Abstract
Objective:
To assess whether smoking cessation after an ischemic stroke or TIA improves outcomes compared to continued smoking.
Methods:
We conducted a prospective observational cohort study of 3,876 nondiabetic men and women enrolled in the Insulin Resistance Intervention After Stroke (IRIS) trial who were randomized to pioglitazone or placebo within 180 days of a qualifying stroke or TIA and followed up for a median of 4.8 years. A tobacco use history was obtained at baseline and updated during annual interviews. The primary outcome, which was not prespecified in the IRIS protocol, was recurrent stroke, myocardial infarction (MI), or death. Cox regression models were used to assess the differences in stroke, MI, and death after 4.8 years, with correction for adjustment variables prespecified in the IRIS trial: age, sex, stroke (vs TIA) as index event, history of stroke, history of hypertension, history of coronary artery disease, and systolic and diastolic blood pressures.
Results:
At the time of their index event, 1,072 (28%) patients were current smokers. By the time of randomization, 450 (42%) patients had quit smoking. Among quitters, the 5-year risk of stroke, MI, or death was 15.7% compared to 22.6% for patients who continued to smoke (adjusted hazard ratio 0.66, 95% confidence interval 0.48–0.90).
Conclusion:
Cessation of cigarette smoking after an ischemic stroke or TIA was associated with significant health benefits over 4.8 years in the IRIS trial cohort.
Stroke is the fifth leading cause of death in the United States and the second leading cause of death worldwide.1 Tobacco use is estimated to be responsible for 12% to 37% of all stroke events and is therefore a leading preventable cause.2,3 Other well-documented harms of smoking include coronary and peripheral artery disease, chronic obstructive pulmonary disease, and lung and bladder cancer. Despite decades of research on these adverse effects, 15% of adults in the United States currently smoke cigarettes.4 Recent American Heart Association guidelines for primary prevention of stroke include Class I recommendations for continued abstinence for nonsmokers and for cessation by current smokers. The recommendation for cessation is based on observational data showing that quitting rapidly reduces a smoker's risk of a first stroke or myocardial infarction (MI) to near that of someone who never smoked.5
Smoking cessation might be particularly beneficial for patients who have already had an ischemic stroke or TIA because they are at very high risk for future cardiovascular events.6 This potential benefit prompted the American Heart Association to issue a Class I recommendation for smoking cessation in the 2014 secondary stroke prevention guidelines, but the level of evidence is only C, meaning that it is based on evaluations of very limited populations, consensus expert opinions, case studies, or accepted standard of care.6 To better quantify the association between smoking after ischemic stroke or TIA and the risk for adverse health outcomes, we examined the effect of smoking cessation among participants enrolled in the Insulin Resistance Intervention After Stroke (IRIS) trial.
METHODS
IRIS study design and procedures.
The methods and main results for the IRIS trial have been previously published.7,8 Briefly, IRIS was a randomized, double-blind, placebo-controlled study in 3,876 insulin-resistant, nondiabetic patients with a recent ischemic stroke or TIA designed to test whether pioglitazone, an insulin-sensitizing drug of the thiazolidinedione class, would reduce the incidence of MI and stroke. From 2005 to 2013, investigators at 179 hospitals and clinics in 7 countries enrolled patients and collected data. Participants were followed up for up to 5 years from randomization or to the last scheduled follow-up contact before August 1, 2015, whichever came first.
Standard protocol approvals, registrations, and patient consents.
Informed consent was signed by all patients. The IRIS study was approved by the review committees at the participating sites.
Potentially eligible patients with a qualifying neurologic event within the prior 6 months underwent a screening interview, physical examination, and phlebotomy. Patients were excluded if they met the 2005 American Diabetes Association criteria for diabetes (i.e., fasting plasma glucose ≥126 mg/dL [7.0 mmol/L], repeated and confirmed) or if their hemoglobin A1c was ≥7.0% at the screening blood test. Insulin resistance was defined by a score >3.0 on the homeostasis model assessment–insulin resistance measure.9 Other major exclusions included history of heart failure or bladder cancer and predicted survival <4 years.
