Abstract
Background
The aim of this study was to investigate the clinical and prognostic significance of pathological and inflammatory marker in mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery.
Material/Methods
We retrospectively evaluated the patient records of mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery at Liaoning Cancer Hospital and Institute from January 2006 to December 2013. The relationship between overall survival (OS) and clinicopathologic variables, pretreatment neutrophil-to-lymphocyte ratio (NLR), pretreatment platelet-to-lymphocyte ratio (PLR), pretreatment lymphocyte-to-monocyte ratio (LMR), and other biomarkers were analyzed by using Kaplan-Meier analysis and log-rank testing. Subsequently a Cox proportional hazard model was used to calculate hazard ratios for the risk of death.
Results
A total of 100 mucinous rectal cancer patients were included for analysis during the study period. The median age at presentation was 60.5 years (range, 26–81 years). The median overall survival (OS) for the whole group was 94 months. On univariate analysis, time interval from CCRT to operation (HR 0.37, p=0.03), lymphovascular invasion (HR 3.23, p=0.009), pretreatment NLR (HR 3.87, p=0.012), and LMR (HR 0.31, p=0.002) were significant prognostic factors for OS. In a multivariate analysis, pretreatment LMR was found to be an independent prognostic factor for overall survival (HR, 0.43; 95%CI, 0.18 to 1.00, p=0.045).
Conclusions
Pretreatment lymphocyte-to-monocyte ratio is a useful prognostic marker of OS in patients with mucinous rectal carcinoma treated with neoadjuvant chemoradiotherapy and curative surgery.
MeSH Keywords: Adenocarcinoma, Mucinous; Chemoradiotherapy; Neoadjuvant Therapy; Prognosis
Background
Colorectal cancer is the third most commonly diagnosed cancer in males and the second in females, with over 1.2 million new cancer cases and 608 700 deaths estimated to have occurred in 2008 [1]. Mucinous adenocarcinoma (MAC) is an uncommon and rare histopathological type of colorectal cancer, accounting for 4% to 15% of cases of primary colorectal cancer [2–4]. According to the World Health Organization definition, MAC is diagnosed when the extracellular mucin cover 50% of the lesion or more [5]. Although rectal cancer is a type of colorectal cancer, the management of rectal cancer is different from that of colon cancer due to its higher postoperative recurrence [6–8]. Currently, the standard of care for locally advanced rectal cancer (T3/4 or lymph node-positive) is neoadjuvant chemoradiotherapy (CRT) followed by curative surgery [9,10]. Although the prognosis of locally advanced rectal cancer has been significantly improved, about 5–10% of these patients develop recurrence [11,12], and there is marked heterogeneity in the duration of survival among these patients. Therefore, it is necessary to identify patients at greatest risk of worse outcomes by using clinical, inflammatory, and molecular biomarkers.
For the past decade, there has been a growing consensus that inflammation is involved in the development and progression of malignant tumors, including colorectal carcinoma [13]. An inflammatory microenvironment promotes the development of tumors by promoting angiogenesis and metastasis, subverting adaptive immune responses, and altering responses to hormones and chemotherapeutic agents [14,15]. Recently, the markers of systematic inflammatory response, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), have been reported to be associated with pathological complete response [16–19] and prognosis of colorectal cancer [20–24]. However, all of these reports included all histopathological types of colorectal cancer, and there have been no reports on the predictive role of systematic inflammatory response in mucinous rectal cancer. The aim of this retrospective study was to evaluate the prognostic significance of pathological and inflammatory markers in mucinous rectal cancer patients receiving neoadjuvant chemoradiotherapy and curative surgery.
Ethics statement
The Liaoning Cancer Hospital and Institute Ethics Institution Office approved this study. This retrospective study posed no potential risk to the subjects, and the subjects’ personal privacy was protected. This study strictly conformed to the principles outlined in the Declaration of Helsinki.
Material and Methods
Patients
The medical records of patients who underwent neoadjuvant chemoradiotherapy and surgical resection at Liaoning Cancer Hospital and Institute from 2006 to 2013 with a diagnosis of mucinous rectal cancer were retrieved from our department. Inclusion criteria were: patients aged 18 years or older with histologically confirmed clinical stage T3/4 or node-positive disease, located within 10 cm of the anal verge, rectal cancer as a single primary tumor, completed neoadjuvant chemoradiotherapy, and received radical surgery.
