Highlights
-
•
Teriparatide (TPTD) and fracture healing studies in premenopausal women is limited.
-
•
Brief TPTD administration showed a greater anabolic window compared to placebo.
-
•
Bone formation markers increased in TPTD- vs. placebo-treated group (both p ≤ 0.01).
-
•
TPTD (83.3%) vs. placebo (57.1%) showed improved stress fractures on MRI (p = 0.18).
Keywords: Teriparatide, Pilot study, Premenopausal, Stress fracture, Anabolic window
Abstract
Aims
In this pilot, placebo-controlled study, we evaluated whether brief administration of teriparatide (TPTD) in premenopausal women with lower-extremity stress fractures would increase markers of bone formation in advance of bone resorption, improve bone structure, and hasten fracture healing according to magnetic resonance imaging (MRI).
Methods
Premenopausal women with acute lower-extremity stress fractures were randomized to injection of TPTD 20-µg subcutaneous (s.c.) (n = 6) or placebo s.c. (n = 7) for 8 weeks. Biomarkers for bone formation N-terminal propeptide of type I procollagen (P1NP) and osteocalcin (OC) and resorption collagen type-1 cross-linked C-telopeptide (CTX) and collagen type 1 cross-linked N-telopeptide (NTX) were measured at baseline, 4 and 8 weeks. The area between the percent change of P1NP and CTX over study duration is defined as the anabolic window. To assess structural changes, peripheral quantitative computed topography (pQCT) was measured at baseline, 8 and 12 weeks at the unaffected tibia and distal radius. The MRI of the affected bone assessed stress fracture healing at baseline and 8 weeks.
Results
After 8 weeks of treatment, bone biomarkers P1NP and OC increased more in the TPTD- versus placebo-treated group (both p ≤ 0.01), resulting in a marked anabolic window (p ≤ 0.05). Results from pQCT demonstrated that TPTD-treated women showed a larger cortical area and thickness compared to placebo at the weight bearing tibial site, while placebo-treated women had a greater total tibia and cortical density. No changes at the radial sites were observed between groups. According to MRI, 83.3% of the TPTD- and 57.1% of the placebo-treated group had improved or healed stress fractures (p = 0.18).
Conclusions
In this randomized, pilot study, brief administration of TPTD showed anabolic effects that TPTD may help hasten fracture healing in premenopausal women with lower-extremity stress fractures. Larger prospective studies are warranted to determine the effects of TPTD treatment on stress fracture healing in premenopausal women.
Introduction
Stress fractures are the most common overuse injuries of the lower extremities and occur five times more frequently in women than in men [1], [2]. In a large study of Finnish military conscripts involving 102,515 person-years, the strongest risk factor identified for stress fracture injuries was female gender (hazard ratio = 8.2) [3]. Vigorous training is associated with a higher risk of stress fractures, with average healing time of up to 12 weeks or more, depending on the location and severity of the stress injury [4]. Although stress fractures and injuries cause chronic pain and disability, there are currently no systemic medical treatments available that may hasten fracture repair and recovery.
Stress fractures are typically diagnosed by clinical signs and symptoms, which may be difficult to evaluate due to the subclinical and asymptomatic nature of stress fractures [5], [6]. In a clinical setting, imaging techniques are used to confirm and assess the extent of the injury and monitor fracture healing. An imaging comparison study showed that the sensitivity of magnetic resonance imaging (MRI), computed tomography (CT), and bone scans of 50 tibial stress fractures were 88%, 42%, and 74%, respectively [7]. The specificity and positive and negative predictive values for MRI were 100%, 100%, and 62%, respectively, and for CT 100%, 100%, and 26%, respectively. Thus, MRI has become the imaging modality of choice to detect early and progressive stress fractures because of its high sensitivity and specificity [8]. Fredericson et al. applied a diagnostic approach using a MRI classification system to correlate clinical symptoms with the severity of stress injuries [9]. Accurate assessment of stress fractures is imperative to guide treatment recommendations. Current therapeutic approaches for lower-extremity stress fractures include: protective weight-bearing methods, non-weight bearing interventions, immobilization with boot, and/or ultrasound [10]; however, no systematic medical therapies to improve stress fracture healing are available at this time.
Daily administration of human recombinant parathyroid hormone (PTH)(1–34) or teriparatide (TPTD) is currently the only anabolic therapy approved by the Food and Drug Administration (FDA) for the treatment of osteoporosis in postmenopausal women and men, and reduction of risk for vertebral and non-vertebral fractures in postmenopausal women [11], [12], [13]. When TPTD is administered daily, a rapid rise in bone formation biomarkers and a delayed increase in bone resorption biomarkers occur [14], [15]. This initial increase in bone formation over resorption biomarkers creates an “anabolic window” [16], [17]. Additionally, emerging data suggest that PTH may have important effects on fracture repair. A placebo-controlled trial in rodents with closed fractures showed an increase in anabolic activity and improved mineralization after daily TPTD therapy [18]. In animal studies, PTH improved bone structure, torsion strength, callus formation, and fracture repair, with a sustained benefit after discontinuation that may be important in the healing of lower-extremity stress fractures [19], [20], [21].