Standardized procedures were used to collect data on each participant's medical history and clinical features associated with increased cardiovascular risk. At baseline, patients were asked about their smoking history, including age at initiation, average number of cigarettes smoked daily, current status (smoking, not smoking), and, for former smokers, the time since most recent cessation. At each annual contact, participants were asked about current smoking status. Adherence to standard preventive therapies for secondary stroke prevention was also ascertained at baseline and annually, including measurement of blood pressure and a medication history. Quarterly interviews inquired about interim health events, including stroke and heart attack. All reported events were submitted to external reviewers for adjudication.
Statistical methods.
Study size for the original IRIS trial was determined by a power analysis for its primary research aim.
For this prospective observational cohort study, IRIS participants were classified according to smoking status at the time of randomization: never smokers, former smokers (i.e., stopped smoking before the index stroke or TIA event), quitters (i.e., quit after the index event and not smoking at the time of randomization), or continuing smokers. The primary aim of our analysis was to compare the risk of the composite outcome of stroke, MI, or death in patients who quit smoking vs patients who continued to smoke after their index event. We also examined risk for the components of this outcome (i.e., stroke alone, MI alone, and all-cause mortality). Participants without outcomes were censored at the time of their last completed follow-up contact. These analyses were not prespecified in the IRIS research protocol or data analysis plan. Rather, the analysis was designed after completion of the IRIS trial because of observational research showing that smoking cessation in patients with established coronary heart disease reduced subsequent all-cause mortality10 or recurrent cardiovascular events.11,12
Cumulative probabilities of outcome-free survival over time by smoking status were calculated by the Kaplan-Meier method,13 and differences were tested by the log-rank statistic using an α of 0.05 (2 sided). The effect of smoking status on risk was quantified by hazard ratios (HRs; with 95% confidence intervals [CIs]) from Cox proportional hazards models14 that included baseline cardiovascular risk features and treatment assignment (pioglitazone or placebo). The risk features included in the Cox models were those prespecified as adjustment variables for the IRIS trial and included the following: age, sex, stroke (vs TIA) as the index event, history of stroke, history of hypertension, history of coronary artery disease, systolic blood pressure, and diastolic blood pressure. All adjustment variables were obtained by self-report from participants except for type of index event (determined by site investigators) and blood pressure (measured at screening blood test). Participants missing information on any of these features are excluded from the adjusted analyses.
