Abstract
Muscle loss associated with disease (cachexia) or with aging (sarcopenia) is common in dogs, but clinically relevant methods for quantifying muscle loss are needed. We previously validated an ultrasound method of quantifying muscle size in dogs in a single breed. The goal of this study was to assess the variability and reproducibility of the Vertebral Epaxial Muscle Score (VEMS) in other dog breeds. Static ultrasound images were obtained from 38 healthy, neutered dogs of 5 different breeds between 1- and 5-years-old. The maximal transverse right epaxial muscle height and area at the level of the 13th thoracic vertebra (T13) were measured. Length of the 4th thoracic vertebra (T4) was measured from thoracic radiography. Ratios of the muscle height and area to vertebral length (height/T4 and area/T4, respectively) were calculated to account for differences in body size among breeds. Reproducibility testing was performed on 2 dogs of each breed (26% of the total) to determine intra- and inter-investigator reproducibility, as well as intra-class correlation. Mean height/T4 = 1.02 ± 0.18 and mean area/T4 = 3.32 ± 1.68. There was no significant difference for height/T4 (P = 0.10) among breeds, but breeds were significantly different in area/T4 (P < 0.001). Intra-class correlation ranged from 0.80 to 0.99. Testing showed better reproducibility for height/T4 compared to area/T4. The VEMS using height/T4 was valid and reproducible for healthy dogs of different sizes and body conformations. Studies assessing this technique in dogs with congestive heart failure and other diseases associated with muscle loss are warranted.
Résumé
La perte musculaire associée à la maladie (cachexie) ou à l’âge (sarcopénie) est fréquente chez les chiens, mais des méthodes appropriées pour quantifier en clinique la perte de muscle sont requises. Nous avons validé précédemment une méthode par échographie pour quantifier la taille musculaire chez des chiens d’une seule race. L’objectif de la présente étude était d’évaluer la variabilité et la reproductibilité du pointage du muscle épi-axial vertébral (PMEV) chez d’autres races de chien. Des images échographiques statiques furent obtenues de 38 chiens stérilisés en santé de cinq races différentes et âgés entre 1 et 5 ans. La hauteur transverse maximale du muscle épi-axial droit et la surface à la hauteur de la 13e vertèbre thoracique (T13) ont été mesurées. La longueur de la 4e vertèbre thoracique (T4) a été mesurée à partir de radiographies thoraciques. Les ratios de la hauteur du muscle et de la surface à la longueur de la vertèbre (hauteur/T4 et surface/T4, respectivement) ont été calculés pour tenir compte des différences de la taille entre les différentes races. Un test de reproductibilité a été effectué chez deux chiens de chaque race (26 % du total) afin de déterminer la reproductibilité intra- et inter-investigateur, ainsi que la corrélation intra-classe. La moyenne du ratio hauteur/T4 était de 1,02 ± 0,18 et la moyenne du ratio surface/T4 était de 3,32 ± 1,68. Il n’y avait pas de différence significative parmi les races pour le ratio hauteur/T4 (P = 0,10), mais les races différaient significativement pour ce qui est du ratio surface/T4 (P < 0,001). Les corrélations intra-classes variaient entre 0,80 et 0,99. Les tests ont montré une meilleure reproductibilité pour le ratio hauteur/T4 comparativement au ratio surface/T4. Le PMEV utilisant le ratio hauteur/T4 était valide et reproductible pour des chiens en santé de différentes tailles et conformations corporelles. Des études évaluant cette technique chez de chiens avec une défaillance cardiaque congestive et autres maladies associées avec de la perte musculaire sont justifiées. (Traduit par Docteur Serge Messier)
Introduction
Cachexia, a loss of muscle, occurs in various diseases, including congestive heart failure, cancer, and chronic kidney disease (1). Sarcopenia is the muscle loss that occurs with aging but, unlike cachexia, occurs in the absence of disease (1). In humans, and likely also in companion animals, this muscle loss can affect strength, immune function, wound healing, and is an independent predictor of survival (1–4). Given the high prevalence and serious consequences of cachexia, drugs are being developed for humans and companion animals with this condition. However, one of the main challenges of studying cachexia and sarcopenia is the accurate assessment of the degree of muscle loss. When severe muscle loss is present, cachexia and sarcopenia are easy to identify (although still difficult to quantify) but interventions are less likely to be successful at this stage. Therefore, earlier identification of cachexia and sarcopenia is desirable, as is a clinically relevant method of measuring the degree of muscle loss.
