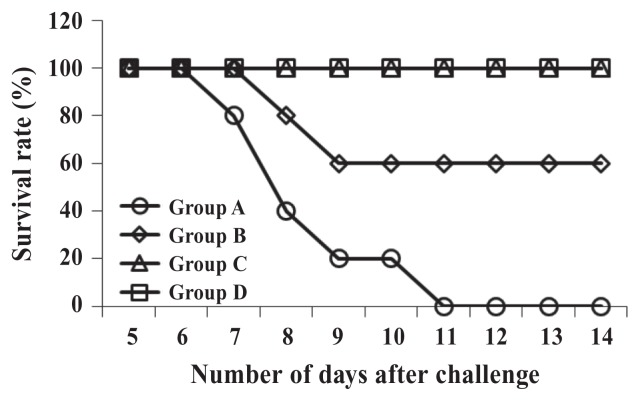
As shown in Figure 4, all the mice in groups C and D survived until the end of the study; however, the mice in the control group started dying 7 d after the challenge with strain HJL456 and were all dead by day 11, 2 mice having died between 8 and 9 d after the challenge.
Figure 4.
Survival rates after challenge with S. Typhimurium strain HJL456 in the same 4 groups of mice 4 wk after primary vaccination.
Discussion
Salmonella Typhimurium affects different organ systems as it penetrates the mucosal barrier and spreads to the phagocytic system (5,33–36). Therefore, an efficient salmonellosis vaccine must produce CMI (34,35). Several research teams have used live, attenuated S. Typhimurium strains as vaccine candidates (9,10,37–39); however, inherent safety risks such as reversion to virulence may obstruct the use of these strains for humans. Similar to S. Typhimurium, S. Gallinarum affects different organs and causes a well-recognized septicemic disease of poultry, called fowl typhoid (40,41). An efficient S. Gallinarum vaccine must produce CMI (41). A commercial live vaccine has been used to prevent fowl typhoid; however, again, reversion to virulence may occur (42). Recently, some researchers reported that an S. Gallinarum ghost vaccine candidate could induce CMI and protective antibody in chickens inoculated via an intramuscular, oral, or intraperitoneal route (14,43). The methodology represents a comparatively innovative approach to the improvement of vaccine technology but has seldom been used for S. Typhimurium. Construction of this S. Gallinarum ghost vaccine candidate was complex. Briefly, at least 1 essential gene from the bacterium had to be deleted to maintain the plasmid containing the E-lyse gene for inducing the ghost vaccine, and 2-step culture was necessary for induction (14,43).
Our new method of producing an S. Typhimurium ghost vaccine was based on S. Typhimurium lysis by means of a recombinant lysozyme–PMAP36 fusion protein, the lysozyme being derived from Salmonella bacteriophage p22 and an AMP for the formation of pores in cell envelopes. Therefore, the fusion protein was used to improve the lysis of S. Typhimurium. The bacterial ghost vaccine lacked all cytoplasmic contents; however, its outer membrane structure was preserved, and therefore the vaccine had high immunogenicity. The effectiveness of the ghost vaccine was demonstrated by TEM.
Live, attenuated S. Typhimurium as well some of its recombinant proteins have been studied as potential candidate vaccines to prevent salmonellosis (19,44–47). Recombinant proteins and inactivated vaccine candidates usually require multiple doses and the use of an effective adjuvant to induce efficient protective immune responses against Salmonella (19,46). Live, attenuated vaccine candidates have the risk of reverting to a virulent strain, although they can induce strong protective immune responses (41,42). In this study, we investigated whether IM vaccination with only the S. Typhimurium ghost vaccine induced with the recombinant fusion protein could protect mice against challenge with virulent S. Typhimurium. At the mucosal surface a vaccine producing a protective immune response can inhibit the entry and colonization of pathogens (19,48). In our study the serum IgG and fecal IgA titers were significantly higher in the vaccinated mice than in the control group. These results show that IM vaccination with the ghost vaccine powerfully enhanced humoral immune responses.