Clinical variables and definitions
Data on sex, age at diagnosis, date of diagnosis, pathological diagnosis, neoadjuvant chemotherapy, dose of radiation, time interval from concurrent chemoradiotherapy to operation, preoperative carcinoembryonic antigen (CEA) levels, preoperative α-fetoprotein (AFP) levels, and pathologic features (lymphovascular and perineural invasion) were obtained by reviewing the medical records. All tumors were staged according to the TNM staging system of the American Joint Committee on Cancer (7th version, 2009). Routine laboratory measurements were performed before neoadjuvant chemoradiotherapy. Data on hemoglobin (HB), neutrophil count, lymphocyte count, and platelet count were used to calculate NLR, PLR, and LMR. The cut-off CEA and AFP concentrations were 5.0 and 10 ng/d, respectively, which in our laboratory was the CEA concentration considered abnormal. NLR was defined as a simple ratio of the absolute neutrophil count over lymphocyte count. PLR was defined as the ratio of the platelet count over the absolute lymphocyte count. LMR was defined as the ratio of the absolute lymphocyte count over the monocyte count. Written informed consent was obtained from all patients and the study procedures were approved by the Ethics Committee of Liaoning Cancer Hospital and Institute.
Follow-up
Patients were followed at 3-month intervals for 2 years, at 6-month intervals for the next 3 years, and annually thereafter. The date of last follow-up was March 2016, which was mainly made with telephone calls. Recurrence was determined by clinical and radiologic examination or histologic confirmation. The main pattern of recurrence was recorded as the first site of detectable failure during the follow-up period. Disease-free survival (DFS) was the time from the surgery to the local or distant failure. Overall survival (OS) was calculated from neoadjuvant chemoradiotherapy to death induced by all causes, or end of follow-up.
Statistical analysis
Survival analysis was conducted using the Kaplan-Meier method. The comparison of the survival curves was performed by log-rank test. A multivariable Cox regression analysis was performed to identify predictive factors of overall survival. Every variable was analyzed by univariate analysis to cover all potentially important predictors, then variables with P≤0.10 in univariate analysis were included in multivariable analysis. This level was chosen to incorporate all potentially important predictor variables in the final modeling process. All sets of variables were analyzed: sex, clinical T stage, clinical N stage, AJCC stage, tumor size, interval from CCRT to operation, total dose of radiation, lymphovascular invasion, perineural invasion, preoperative HB, CEA and AFP levels, and preoperative NLR, PLR, and LMR levels. The optimal cut-off values of NLR, PLR, and LMR were determined by receiver operating characteristic (ROC) analysis, using OS as the end-point. Statistical analysis was performed by using SPSS16.0 software (SPSS Inc., Chicago, IL). P<0.05 was considered statistically significant.
Results
Patient characteristics
The clinical and pathological characteristics of 100 patients (male-to-female ratio: 2.33: 1) are summarized in Table 1. The median age was 60.5 (26–81 years). The mean preoperative CEA and AFP level was 10.0 U/ml (range 0.48–184 U/ml) and 3.75 U/ml (1.05–22.2), including 18 patients with stage II disease and 82 patients with stage III disease. All patients received intensity-modulated radiation therapy to the pelvis of 45–50 Gy and a concomitant boost of 5 Gy to the primary tumor in 25 fractions. A total of 30 (30%) patients received oral capecitabine alone concurrent with radiotherapy, 65 (65%) patients received both capecitabine and oxaliplatin, and 5 (5%) patients received both oxaliplatin and irinotecan. The median interval between the completion of neoadjuvant chemoradiotherapy and surgery was 7 weeks (range 2–12 weeks) (Table 1).
Table 1.
Clinicopathologic characteristics of 100 mucinous rectal cancer patients.