Subjective muscle condition scoring systems have been developed (5–8). There are methods that can provide quantification of total muscle mass (e.g., dual-energy X-ray absorptiometry) or of muscle mass in a certain area [e.g., computed tomography (CT)]. However, these methods typically require general anesthesia or heavy sedation and have limitations that may make them less accurate for measurement of muscle (9). To more effectively identify and quantitatively monitor treatment of cachexia, it will be necessary to have a sensitive, accurate, and non-invasive method to assess muscle loss. Our group evaluated an ultrasound method of measuring muscle in young and old Labrador retrievers compared to CT (10). In that study, both CT (young: 1.3 ± 0.2 versus old: 1.1 ± 0.2: P = 0.04) and ultrasound (young: 0.8 ± 0.2 versus old: 0.6 ± 0.1; P = 0.007) of the epaxial muscles could identify differences in muscle between healthy young and old dogs when the muscle measurement was normalized for body size using the height of the 13th thoracic vertebra (T13). In a clinical situation, using ultrasound is preferable to CT because ultrasound does not require sedation/anesthesia or the requirement for access to CT. Therefore, ultrasound assessment of muscle mass could be useful in clinical research. However, our previous study was performed only in a single dog breed and so it is important to assess whether a similar technique is also valid in dogs of different breeds and sizes. In addition, all measurements in the previous study were obtained by a single observer, so inter-observer reproducibility is unknown.
The primary objective of this study, therefore, was to assess the validity of this ultrasound technique for assessment of muscle mass in healthy dogs of varying sizes and shapes. A secondary objective was to evaluate the variability and reproducibility of this ultrasound method within and between observers in a subpopulation of dogs. Evaluation of this non-invasive method to assess muscle in dogs will enable future research on potential medical and nutritional treatments for the important syndromes of cachexia and sarcopenia.
Materials and methods
Subjects
Client-owned dogs between 1 and 5 y of age and of 5 different breeds (boxer, Cavalier King Charles spaniel, Chihuahua, dachshund, and Doberman pinscher) were included in the study. The breeds chosen for the study were selected in order to have a wide range of body sizes and conformations, and to represent breeds predisposed to cardiac disease that might be evaluated in future studies. Only neutered dogs were included in the study to reduce variation due to hormonal influences on muscle mass and dogs were enrolled so that there was an approximately equal percentage of male and female dogs in each breed group. All dogs were reported to be healthy by their owners and this was confirmed via physical examination and basic laboratory testing (i.e., packed cell volume, total solids, blood urea nitrogen, glucose). Owners signed an informed consent form before enrollment. The study was approved by the Cummings School of Veterinary Medicine at Tufts University Clinical Studies Review Committee and the Tufts University Institutional Animal Care and Use Committee.
Each dog was weighed and a physical examination was performed. Dogs were also assigned a body condition score (BCS; on a 9-point scale) (11) and muscle condition score (on a 4-point scale) (8) by a single investigator. Thoracic radiography [which included the 4th thoracic vertebra (T4)] was performed using a computed radiography system (Kodak CR800; Carestream Health, Rochester, New York, USA) with the dogs in right lateral recumbency. The 4th vertebra was selected as it is less subject to distortion in ascertaining size from a radiograph than other vertebrae and because T4 is also employed in assessing cardiac size with the Vertebral Heart Scale (12). The length of T4 was measured 3 times by a single researcher (JSS) and the results averaged together for a single value using the line tool and magnification features of a Digital Imaging and Communications in Medicine workstation (Carestream Vue PACS; Carestream Health, Rochester, New York, USA).