Causes of death for patients who died during follow-up were classified by an independent committee blinded to treatment assignment and tabulated with standard Centers for Disease Control and Prevention categories,15 and differences across smoking strata for major categories were tested by the χ2 statistic.
RESULTS
Study population.
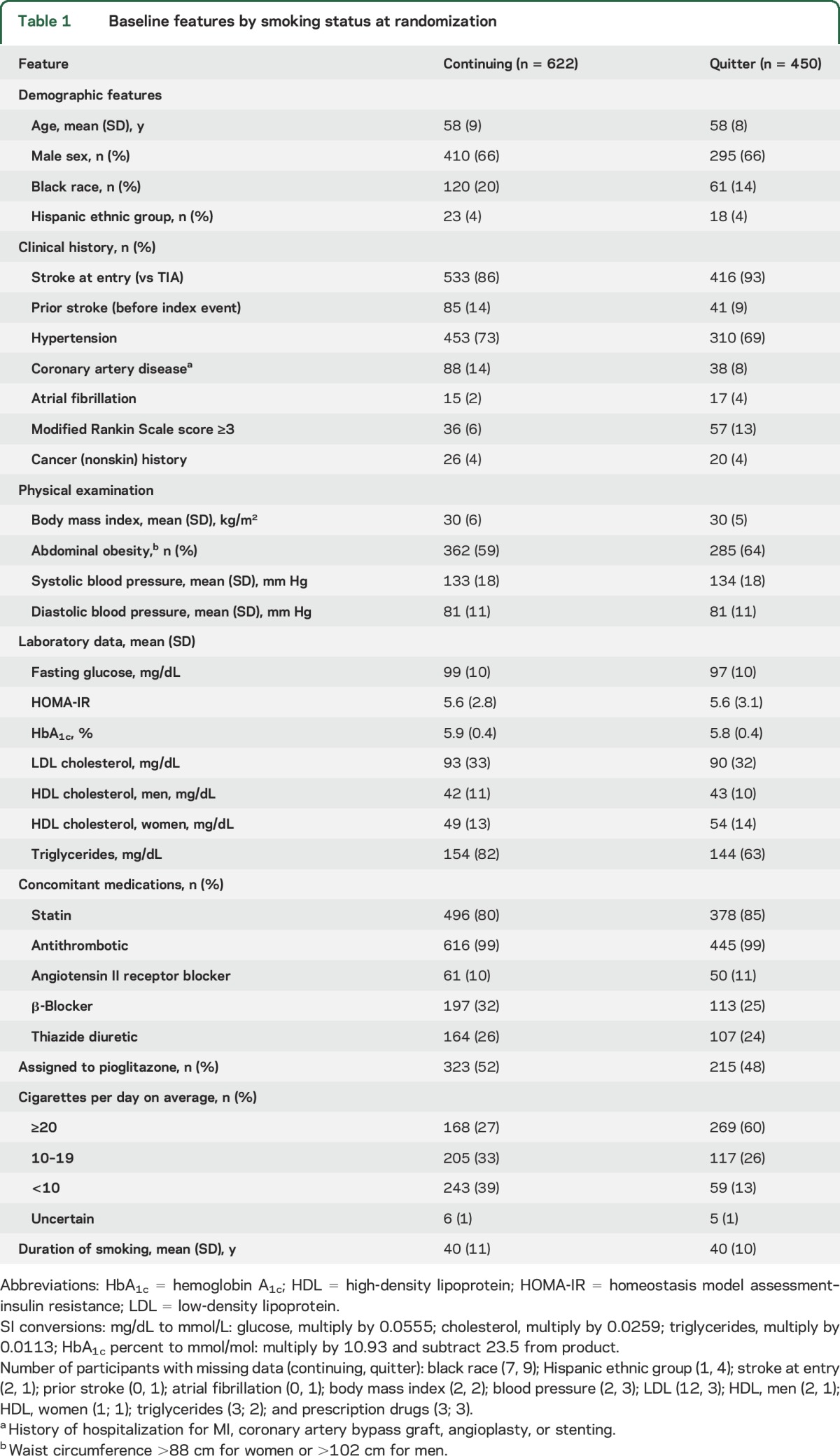
The study cohort was made up of 3,871 IRIS study participants randomized between February 2005 and January 2013 (5 participants were excluded because of missing smoking information) (figure e-1 at Neurology.org). At the baseline interview, 1,309 participants were classified as never smokers, 1,490 as former smokers, 450 as quitters since the index event, and 622 as continuing smokers. Baseline features for quitters and continuing smokers are displayed in table 1. (Features for all participants by smoking status are shown in table e-1.) Some baseline differences between the 2 smoking groups would be expected to reduce risk for vascular outcomes in quitters compared with continuing smokers (i.e., quitters were less likely to report a history of stroke or coronary artery disease before the index event and were more likely to use statin therapy).16,17 Other baseline differences would be expected to increase risk in quitters (i.e., quitters were more likely to enter with a stroke [vs TIA] and be assigned to receive placebo).17 On laboratory testing, quitters had lower fasting glucose, low-density lipoprotein cholesterol, and triglycerides compared to continuing smokers. Among women, quitters had higher high-density lipoprotein cholesterol compared to continuing smokers, but this difference was not as large in men. Quitters more often reported being heavy smokers (i.e., ≥20 cigarettes daily) at the time of the index event (60%) compared to continuing smokers at randomization (27%). However, we do not know if this reflects a true difference in the intensity of smoking between quitters and continuing smokers at the time of the index event or a reduction in smoking (or reported smoking) in the latter group between the event and trial entry. The mean reported duration of smoking was 40 years in both groups. A total of 32 participants (10 never smokers, 13 former smokers, 5 quitters, and 4 continuing smokers) did not have complete data on the 8 specified adjustment features and are excluded from the adjusted analyses; 3 outcomes were excluded (figure e-1).