| Variable | Value |
|---|---|
| Median age (range), y | 60.5 (26–81) |
| Gender, n | |
| Male | 70 |
| Female | 30 |
| Tumor size, cm | |
| ≥5 | 9 |
| <5 | 91 |
| T stage, n | |
| T3 | 58 |
| T4 | 42 |
| Lymph node involvement, n | |
| N(−) | 19 |
| N(+) | 81 |
| Stage, n | |
| II | 18 |
| III | 82 |
| Interval from CCRT to operation (wk) | |
| <7 | 64 |
| ≥7 | 36 |
| Total dose of radiotherapy (Gy) | |
| <50 | 23 |
| ≥50 | 77 |
| Chemotherapy regimens | |
| Capecitabine | 30 |
| L-OHP+ capecitabine | 65 |
| CPT-11+capecitabine | 5 |
| Initial HB | |
| <10 | 8 |
| ≥10 | 92 |
| Preoperative CEA levels | |
| Mean (range), U/ml | 10 (0.48–184) |
| Preoperative AFP levels | |
| Mean (range), U/ml | 3.75 (1.05–22.2) |
| Lymphovascular invasion, n | |
| Positive | 12 |
| Negative | 88 |
| Perineural invasion, n | |
| Positive | 14 |
| Negative | 86 |
The optimal cut-off value for inflammation-based scores
ROCs were performed, and the optimal threshold of inflammation-based score was obtained when the Youden index was maximal. The optimal cut-off points of NLR, PLR, and LMR were 2.25 (Youden index, 0.289), 155 (Youden index, 0.257) and 3 (Youden index, 0.371), respectively. Patients were divided into low and high groups based on these cut-off values. In terms of the LMR, 32 (32%) patients had a low LMR and 68 (68%) had a high LMR. For the NLR, 34 patients (34%) were in the low group, whereas 66 (66%) were in the high group. For the PLR, 50 (52%) patients were in the low group, whereas 48 (48%) were in the high group.
Pattern of failure
After a median follow-up of 45.5 months, a total of 34 mucinous rectal cancer developed recurrence. The specific sites of recurrence were listed in Table 2. Estimated 5-year DFS rate was 63.3±4.9%. The most common site was lung metastasis (35.3%) and local recurrence (26.5%).
Table 2.
Pattern of recurrence.
| Recurrence sites | MAC, n (%) |
|---|---|
| Total | 34* |
| Local recurrence | 9 |
| Liver | 7 |
| Lung | 12 |
| Bone | 2 |
| Lymph node | 6 |
One MAC patient relapsed with lymph node and liver metastasis; another MAC patient relapsed with liver and lung metastasis.
Treatment outcome
The median overall survival (OS) for the whole series was 94 months. The estimated 5-year OS rate was 76.8±4.6%. The cumulative 5-year survival was 81.4% for the lymphovascular invasion-positive group and 42.9% for lymphovascular invasion-negative group (p=0.005). Additionally, estimated 5-year OS for interval from CCRT to operation more than 7 weeks was also significantly higher than for interval from CCRT to operation less than 7 weeks (82.9% versus 42.9%, p=0.023).
Analysis of prognostic factors for overall survival
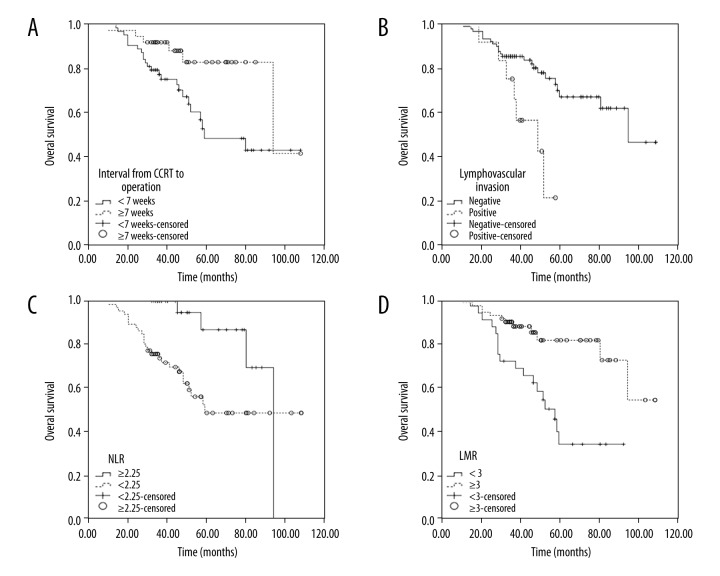
On univariate analysis, interval from CCRT to operation (Figure 1B), lymphovascular invasion (Figure 1A), NLR (Figure 1C), and LMR (Figure 1D) were significantly predictive for longer survival (Table 3), whereas sex, tumor size, and T stage were insignificant variables.
Figure 1.
Univariate analyses for prognostic variable of overall survival: (A) Interval from CCRT to operation (<7 weeks versus ≥7 weeks); (B) Lymphovascular invasion (negative versus positive); (C) Pretreatment NLR (<2.25 versus ≥2.25); (D) LMR (<3 versus ≥3).