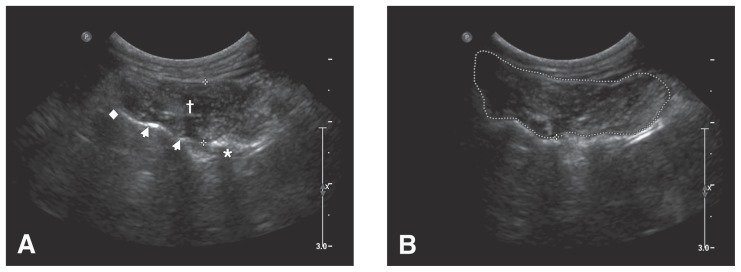
Ultrasonic measurements were performed at the level of T13 with an 8- to 5-MHz curvilinear transducer (Philips iU 22; Philips Medical Systems, Bothel, Washington, USA). Dogs were in standing position and unshaved skin was prepared with isopropyl alcohol to thoroughly wet the hair and skin and ultrasonography gel. The transducer was located at T13 with the beam angle perpendicular to the axis of the spine and using minimal pressure to avoid distorting the shape of the tissues. Measurements were obtained at the level of the articulation of the transverse process of the vertebra with the rib (i.e., the costotransverse joint). Three measurements of the maximal right epaxial muscle height (cm) were made and the mean of the 3 measurements was used as the final muscle height value. Height measurements were obtained using the distance calipers of the ultrasound machine with the first cursor being placed at the junction of the T13 lamina and transverse process at the bone-muscle interface and the second placed dorsolaterally at the muscle-subcutaneous tissue interface, providing the smallest short-axis dimension possible (Figure 1A). For epaxial muscle area, the freehand area tool on the ultrasound machine was used to draw a region around the epaxial musculature. The muscle margins were defined by the spinous process and lamina of T13 (medial margin), the 13th rib (ventral margin), and subcutaneous fat (dorsal and lateral margins; Figure 1B). Three measurements were taken of the maximal right epaxial muscle cross-sectional area in cm2 and the mean of the 3 measurements was used for the final muscle area value. Muscle height and area were measured on all dogs by one of the investigators (JSS) and these measurements were used to conduct analyses across the 5 different breeds.
Figure 1.
Transverse ultrasound images of the right epaxial muscles at the level of the 13th thoracic vertebra (T13) of a 1-year-old spayed female Cavalier King Charles spaniel. A — measurement of epaxial muscle height. * denotes the right transverse process of T13 and the articulation with the 13th rib. † is centered in the hypoechoic epaxial muscles. Arrowheads indicate the hyperechoic interface of the T13 lamina. The diamond is at the ventral aspect of the spinous process. The ultrasound calipers (crosses) measured a muscle height of 0.98 cm. B — measurement of the epaxial muscle area in the same dog. The dotted line estimates the muscle margins. The measured area was 2.63 cm2.
Additional measurements were taken in 2 dogs of each breed (n = 10), by the first radiologist (JSS), and by a second radiologist (AS) in order to evaluate the reproducibility of the measurements [left versus right side (same probe placement), intra-investigator (same and separate probe placement), and inter-investigator reproducibility (same and separate probe placement)]. Intra-investigator reproducibility was tested by having each of the 2 radiologists perform 3 measurements of the right epaxial muscle at the level of T13 with same-probe placement (3 measurements using the same image, with the mean of the 3 measurements used as the final value) and separate probe placement (3 measurements using separate probe placement, with the mean of the 3 measurements used as the final value). For the separate probe placement, between each transducer placement, the ultrasound transducer was removed from the dog, the imaging site (T13) was identified again, and the height and area of the muscle were re-measured. Inter-investigator reproducibility was tested by comparing the same and separate probe placement measurements of area and height of the right epaxial muscle between investigators. The length of T4 was used to calculate a ratio of the muscle area at T13 to T4 length (area/T4) and the muscle height at T13 to T4 length (height/T4). These ratios were used to standardize muscle measurements across dogs of varying sizes and shapes. Vertebral length was used instead of vertebral height as the margins are easier to define and less likely to be distorted.