Table 1.
Baseline features by smoking status at randomization
After a median follow-up of 4.8 years, stroke, MI, or death had occurred in 60 patients in the quitter group and in 121 in the continuing smoking group (5-year risk 15.7% vs 22.6%, adjusted HR 0.66, 95% CI 0.48–0.90). Among secondary outcomes, there were nonsignificant reductions in the incidence of stroke and MI during follow-up in quitters compared to continuing smokers (table 2, figure 1, and table e-2). Death occurred in 23 quitters and 66 continuing smokers (5-year risk 6.1% vs 13.1%, adjusted HR 0.49, 95% CI 0.30–0.79). Among major causes of death, the largest difference was observed for cancer: 7 deaths among quitters were attributable to cancer compared to 21 among continuing smokers (1.5% vs 3.4%, p = 0.07) (table e-3). A lower percentage of deaths was also observed among quitters resulting from cerebrovascular disease (0.2% vs 1.6%, p = 0.03), heart disease (0.7% vs 1.6%, p = 0.16), and unknown cause (1.8% vs 2.9%, p = 0.24).
Table 2.
Risk of outcomes by smoking status at randomization

Figure 1. Time to outcome events.
Time to outcome event by smoking status (quitter = blue line, continuing smoker = red line). (A) Stroke, MI, or death; (B) stroke; (C) MI; and (D) death resulting from any cause. MI = myocardial infarction.
At baseline, quitters were slightly more likely than continuing smokers to achieve their preventive health goals: 63% of quitters vs 61% of continuing smokers had blood pressure <140/90 mm Hg; 99% of quitters vs 99% of continuing smokers were on antithrombotics; 85% of quitters vs 80% of continuing smokers were on statins; and 54% of quitters vs 49% of continuing smokers had achieved all 3 of these preventive health goals. During 5 years of follow-up, quitters continued to be more likely to meet their preventive health goals (table e-4). Of note, 145 of 450 quitters reported resuming smoking at ≥1 annual time points during follow-up, and 190 of 622 continuing smokers reported having quit (data not shown).
DISCUSSION
The results of this study suggest that quitting smoking within 6 months after an ischemic stroke or TIA will significantly reduce the likelihood of stroke, MI, or death in the next 4.8 years. The observed relative risk reduction (RRR) (34%) and absolute risk reduction (ARR) (6.9%) are comparable to those of most other medical treatments for secondary prevention after stroke, including antiplatelet therapy,18 statin therapy,16 blood pressure reduction,19 and pioglitazone.7 The exception is anticoagulation for atrial fibrillation,20 which is associated with substantially greater reductions in relative and absolute risk. The present study supports current guidelines for smoking cessation after stroke or TIA6 and suggests that cessation may be one of the most important single interventions for smokers with an ischemic stroke or TIA.
We are aware of only 1 other study that has examined the effect of smoking cessation immediately after stroke. This observational study of 240 smokers with cerebrovascular disease observed a nonsignificant reduction in mortality over 14 months in quitters compared to continuing smokers.21 Other studies have classified smoking status at the time of the stroke event and were not designed to examine the effect of quitting.22–24 There have been no clinical trials of smoking cessation after stroke or TIA.