Table 3.
Predictive factors for overall survival by univariate and multivariate analyses of the cohort (n=100).
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender | ||||||
| Female | 1 | – | ||||
| Male | 1.13 | 0.55–2.33 | 0.74 | – | – | – |
| T stage | ||||||
| T3 | 1 | – | ||||
| T4 | 1.13 | 0.55–2.33 | 0.74 | – | – | – |
| N stage | ||||||
| N(−) | 1 | – | ||||
| N(+) | 0.85 | 0.36–1.99 | 0.70 | – | – | – |
| Stage | ||||||
| II | 1 | – | ||||
| III | 0.96 | 0.39–2.35 | 0.92 | – | – | – |
| Preoperative HB level | ||||||
| <10 | 1 | – | ||||
| ≥10 | 1.47 | 0.35–6.21 | 0.60 | – | – | – |
| Tumor size, cm | ||||||
| <5 | 1 | – | ||||
| ≥5 | 1.75 | 0.61–5.03 | 0.30 | – | – | – |
| Interval from CCRT to operation, wk | ||||||
| <7 | 1 | 1 | ||||
| ≥7 | 0.37 | 0.15–0.91 | 0.03 | 0.49 | 0.20–1.23 | 0.13 |
| Total dose of RT, Gy | ||||||
| <50 | 1 | – | ||||
| ≥50 | 0.90 | 0.41–1.95 | 0.79 | – | – | – |
| Preoperative AFP levels, U/ml | ||||||
| <10 | 1 | – | ||||
| ≥10 | 0.91 | 0.12–6.72 | 0.93 | – | – | – |
| Preoperative CEA levels, U/ml | ||||||
| <5 | 1 | – | ||||
| ≥5 | 1.69 | 0.81–3.55 | 0.17 | – | – | – |
| Lymphovascular invasion | ||||||
| Negative | 1 | 1 | ||||
| Positive | 3.23 | 1.35–7.75 | 0.009 | 2.29 | 0.92–5.74 | 0.076 |
| Perineural invasion | ||||||
| Negative | 1 | – | ||||
| Positive | 1.68 | 0.68–4.12 | 0.26 | – | – | – |
| NLR | ||||||
| Low (<2.25) | 1 | 1 | ||||
| High (≥2.25) | 3.87 | 1.35–11.11 | 0.012 | 2.87 | 0.87–9.43 | 0.082 |
| PLR | ||||||
| Low (<150) | 1 | 1 | ||||
| High (≥150) | 2.00 | 0.93–4.30 | 0.074 | 0.70 | 0.27–1.80 | 0.46 |
| LMR | ||||||
| Low (<3) | 1 | 1 | ||||
| High (≥3) | 0.31 | 0.14–0.64 | 0.002 | 0.43 | 0.18–1.00 | 0.045 |
In Cox proportional hazard model, pretreatment LMR was found to be an independent prognostic factor for overall survival (hazard ratio, 0.43; 95%CI, 0.18 to 1.00, p=0.045) (Table 3).
Discussion
It has been recognized that MAC of the rectum is a rare and distinctive type of cancer. Previous studies demonstrated that mucinous carcinoma is associated with a larger diameter, higher T classification, and extrahepatic localization of metastases [25–27], and the prognosis of patients with MAC was worse than that of non-MAC colorectal cancer. Consistent with previous studies, the estimated 5-year DFS and OS rates were 63.3% and 76.8%, respectively. We then analyzed clinical factors or treatment variables that are associated with overall survival in mucinous rectal cancer patients who received neoadjuvant chemoradiotherapy and curative surgery. Our univariate results show that the interval from CCRT to operation (HR 0.37, p=0.03), lymphovascular invasion (HR 3.23, p=0.009), pretreatment NLR (HR 3.87, p=0.012), and LMR (HR 0.31, p=0.002) are significant prognostic factors for OS. Multivariate analysis results show pretreatment LMR is an independent prognostic factor for overall survival (hazard ratio, 0.43, p=0.045).