Statistical analysis
The initial adaptive study design was for enrollment of 10 dogs for each of the 5 breeds for a total of 50 dogs, with interim analysis and a subsequent adjustment to the enrollment plan. However, enrollment was slower than anticipated, so an interim analysis was completed and enrollment was discontinued after 38 dogs. Data were examined graphically for normality. Data that were normally distributed are presented as mean ± SD and skewed data are presented as medians (range). Analysis of variance was used to compare muscle height/T4 and area/T4 across breeds. To compare the measures for the right side to those for the left, independent t-tests were performed with an additional sensitivity analysis using the non-parametric Wilcoxon rank-sum test to determine if, due to small sample sizes, any undetected departures from normality had substantial impact on significance of differences. For intra-investigator measures of variability, coefficients of variation (CV) were calculated for each measure using the replicates for each measure performed by each investigator. Intra-class correlations (ICC) were also generated using a linear mixed effects model (with replicates within observer as the repeated measure) for each measure, calculating the ICC as the common covariance (from a compound symmetry covariance structure) divided by the common covariance plus the residual variance (13). An ICC ≥ 0.80 indicated a high correlation between measurements, whereas an ICC from 0.50 to 0.79 indicated a moderate correlation, and an ICC from 0.20 to 0.49 indicated only a slight correlation (14). Bland-Altman plots were generated to investigate the agreement between the 2 investigators and to determine if there was any systematic bias. Commercial statistical software was used for data analysis (SAS Version 9.3; SAS Institute, Cary, North Carolina, USA). P-values < 0.05 were considered significant.