We found that the benefit of smoking cessation emerged early (i.e., within 5 years) after an acute ischemic stroke or TIA, which is consistent with prior research on the vascular effects of smoking. Smoking increases the risk for vascular disease by 2 major mechanisms: induction of a procoagulant state and acceleration of atherosclerosis. The procoagulant state is characterized by an increase in platelet aggregation, increased fibrinogen concentration, decrease in fibrinolysis, polycythemia, and high blood viscosity25 and is rapidly reversible within days of smoking cessation.26 Smoking accelerates atherosclerosis through several pathways, including impaired endothelial function (with decreases in nitric oxide), increased inflammation (through an increase in peripheral leukocytes and inflammatory markers), and lipid modification (increased cholesterol, triglycerides, and low-density lipoprotein; decreased high-density lipoprotein; and oxidation of low-density lipoprotein).25 Although the atherogenic effects of smoking likely take longer to dissipate, several studies have observed that stroke risk declines exponentially after smoking cessation and returns to baseline risk within 5 years of quitting.27,28
Smoking cessation in our study had a particularly large effect on all-cause mortality (RRR 51%, ARR 7.0%). This finding suggests that smoking cessation may be distinct from other medical interventions for secondary stroke prevention that are associated with no significant improvement in survival despite reductions in risk for cardiovascular events.7,16,19,20 The 1 exception is antiplatelet therapy after a TIA or stroke, which resulted in a smaller but still significant decrease in risk for all-cause mortality after 3 years of therapy (RRR 12%, ARR 1.5%).29
The most common cause of death among IRIS participants was cancer, followed by stroke, heart disease, and respiratory infection. All these causes were reduced among quitters, but only stroke (p = 0.03) and cancer (p = 0.07) reached or approached statistical significance. The decreased rate of cancer death observed in quitters compared to continuing smokers is most likely attributable to the beneficial effects of smoking cessation on case fatality. At baseline, quitters and continuing smokers had similar reported cancer histories and similar years of exposure to tobacco. However, we observed a quantitatively large reduction in cancer case fatality in quitters compared with continuing smokers (7 of 31 = 23% vs 21 of 49 = 43%, χ2 p value = 0.06). The finding that quitters have lower rates of cancer death relative to continuing smokers is consistent with other research showing that smoking cessation in patients who already have a cancer diagnosis is associated with a rapid decrease in case fatality.30,31 Mechanisms for the rapid decrease may include enhanced sensitivity to radiation therapy32 and chemotherapy,33 elimination of stimulation of tumor growth by nicotine,34,35 and prevention of death among cancer patients resulting from comorbid pulmonary and cardiovascular disease.36,37
The results of this study should be considered in the context of 2 potential sources of bias. Prevention bias refers to the tendency of individuals who make 1 healthy choice (e.g., quitting smoking) to make others as well (e.g., taking medication as prescribed, seeing a doctor regularly, exercising) that could improve outcomes.38 Consistent with a prevention bias, quitters were slightly more likely than continuing smokers to have achieved their preventive health goals at baseline and during follow-up. This small difference between groups may have increased the benefit attributed to smoking cessation in this study but is probably not large enough to account for the full difference in outcome rates. A second source of bias may have resulted from selective loss of patients from the study cohort between the index event and randomization. If patients who quit smoking after their stroke/TIA and patients who continued to smoke had differing short-term survival or differentially agreed to participate in IRIS on the basis of features associated with prognosis, the randomized patients may not reflect the true association between smoking and outcomes in the underlying population. In addition to these potential sources of bias, this analysis of smoking was an unplanned, secondary analysis of data from a randomized clinical trial, and the findings, although compelling, must be regarded as hypothesis generating only. Finally, the IRIS trial enrolled insulin-resistant, nondiabetic patients, and our results may not be generalizable to all stroke patients who smoke.
This study also had several notable strengths, including detailed data on smoking, close follow-up of participants, and careful adjudication of outcomes. In addition, quitters and continuing smokers were well balanced in most demographic and clinical characteristics.