Because Salmonella is a facultative intracellular bacterium that survives in macrophages, CMI is vital for clearance (34,35,41). In our study, CMI was analyzed by ELISA evaluation of induced cytokines prepared from splenocytes collected from mice inoculated with the ghost vaccine and restimulated in vitro with the ghost vaccine. In general, mucosal IgA is strongly induced by Th2-type immunity. In particular, IL-10 is one of the main cytokines that enhances IgA production (48,49). Our results demonstrate that cytokine secretions, which are associated with an enhanced IgA response, were powerfully strengthened by our vaccine candidate. In addition, the splenocytes displayed high IFN-γ concentrations, providing an indication of Th1-type immunity. Thus, IM vaccination with an S. Typhimurium ghost vaccine produces sufficient cytokines, which are associated with CMI. In accordance with our original hypothesis, the antibody titers and CMI responses of the mice vaccinated with the ghost vaccine were significantly greater than those of the unvaccinated mice.
All the mice in groups C and D were completely protected against salmonellosis after challenge with a virulent wild-type S. Typhimurium strain, whereas all the control mice and 80% of the mice in groups A and B died after the challenge. This result suggests that IM vaccination with the S. Typhimurium ghost vaccine can elicit both types of immune response and effectively protect against salmonellosis in mouse models.
In this study S. Typhimurium was lysed by the recombinant lysozyme–PMAP36 fusion protein, to be used as a ghost vaccine. The vaccine, at doses of approximately 1 × 104, 1 × 105, and 1 × 106 cells, induced robust humoral and cell-mediated immune responses in mice and conferred protection against virulent S. Typhimurium infection in all the mice vaccinated with 1 × 105 or 1.0 × 106 cells and 20% of those vaccinated with 1 × 104 cells. Therefore, we conclude that IM vaccination with 1 × 105 cells of the ghost vaccine is effective against salmonellosis in a murine model.
Acknowledgments
This work was supported by the National Research Foundation of Korea and a grant (2013R1A4A1069486) from the Korean government.
References
- 1.Matsui M, Takaya A, Yamamoto T. σ32-mediated negative regulation of Salmonella pathogenicity island 1 expression. J Bacteriol. 2008;190:6636–6645. doi: 10.1128/JB.00744-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norimatsu M, Chance V, Dougan G, Howard CJ, Villarreal-Ramos B. Live Salmonella enterica serovar Typhimurium (S. Typhimurium) elicit dendritic cell responses that differ from those induced by killed S. Typhimurium. Vet Immunol Immunopathol. 2004;98:193–201. doi: 10.1016/j.vetimm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittrücker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 5.Abd El Ghany M, Jansen A, Clare S, et al. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect Immun. 2007;75:1835–1842. doi: 10.1128/IAI.01655-06. Epub 2007 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roesler U, Heller P, Waldmann KH, Truyen U, Hensel A. Immunization of sows in an integrated pig-breeding herd using a homologous inactivated Salmonella vaccine decreases the prevalence of Salmonella typhimurium infection in the offspring. J Vet Med B Infect Dis Vet Public Health. 2006;53:224–228. doi: 10.1111/j.1439-0450.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- 7.Thatte J, Rath S, Bal V. Immunization with live versus killed Salmonella typhimurium leads to the generation of an IFN-gamma dominant versus an IL-4-dominant immune response. Int Immunol. 1993;5:1431–1436. doi: 10.1093/intimm/5.11.1431. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein TK, Killar LM, Sultzer BM. Immunity to infection with Salmonella typhimurium: Mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984;150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 9.Hur J, Kim MY, Lee JH. Evaluation of efficacy of a new live Salmonella Typhimurium vaccine candidate in a murine model. Comp Immunol Microbiol Infect Dis. 2011;34:171–177. doi: 10.1016/j.cimid.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Hur J, Lee JH. Immunization of pregnant sows with a novel virulence gene deleted live Salmonella vaccine and protection of their suckling piglets against salmonellosis. Vet Microbiol. 