Results
Thirty-eight dogs were enrolled in the study between April, 2013 and March, 2016. This included boxers (n = 10), Cavalier King Charles spaniels (n = 10), Chihuahuas (n = 7), dachshunds (n = 6), and Doberman pinschers (n = 5). Nineteen of the dogs were castrated males and 19 were spayed females. Mean age for all 38 dogs was 3.1 ± 1.5 y. Median body weight was 9.0 kg (range: 1.5 to 38.8 kg), with a mean BCS of 6 ± 1. All dogs had a normal muscle condition score. Although body weight was significantly different among breeds (P < 0.001), there were no significant differences in gender, age, or BCS (Table I).
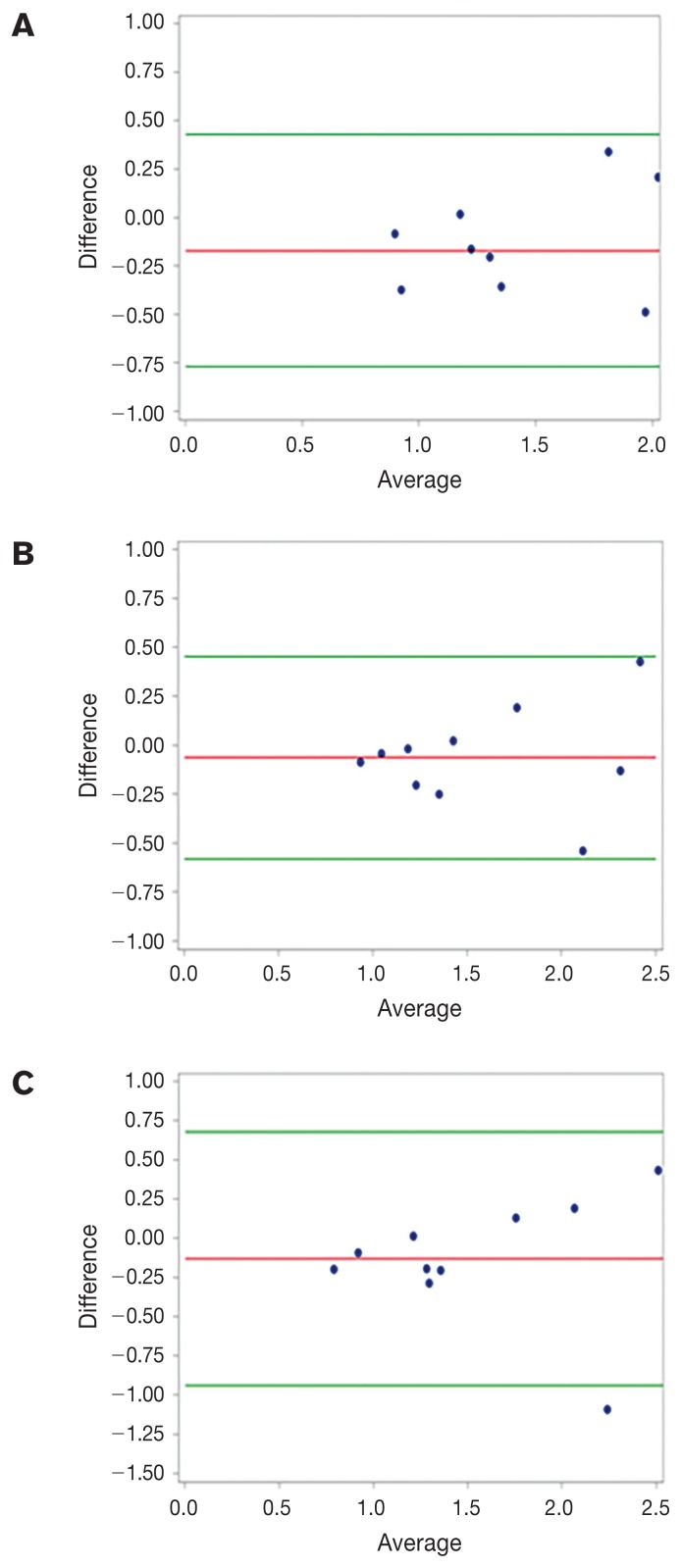
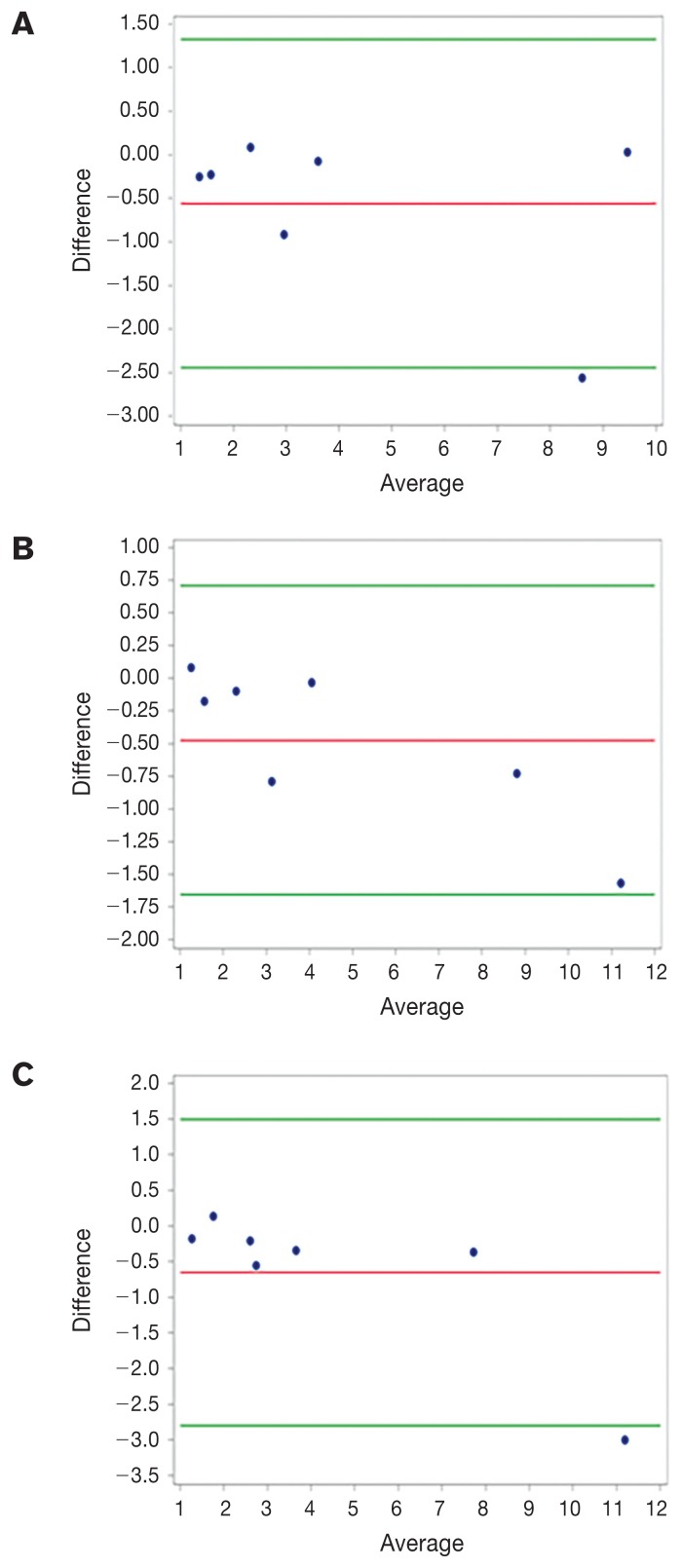
Mean (± SD) muscle height/T4 for all dogs combined was 1.02 ± 0.18 and mean muscle area/T4 was 3.32 ± 1.68 (Table I). There was no significant difference for height/T4 (P = 0.10) among breeds, but breeds were significantly different in area/T4 (P < 0.001). There were no significant differences between right and left side measurements for either muscle height or muscle area. For intra-investigator reproducibility testing, CVs were larger for muscle area (intra-observer CV = 67.3 to 78.0; total CV = 69.8 to 74.9) than for muscle height (intra-observer CV = 32.0 to 40.8; total CV = 33.7 to 38.5). Intra-class correlations ranged from 0.80 to 0.99. Bland-Altman plots illustrating inter-observer comparisons for muscle height and area are shown in Figures 2 and 3, respectively.