Healthcare providers have a unique opportunity to counsel patients after they have an ischemic stroke or TIA. This article provides a quantitative estimate for the benefits of smoking cessation in this population. Among 100 patients who continue to smoke after an ischemic stroke or TIA, 23 may be expected to have a stroke, MI, or death within 5 years compared to only 16 of 100 who quit. In simple terms, for every 100 of these patients who manage to quit cigarettes, fully 7 additional patients will survive 5 years without MI or recurrent stroke than otherwise would have. If confirmed, our results suggest that healthcare providers should give very high priority to helping patients quit smoking cigarettes after an ischemic stroke or TIA.
Supplementary Material
GLOSSARY
- ARR
absolute risk reduction
- CI
confidence interval
- HR
hazard ratio
- IRIS
Insulin Resistance Intervention After Stroke
- MI
myocardial infarction
- RRR
relative risk reduction
Footnotes
Supplemental data at Neurology.org
Editorial, page 1656
Contributor Information
Collaborators: IRIS Trial Investigators, Christopher Bladin, Stephen Davis, Tissa Wijeratne, Christopher Levi, Mark Parsons, Amy Brodtmann, Steven Ng, John Archer, Candice Delcourt, Toni R. Winder, Leo Berger, Jean‐Martin Boulanger, Richard K. Chan, J. David Spence, Andre Durocher, Ariane Mackey, Steve Verreault, Jeffrey Minuk, Andrew M. Penn, Ashfaq Shuaib, Robert Cote, Daniel Selchen, Neville Bayer, Margaret Sweet, Salim Malik, Grant Stotts, Bernd Griewing, Hassan Soda, Renate Weinhardt, Jörg Berrouschot, Anett Stoll, Otto W. Witte, Albrecht Günther, Ulf Bodechtel, Ulf Schminke, Carsten Hobohm, Andreas Hetzel, Johann Lambeck, Katja E. Wartenberg, Hagen Huttner, Ralf Dittrich, Darius G. Nabavi, Klaus Gröschel, Gotz Thomalla, M. Rosenkranz, Sebastian Jander, Andreas Meisel, Albert Ludolph, Katharina Althaus, R. Huber, Matthias Lorenz, David Tanne, Oleg Merzlyak, Natan M. Bornstein, Gregory Telman, Yair Lampl, Jonathan Streifler, Boaz Weller, Gal Ifergane, Y. Wirgin, Antonio Carolei, Danilo Toni, Paolo Stanzione, Giuseppe Micieli, Giancarlo Agnelli, Valeria Caso, Carlo Gandolfo, Giancarlo Comi, Domenico Consoli, Maurizia Rasura, Vincenzo Di Lazzaro, Anand Dixit, Becky Jupp, Louise Shaw, Isam Salih, Bernard Esisi, Michael Power, William D. Strain, Salim Elyas, Dulka Manawadu, Lalit Kalra, Eoin O'Brien, Elizabeth Warburton, Kausik Chatterjee, David R. Hargroves, Adrian Blight, Barry Moynihan, Hugh S. Markus, Mary Joan Macleod, David Lance Broughton, Helen Rodgers, Thant Hlaing, Scott Muir, Mahmud Sajid, Philip M.W. Bath, Christopher Price, Lakshmanan Sekaran, Djamil Vahidassr, Keith W. Muir, James McIlmoyle, Prabal K. Datta, Richard Davey, Peter Langhorne, David Stott, Prabal K. Datta, Timothy John England, K. Muhidden, Janice Elizabeth O'Connell, Nikhil Majmudar, Joseph Schindler, Wayne M. Clark, Pramodkumar Sethi, Guy Rordorf, Dawn O. Kleindorfer, Scott