2010;143:270–276. doi: 10.1016/j.vetmic.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Reyes AW, Simborio HL, Hop HT, Arayan LT, Kim S. Molecular cloning, purification and immunogenicity of recombinant Brucella abortus 544 malate dehydrogenase protein. J Vet Sci. 2016;17:119–122. doi: 10.4142/jvs.2016.17.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hur J, Lee JH. Immune responses to new vaccine candidates constructed by a live attenuated Salmonella Typhimurium delivery system expressing Escherichia coli F4, F5, F6, F41 and intimin adhesin antigens in a murine model. J Vet Med Sci. 2011;73:1265–1273. doi: 10.1292/jvms.11-0087. [DOI] [PubMed] [Google Scholar]
- 13.Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: Production and applications. Bioeng Bugs. 2010;1:326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhari AA, Jawale CV, Kim SW, Lee JH. Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective efficacy against fowl typhoid in chickens. Vet Res. 2012;43:44. doi: 10.1186/1297-9716-43-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang HY, Srinivasan J, Curtiss R., 3rd Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect Immun. 2002;70:1739–1749. doi: 10.1128/IAI.70.4.1739-1749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguchi M, Sekiya Y, Suzuki M, Yamamoto T, Matsui H. An oral Salmonella vaccine promotes the down-regulation of cell surface Toll-like receptor 4 (TLR4) and TLR2 expression in mice. FEMS Inmunol Med Microbiol. 2007;50:300–308. doi: 10.1111/j.1574-695X.2007.00240.x. Epub 2007 Apr 20. [DOI] [PubMed] [Google Scholar]
- 17.Negi VD, Singhamahapatra S, Chakravortty D. Salmonella enterica serovar Typhimurium strain lacking pmrG-HM-D provides excellent protection against salmonellosis in murine typhoid model. Vaccine. 2007;25:5315–5323. doi: 10.1016/j.vaccine.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Galen JE, Gomez-Duarte OG, Losonsky GA, et al. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine. 1997;15:700–708. doi: 10.1016/s0264-410x(96)00227-7. [DOI] [PubMed] [Google Scholar]
- 19.Risso GS, Carabajal MV, Bruno LA, et al. U-Omp19 from Brucella abortus is a useful adjuvant for vaccine formulations against Salmonella infection in mice. Front Immunol. 2017;8:171. doi: 10.3389/fimmu.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boman HG. Antibacterial peptides: Basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 21.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 22.Gazit E, Boman A, Boman HG, Shai Y. Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- 23.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 24.Wilmes M, Sahl HG. Defensin-based anti-infective strategies. Int J Med Microbiol. 2014;304:93–99. doi: 10.1016/j.ijmm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Lv Y, Wang J, Gao H, et al. Antimicrobial properties and membrane-active mechanism of a potential α-helical antimicrobial derived from cathelicidin PMAP-36. PLoS One. 2014;9:e86364. doi: 10.1371/journal.pone.0086364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Düring K, Porsch P, Mahn A, Brinkmann O, Gieffers W. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999;449:93–100. doi: 10.1016/s0014-5793(99)00405-6. [DOI] [PubMed] [Google Scholar]
- 28.Rennell D, Poteete AR. Phage P22 lysis genes: Nucleotide sequences and functional relationships with T4 and lambda genes. Virology. 1985;143:280–289. doi: 10.1016/0042-6822(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 29.Storici P, Scocchi M, Tossi A, Gennaro R, Zanetti M. Chemical synthesis and biological activity of a novel antibacterial peptide deduced from a pig myeloid cDNA. FEBS Lett. 1994;337:303–307. doi: 10.1016/0014-5793(94)80214-9. [DOI] [PubMed] [Google Scholar]