Figure 2.
Bland-Altman plots for muscle height at the level of the 13th thoracic vertebra (T13) comparing measurements between 2 investigators under 3 separate conditions: A — left side with the same probe placement, B — right side with the same probe placement, and C — right side with separate probe placement. The Y-axis represents investigator bias (both fixed and proportional) as the difference between the 2 investigators as a function of the average measurement (i.e., the best estimate of the real value) on the X-axis. The green lines represent the standard deviation of the differences which measures the random fluctuations around the mean difference.
Figure 3.
Bland-Altman plots for muscle area at the level of the 13th thoracic vertebra (T13) comparing measurements between 2 investigators under 3 separate conditions: A — left side with the same probe placement, B — right side with the same probe placement, and C — right side with separate probe placement. See Figure 2 legend for additional details.
Discussion
To our knowledge, this is the first published study evaluating a non-invasive, clinically applicable technique for measuring muscle in dogs of multiple breeds and sizes. The technique was developed and previously compared to CT in a single breed of dog (10), but it required some further refinements and validation in multiple breeds and with different investigators. In the previous study of Labrador retrievers, the ratio of the epaxial muscle area to vertebral size correlated well with CT results and identified significant differences between young and old dogs. However in the current study, there was significant variability in the muscle area/T4 among different breeds and insufficient reproducibility between and within investigators to support its use. The muscle area was subjectively more difficult to measure in large dogs due to the inability to reliably include both the dorsomedial and lateral aspects of the epaxial muscles on one ultrasound image.
Using a ratio of the muscle height/T4 length appears to normalize the muscle height across breeds and we propose the term, Vertebral Epaxial Muscle Score (VEMS) to represent this ultrasound technique. Additional studies are needed in larger groups and with more breeds but the VEMS may be a useful method in dogs. It would also be important to test the VEMS to determine whether it can identify differences in dogs of various breeds with sarcopenia or cachexia (e.g., cardiac cachexia, cancer cachexia) compared to healthy controls. Although this method assesses only a single location (the epaxial muscles), clinically, this appears to be the area affected earliest and most universally by muscle loss (1). It is important to note that neither CT nor the VEMS can assess “functional” muscle height or area since they cannot assess fatty infiltration of the muscle. Even MRI, which can assess fatty infiltration, does not truly measure muscle function. In humans, muscle function is an important component of cachexia and sarcopenia, and is assessed by tests such as grip strength or the 6-minute walk test (15). Additional research on methods of assessing muscle quality and function are warranted. The VEMS requires a thoracic radiograph to measure the length of T4, which is commonly acquired in many animals, especially those with cardiac disease. This method could be refined by identifying landmarks that could be measured without the need for a radiograph. However, ensuring that measurements would not be affected by overweight or obese body condition or other factors will be important. Additional work also is needed to optimize training so that users of the VEMS are measuring the muscle in the same place and using the same methods to ensure that results are comparable across studies. Even in the current study, there was modest intra- and inter-observer variability in the measurement of muscle height. Investigators experienced a learning curve for the method and measurements took less time with more practice, so adequate training is important to ensure reproducible results. The most difficult aspects were with measurement of the muscle area, particularly for the larger dogs in which the muscle often extended to the edge or just beyond the edge of the field of view. Some human studies have used the panoramic image function to address this issue, which could be considered in future canine studies.