L. Silliman, Mark Gorman, Michael A. Kelly, Lafayette Singleton, Brett C. Meyer, Christy Jackson, James Walker, As'ad Ehtisham, Hewitt C. Goodpasture, David Wang, Pierre Fayad, Steve Cordina, Dean Naritoku, David Chiu, Timothy Lukovits, Richard Goddeau, Robin Clark‐Arbogast, Richard Leigh, Robert J. Wityk, L. Creed Pettigrew, Ashis H. Tayal, Judy Jarouse, Gary H. Friday, Souvik Sen, Anthony S. Kim, S. Claiborne Johnston, Jacob S. Elkins, Anna M. Barrett, Enrique C. Leira, Adam Kelly, S. Burgin, David A. Rempe, Michael R. K. Jacoby, Dr. Bruce Hughes, Jennifer Majersik, Elaine J. Skalabrin, Jin‐Moo Lee, Chung Hsu, Sophia Sundararajan, Andrew Slivka, Alireza Minagar, Radica Alicic, Madeleine Geraghty, Carlos S. Kase, Maartan Lansberg, Greg Albers, Dennis W Dietrich, Joseph P. Hanna, Nina T. Gentile, Fernando Santiago, Irene Katzan, Marilou Ching, Sawyer, Tanya Warwick, Engin Yilmaz, Laura Pedelty, Michael J. Schneck, Bruce M. Coull, Nina J. Solenski, Karen Johnston, Vivien Lee, Shyam Prabhakaran, Mark D. Johnson, Isaac E. Silverman, Miran W. Salgado, Robert Birkhahn, Richard Strawsburg, Irfan Altafullah, Daniel Aaron Cohen, Richard Zweifler, Peterkin Lee Kwen, Maxim D. Hammer, Nirav Vora, Gretchen E. Tietjen, Erfan Albakri, Bhuvaneswari (Bo) K. Dandapani, Glen Jickling, Piero Verro, Matthew J. Roller, Richard L. Hughes, Jennifer Simpson, Thomas R. Vidic, Stephanie Lash, Bruce Sigsbee, Daniel Rosenbaum, Pasquale Fonzetti, James D. Fleck, Adrian J. Goldszmidt, Andrei V Alexandrov, James H. Halsey, Robert Hart, Justin A. Sattin, Sandeep Kumar, Diane Book, Michel Torbey, James J. Poock, Molly K. King, Glenn D. Graham, Gene Yong Sung, Thomas Mirsen, Alexander W. Dromerick, Andreas D. Runheim, Christy M. Jackson, Eliahu Feen, Raymond K. Reichwein, Michael F. Waters, Colum Amory, Gary L. Bernardini, Rodney D. Bell, B. Franklin Diamond, Daniel M. Rosenbaum, David Palestrant, Alan Z. Segal, Kathleen Burger, Ronald L. Schwartz, Panayiotis Mitsias, Jeffrey Kramer, Jeffrey Kramer, David Robbins, Brian Silver, J. Donald Easton, Edward Feldmann, Marilyn M. Rymer, Joyce Dorssom, Latisha Ali, Bruce Ovbiagele, and Howard S. Kirshner
AUTHOR CONTRIBUTIONS
Katherine A. Epstein: design of data analyses, interpretation of results, drafting, writing, and revision of report. Catherine M. Viscoli: data acquisition, design of data analyses, performance of statistical analysis, interpretation of results, writing and revision of report. J. David Spence, Lawrence H. Young, Silvio E. Inzucchi, and Mark Gorman: data acquisition, critical revision of the manuscript for intellectual content. Brett Gerstenhaber: critical revision of the manuscript for intellectual content. Peter D. Guarino, Anand Dixit, and Karen L. Furie: data acquisition, critical revision of the manuscript for intellectual content. Walter N. Kernan: data acquisition, design of data analyses, interpretation of results, writing and revision of report.