The benefit of neoadjuvant chemoradiotherapy in rectal cancer is well established in several phase III trials due to its reduced local recurrence [28,29], but the best interval between neoadjuvant therapy and surgical treatment is still undetermined. It seems that the radiotherapy-induced necrosis might be time-dependent; thus, a controlled postponement of surgery would allow a potentiation of the effect, maximizing the benefits of neoadjuvant therapy. Francois et al. [30] demonstrated that a longer interval between neoadjuvant radiation and surgery was associated with improved tumor clinical response and pathologic downstaging. Recently, Wang et al. performed a meta-analysis and also found that there was a significantly increased rate of pathological complete response in rectal cancer patients treated with surgery followed 7 or 8 weeks later by neoadjuvant chemotherapy (RR, 1.45; 95% CI, 1.18–1.78; and p<0.01 and RR, 1.49; 95% CI, 1.15–1.92; and p=0.002, respectively) [31]. In the present, we found that estimated 5-year OS for the interval from CCRT to operation more than 7 weeks is significantly higher than for the interval from CCRT to operation less than 7 weeks (82.9% versus 42.9%, p=0.023). On univariate analysis, the interval from CCRT to operation is a significant predictor for OS, but it became non-significant in multivariate analysis.
Several previous studies have demonstrated that positive lymphovascular invasion is a predictor of poorer OS in colorectal cancer patients [26,27,32]. Consistent with the published data, we also found that lymphovascular invasion is a significant predictor for OS in mucinous rectal cancer in univariate analysis, but it is not an independent prognostic factor for overall survival in multivariate analysis.
More and more evidence indicates that cancer-related inflammation is involved in cancer development and progression [33,34]. Neutrophilia, thrombocytosis, monocytosis, and lymphopenia tend to represent a nonspecific response to cancer-related inflammation and are associated with poor survival in cancers [34,35]. Neutrophils interact with tumor cells by producing cytokines and chemokines, which affects tumor cell proliferation, angiogenesis, and metastases [36]. Tumor-associated macrophages, which arise from blood monocytes, promote tumor progression and metastases [37]. Chan et al. found that the lymphocyte-to-monocyte ratio was an independent predictor of OS in patients with CRC undergoing curative resection [23]. Shibutani et al. [21] demonstrated that postoperative NLR was an independent prognostic factor in patients with CRC who underwent potentially curative surgery. However, the prognostic role of inflammatory indexes in mucinous rectal cancer patients who received neoadjuvant chemoradiotherapy and curative surgery remains undetermined. As inflammation-based indexes are simple and comprise components of blood assay with low cost, the establishment of a predictive model based on inflammation is useful, especially for patients in developing countries. In our study, we show that pretreatment NLR and LMR are significant prognostic factors for OS in these patients. Multivariate analysis results show that pretreatment LMR is an independent prognostic factor for overall survival (hazard ratio, 0.43, p=0.045).
The main advantage of the present study is that it is one of the largest series evaluating the treatment outcomes of mucinous rectal cancer patients who received neoadjuvant chemoradiotherapy and curative surgery. However, there are several limitations in this study. The major limitation is that it is a retrospective study, which could result in various sources of bias. However, it appears difficult to conduct a randomized trial for this rare disease. Secondly, this is a single-institution study; thus, the findings of this study might not be applicable to other cohorts of patients. Additionally, we evaluated patients who were treated over the course of 7 years. The radiotherapy and chemotherapy regimens may have changed over time.
Conclusions
In conclusion, this large retrospective study demonstrates that pretreatment LMR is an independent clinical predictor for overall survival in patients with mucinous rectal carcinoma treated with neoadjuvant chemoradiotherapy and curative surgery. These findings may help clinicians predict the prognosis of mucinous rectal carcinoma and develop individualized treatment strategies.