There are a number of additional limitations to the current study. Dogs were identified as healthy based only on history, physical examination, and minimal laboratory testing. Although all were young and appeared to be healthy, underlying diseases associated with cachexia (i.e., muscle loss) could have been present. Nonetheless, none of the dogs exhibited any muscle loss, so diseases severe enough to affect the dogs’ muscle mass were considered less likely. Sample size also was lower than initially planned due to slow enrollment. Additional dogs could have revealed a significant difference among breeds for the VEMS (muscle height/T4), so additional research on this method is warranted. The limited number of breeds (5) and including only neutered dogs are also important to consider since there could be differences in muscle between other breeds and between neutered and intact animals. However, even if there is some inter-breed variation (e.g., with heavily muscled breeds), this measurement might still be a useful monitoring tool for muscle mass in individual dogs with cancer, chronic kidney disease, and congestive heart failure. Further research is needed to assess the precision of measurements over time in individual animals, particularly those with medical conditions. Dogs also varied in BCS and it is unclear how much this might affect the VEMS. For time and feasibility reasons, reproducibility testing was only performed on a subpopulation of 10 dogs (2 from each of the 5 breeds). Finally, we did not control for dogs’ normal activity level and this may have resulted in the variability in muscle size within breeds. This factor requires additional research to determine how much it can affect the resulting measurements.
Although the VEMS does not quantify the total amount of muscle mass, it may provide a clinically feasible method for detecting and monitoring the severity of muscle loss earlier in dogs with sarcopenia, congestive heart failure, cancer, chronic kidney disease, and other conditions associated with muscle loss. Much additional research is needed, but it may also provide better quantification of muscle loss in clinical cases and research studies, compared to more subjective muscle condition scores (5–8). This could help to identify dogs with muscle loss at an earlier stage and would provide valuable information on efficacy of medical, nutritional, and surgical treatments.
Acknowledgment
This study was supported by the Barkley Fund.
References
- 1.Freeman LM. Cachexia and sarcopenia: Emerging syndromes of importance in dogs and cats. J Vet Intern Med. 2012;26:3–17. doi: 10.1111/j.1939-1676.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos Intl. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: A systematic overview. Pharmacol Therap. 2009;121:227–252. doi: 10.1016/j.pharmthera.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Freeman LM, Rush JE, Kehayias JJ, et al. Nutritional alterations and the effect of fish oil supplementation in dogs with heart failure. J Vet Intern Med. 1998;12:440–448. doi: 10.1111/j.1939-1676.1998.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 6.Michel KE, Sorenmo K, Shofer FS. Evaluation of body condition and weight loss in dogs presented to a veterinary oncology service. J Vet Intern Med. 2004;18:692–695. doi: 10.1892/0891-6640(2004)18<692:eobcaw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Michel KE, Anderson W, Cupp C, Laflamme DP. Correlation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometry. Br J Nutr. 2011;106( Suppl 1):S57–59. doi: 10.1017/S000711451100050X. [DOI] [PubMed] [Google Scholar]
- 8.World Small Animal Veterinary Association Global Nutrition Committeee. Muscle condition score chart. 2013. [Last accessed August 1, 2016]. Available from: http://www.wsava.org/nutrition-toolkit.
- 9.Freeman LM, Kehayias JJ, Roubenoff R. Letter to the editor. J Vet Intern Med. 1996;10:99–100. doi: 10.1111/j.1939-1676.1996.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson D, Sutherland-Smith J, Watson AL, Freeman LM. Assessment of methods of evaluating sarcopenia in old dogs. Am J Vet Res. 2012;73:1794–1800. doi: 10.2460/ajvr.73.11.1794. [DOI] [PubMed] [Google Scholar]
- 11.Laflamme DP. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10–15. [Google Scholar]
- 12.Buchanan JW, Bücheler J. Vertebral scale system to measure canine heart size in radiographs. J Am Vet Med Assoc. 1995;206:194–199. [PubMed] [Google Scholar]
- 13.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York, New York: Academic Press; 1977. [Google Scholar]
- 15.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report on the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]