STUDY FUNDING
This work (and the original IRIS trial) was supported by a grant (U01NS044876) from the National Institute of Neurological Disorders and Stroke (NINDS), NIH. Pioglitazone and placebo for the original IRIS trial were provided by Takeda Pharmaceuticals. The NINDS and Takeda Pharmaceuticals had no role in data collection, data analysis, data interpretation, or writing of this report.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Bonita R, Scragg R, Stewart A, Jackson R, Beaglehole R. Cigarette smoking and risk of premature stroke in men and women. Br Med J (Clin Res Ed) 1986;293:6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–775. [DOI] [PubMed] [Google Scholar]
- 4.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults—United States, 2005–2015. MMWR Morb Mortal Wkly Rep 2016;65:1205–1211. [DOI] [PubMed] [Google Scholar]
- 5.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 7.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viscoli CM, Brass LM, Carolei A, et al. Pioglitazone for secondary prevention after ischemic stroke and transient ischemic attack: rationale and design of the Insulin Resistance Intervention after Stroke Trial. Am Heart J 2014;168:823–829.e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 10.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 11.Rallidis LS, Sakadakis EA, Tympas K, et al. The impact of smoking on long-term outcome of patients with premature (</=35years) ST-segment elevation acute myocardial infarction. Am Heart J 2015;169:356–362. [DOI] [PubMed] [Google Scholar]
- 12.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med 2002;137:494–500. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J R Stat Soc B 1972;34:187–220. [Google Scholar]
- 15.Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep 2016;65:1–95. [PubMed] [Google Scholar]
- 16.Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559.16899775 [Google Scholar]
- 17.Kernan WN, Viscoli CM, Brass LM, et al. The Stroke Prognosis Instrument II (SPI-II): a clinical prediction instrument for patients with transient ischemia and nondisabling ischemic stroke. Stroke 2000;31:456–462. [DOI] [PubMed] [Google Scholar]
- 18.Canadian Cooperative Study Group. A randomized trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med 1978;299:53–59. [DOI] [PubMed] [Google Scholar]
- 19.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041. [DOI] [PubMed] [Google Scholar]
- 20.European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255–1262. [PubMed] [Google Scholar]
- 21.Alvarez LR, Balibrea JM, Surinach JM, et al. Smoking cessation and outcome in stable outpatients with coronary, cerebrovascular, or peripheral artery disease. Eur J Prev Cardiol 2013;20:486–495. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai N, Okuhara Y, Iiyama T, et al. Effects of smoking on outcomes after acute atherothrombotic stroke in Japanese men. J Neurol Sci 2013;335:164–168. [DOI] [PubMed] [Google Scholar]
- 23.Ovbiagele B, Weir CJ, Saver JL, Muir KW, Lees KR; IMAGES Investigators. Effect of smoking status on outcome after acute ischemic stroke. Cerebrovasc Dis 2006;21:260–265. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Gall SL, Dewey HM, Macdonell RA, Sturm JW, Thrift AG. Baseline smoking status and the long-term risk of death or nonfatal vascular event in people with stroke: a 10-year survival analysis. Stroke 2012;43:3173–3178. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–1737. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell M, Rampling MW, Cholerton S, Sever PS. Haemorheological changes in the very short term after abstention from tobacco by cigarette smokers. Br J Haematol 1991;79:500–503. [DOI] [PubMed] [Google Scholar]
- 27.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and decreased risk of stroke in women. JAMA 1993;269:232–236. [PubMed] [Google Scholar]
- 28.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke: the Framingham Study. JAMA 1988;259:1025–1029. [PubMed] [Google Scholar]
- 29.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobson Amato KA, Hyland A, Reed R, et al. Tobacco cessation may improve lung cancer patient survival. J Thorac Oncol 2015;10:1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 1993;328:159–163. [DOI] [PubMed] [Google Scholar]
- 33.Johnston-Early A, Cohen MH, Minna JD, et al. Smoking abstinence and small cell lung cancer survival: an association. JAMA 1980;244:2175–2179. [PubMed] [Google Scholar]
- 34.Chernyavsky AI, Shchepotin IB, Galitovkiy V, Grando SA. Mechanisms of tumor-promoting activities of nicotine in lung cancer: synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer 2015;15:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobus SL, Warren GW. The biologic effects of cigarette smoke on cancer cells. Cancer 2014;120:3617–3626. [DOI] [PubMed] [Google Scholar]
- 36.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013;309:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimenez-Ruiz CA, Andreas S, Lewis KE, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J 2015;46:61–79. [DOI] [PubMed] [Google Scholar]
- 38.Barrett-Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med 1991;115:455–456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