Footnotes
Source of support: This work was supported by the National Natural Science Fund from the National Natural Science Foundation of China (grant no. 81672427)
Conflicts of interest statement
All authors declare that they have no potential conflicts of interests.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chew MH, Yeo SA, Ng ZP, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis. 2010;25(10):1221–29. doi: 10.1007/s00384-010-1033-3. [DOI] [PubMed] [Google Scholar]
- 3.Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer. 2006;107(5 Suppl):1128–41. doi: 10.1002/cncr.22010. [DOI] [PubMed] [Google Scholar]
- 4.Du W, Mah JT, Lee J, et al. Incidence and survival of mucinous adenocarcinoma of the colorectum: A population-based study from an Asian country. Dis Colon Rectum. 2004;47(1):78–85. doi: 10.1007/s10350-003-0014-9. [DOI] [PubMed] [Google Scholar]
- 5.Bosman FT. In: WHO Classification of Tumors of the Digestive System. 4th ed. Carneiro HRH, Theise ND, editors. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 6.Boland PM, Fakih M. The emerging role of neoadjuvant chemotherapy for rectal cancer. J Gastrointest Oncol. 2014;5(5):362–73. doi: 10.3978/j.issn.2078-6891.2014.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen LB, Wille-Jorgensen P. National and international guidelines for rectal cancer. Colorectal Dis. 2014;16(11):854–65. doi: 10.1111/codi.12678. [DOI] [PubMed] [Google Scholar]
- 8.Salem ME, Hartley M, Unger K, et al. Neoadjuvant combined-modality therapy for locally advanced rectal cancer and its future direction. Oncology (Williston Park) 2016;30(6):546–62. [PubMed] [Google Scholar]
- 9.Li Y, Wang J, Ma X, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci. 2016;12(8):1022–31. doi: 10.7150/ijbs.15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Peng Y, Wang LA, et al. The influence of neoadjuvant therapy for the prognosis in patients with rectal carcinoma: A retrospective study. Tumour Biol. 2016;37(3):3441–49. doi: 10.1007/s13277-015-4153-0. [DOI] [PubMed] [Google Scholar]
- 11.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 12.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–25. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 13.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 14.Nozoe T, Matsumata T, Kitamura M, et al. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176(4):335–38. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 15.Guillem-Llobat P, Dovizio M, Alberti S, et al. Platelets, cyclooxygenases, and colon cancer. Sem Oncol. 2014;41(3):385–96. doi: 10.1053/j.seminoncol.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Ryan JE, Warrier SK, Lynch AC, et al. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Colorectal Dis. 2016;18(3):234–46. doi: 10.1111/codi.13207. [DOI] [PubMed] [Google Scholar]
- 17.De Felice F, Izzo L, Musio D, et al. Clinical predictive factors of pathologic complete response in locally advanced rectal cancer. Oncotarget. 2016;7(22):33374–80. doi: 10.18632/oncotarget.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi E, Kim JH, Kim OB, et al. Predictors of pathologic complete response after preoperative concurrent chemoradiotherapy of rectal cancer: A single center experience. Radiat Oncol J. 2016;34(2):106–12. doi: 10.3857/roj.2015.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawfik B, Mokdad AA, Patel PM, et al. The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs. 2016;27(9):879–83. doi: 10.1097/CAD.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 20.Sokolov M, Angelov K, Vasileva M, et al. Clinical and prognostic significance of pathological and inflammatory markers in the surgical treatment of locally advanced colorectal cancer. Onco Targets Ther. 2015;8:2329–37. doi: 10.2147/OTT.S82958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World J Surg Oncol. 2015;13:194. doi: 10.1186/s12957-015-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin MS, Huang JX, Yu H. Prognostic significance of Glasgow prognostic score in patients with stage II colorectal cancer. Int J Clin Exp Med. 2015;8(10):19138–43. [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JC, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539–46. doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J, Zhu Y, Wu W, et al. Prognostic role of neutrophil-to-lymphocyte ratio in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Med Sci Monit. 2017;23:315–24. doi: 10.12659/MSM.902752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitsche U, Zimmermann A, Spath C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258(5):775–82. doi: 10.1097/SLA.0b013e3182a69f7e. discussion 782–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Zhang YC, Yang XY, et al. Prognostic significance of the mucin component in stage III rectal carcinoma patients. Asian Pac J Cancer Prev. 2014;15(19):8101–5. doi: 10.7314/apjcp.2014.15.19.8101. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Huh JW, Park YA, et al. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine (Baltimore) 2015;94(15):e658. doi: 10.1097/MD.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30(31):3827–33. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 29.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 30.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 31.Wang XJ, Zheng ZR, Chi P, et al. Effect of interval between neoadjuvant chemoradiotherapy and surgery on oncological outcome for rectal cancer: A systematic review and meta-analysis. Gastroenterol Res Pract. 2016;2016:6756859. doi: 10.1155/2016/6756859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makela JT, Kiviniemi H. Clinicopathological features of colorectal cancer in patients under 40 years of age. Int J Colorectal Dis. 2010;25(7):823–28. doi: 10.1007/s00384-010-0914-9. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8(8):2553–62. [PubMed] [Google Scholar]
- 34.Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–78. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku JH, Kang M, Kim HS, et al. The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br J Cancer. 2015;112(3):461–67. doi: 10.1038/bjc.2014.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji H, Houghton AM, Mariani TJ, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25(14):2105–12. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 37.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]