- 30.Kwon AJ, Moon JY, Kim WK, Kim S, Hur J. Protection efficacy of the Brucella abortus ghost vaccine candidate lysed by the N-terminal 24-amino acid fragment (GI24) of the 36-amino acid peptide PMAP-36 (porcine myeloid antimicrobial peptide 36) in murine models. J Vet Med Sci. 2016;78:1541–1548. doi: 10.1292/jvms.16-0036. Epub 2016 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adone R, Francia M, Pistoia C, Petrucci P, Pesciaroli M, Pasquali P. Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine. 2012;30:3992–3995. doi: 10.1016/j.vaccine.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Riquelme-Neira R, Retamal-Díaz A, Acuña F, et al. Protective effect of a DNA vaccine containing an open reading frame with homology to an ABC-type transporter present in the genomic island 3 of Brucella abortus in BALB/c mice. Vaccine. 2013;31:3663–3667. doi: 10.1016/j.vaccine.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Kincy-Cain T, Clements JD, Bost KL. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun. 2000;8:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brumme S, Arnold T, Sigmarsson H, et al. Impact of Salmonella Typhimurium DT104 virulence factors invC and sseD on the onset, clinical course, colonization patterns and immune response of porcine salmonellosis. Vet Microbiol. 2007;124:274–285. doi: 10.1016/j.vetmic.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Price JD, Simpfendorfer KR, Mantena RR, et al. Gamma interferon-independent effects of interleukin-12 on immunity to Salmonella enterica serovar Typhimurium. Infect Immun. 2007;75:5753–5762. doi: 10.1128/IAI.00971-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinnear CL, Strugnell RA. Vaccination method affects immune response and bacterial growth but not protection in the Salmonella Typhimurium animal model of typhoid. PLoS One. 2015;10:e0141356. doi: 10.1371/journal.pone.0141356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidalgo AA, Villagra NA, Jerez SA, Fuentes JA, Mora GC. A conditionally lethal mutant of Salmonella Typhimurium induces a protective response in mice. Biochem Biophys Res Commun. 2016;470:313–318. doi: 10.1016/j.bbrc.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 39.Powell DA, Roberts LM, Ledvina HE, Sempowski GD, Curtiss R, 3rd, Frelinger JA. Distinct innate responses are induced by attenuated Salmonella enterica serovar Typhimurium mutants. Cell Immunol. 2016;299:42–49. doi: 10.1016/j.cellimm.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pomeroy BS, Nagaraja KV. Fowl typhoid. Dis Poult. 1991;9:87–99. [Google Scholar]
- 41.Won G, Chaudhari AA, Lee JH. Protective efficacy and immune responses by homologous prime-booster immunizations of a novel inactivated Salmonella Gallinarum vaccine candidate. Clin Exp Vaccine Res. 2016;5:148–158. doi: 10.7774/cevr.2016.5.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon HJ, Cho SH. Pathogenicity of SG 9R, a rough vaccine strain against fowl typhoid. Vaccine. 2011;29:1311–1318. doi: 10.1016/j.vaccine.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 43.Jawale CV, Lee JH. Characterization of a Salmonella Typhimurium ghost carrying an adjuvant protein as a vaccine candidate for the protection of chickens against virulent challenge. Avian Pathol. 2014;43:506–513. doi: 10.1080/03079457.2014.966303. [DOI] [PubMed] [Google Scholar]
- 44.De Benedetto G, Alfini R, Cescutti P, et al. Characterization of O-antigen delivered by generalized modules for membrane antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine. 2017;35:419–426. doi: 10.1016/j.vaccine.2016.11.089. Epub 2016 Dec 18. [DOI] [PubMed] [Google Scholar]
- 45.Galen JE, Buskirk AD, Tennant SM, Pasetti MF. Live attenuated human Salmonella vaccine candidates: Tracking the pathogen in natural infection and stimulation of host immunity. EcoSal Plus. 2016;7(1):1–17. doi: 10.1128/ecosalplus.ESP-0010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebauer J, Kudlackova H, Kosina M, et al. A proteomic approach to the development of DIVA ELISA distingishing pigs infected with Salmonella Typhimurium and pigs vaccinated with a Salmonella Typhimurium-based inactivated vaccine. BMC Vet Res. 2016;12:252. doi: 10.1186/s12917-016-0879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groves PJ, Sharpe SM, Muir WI, Pavic A, Cox JM. Live and inactivated vaccine regimens against caecal Salmonella Typhimurium colonisation in laying hens. Aust Vet J. 2016;94:387–393. doi: 10.1111/avj.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okahashi N, Yamamoto M, Vancott JL, et al. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanCott JL, Staats HF, Pascual DW, et al. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines after oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]