A number of uncontrolled studies and case series in humans have shown that the use of TPTD in adults with delayed and nonunion fractures may hasten bone healing [22], [23], [24], [25], [26], [27]. At present, there is only one randomized-controlled trial after daily treatment of TPTD 20 µg for 8 weeks demonstrated an acceleration in healing of non-weight bearing, distal radius fractures in postmenopausal women [28]. Data related to TPTD use in premenopausal women are limited [29], [30], [31], [32], [33]. Cohen et al. conducted an open-label, pilot study examining effects of daily therapy of TPTD on bone for 18–24 months in premenopausal women with unexplained fragility fractures or idiopathic osteoporosis. Results showed that TPTD increased BMD of spine and hip, improved trabecular microarchitecture and stiffness at the hip, and bone strength in the radius and tibia in premenopausal women [31], [32]. These data, along with animal studies, case reports, uncontrolled and limited controlled studies in humans provide support of the potential benefit of TPTD in fracture healing.
The high incidence of stress fractures in women and extensive time for stress fractures healing, treatment to hasten fracture repair, which may reduce the time for recovery, improve bone structure and healing, and advance fracture care, are of great interest. Therefore, the objectives of this randomized, placebo-controlled study were to examine whether, TPTD injections for 8 weeks (1) increased bone formation biomarkers in advance of resorption biomarkers, thereby generating an anabolic window of bone healing, (2) improved bone structure according to peripheral quantitative computed tomography (pQCT) and (3) hastened fracture healing assessed by MRI in premenopausal women with lower-extremity stress fractures compared to placebo.
Materials and methods
Study subjects
Premenopausal women, aged 21 to 45 years old, with recent lower-extremity stress fractures diagnosed within 4 weeks of the screening visit were eligible for enrollment. Exclusion criteria included open epiphyses, history of radiation therapy, more than 3 months amenorrhea, current pregnancy or breastfeeding, history of disorders associated with low bone mass or fractures, and/or use of medications that affect bone mass. Other inclusion criteria included normal baseline alkaline phosphatase, serum calcium, phosphorus, and thyroid-stimulating hormone levels. All participants provided written informed consent before any study procedure was performed. This study was performed in the Endocrine Division at the Brigham and Women's Hospital (BWH) and in the NIH-sponsored, Harvard Clinical and Translational Science Center. The Partners Institutional Review Board (IRB) approved the protocol (Protocol No. 2006P000740).
Study drug
Teriparatide™ and placebo injection pens were provided by Eli Lilly Corporation (Indianapolis, IN, USA). In this randomized, double-blinded study, injection pens were identical in appearance and dispensed at baseline and 4 weeks after a negative pregnancy was confirmed. Participants were trained on how to self-administer the daily injection.
Study design
Eligible premenopausal women were enrolled and randomized by the investigator pharmacist at BWH in a 1:1 ratio to receive either TPTD 20 µg or matching placebo once daily for 8 weeks. Laboratory tests were collected at baseline, 4 and 8 weeks. Dual-energy X-ray absorptiometry (DXA) was performed at baseline; follow-up DXA was not performed as changes in bone are not generally observed in this short time interval. pQCT was measured at the radius and unaffected tibia at baseline, 8 and 12 weeks. MRI (1.5T) was performed at baseline and 8 weeks to test whether TPTD hastened stress fracture healing. Calcium intakes were calculated and calcium and cholecalciferol were provided to all subjects to achieve intakes of 1000 mg of elemental calcium and 400 IU vitamin D daily. All women were instructed to use barrier or hormonal contraceptives. A baseline physical examination was also completed. Vital signs, heights (Holtain Stadiometer, Great Britain), and digital scale weights (Tanita BWB-800, Illinois, USA) were determined, and body mass index (BMI, kg/m2) was calculated. Study procedures after enrollment included: review of medical history and concomitant medications, laboratory testing for serum electrolytes, thyroid stimulating hormone, and serum human chorionic gonadotropin (HCG) to rule out pregnancy at screening, baseline, 4 and 8 weeks.
Bone biomarkers
Biochemical markers of bone turnover were monitored at baseline, 4 and 8 weeks in all study participants. Bone formation markers serum N-terminal propeptide of type I procollagen (P1NP; normal range 19–83 ug/L) and osteocalcin (OC; 5–25 ng/mL) and bone resorption markers collagen type-1 cross-linked C-telopeptide (CTX; 0.11–0.74 ng/mL) and urinary collagen type 1 cross-linked N-telopeptide (NTX; 5–65 nM BCE/mM Creatinine) were measured. Serum samples were collected after an overnight fast by 10 am for markers of bone formation (P1NP and OC) and bone resorption (CTX) to minimize diurnal and biological variations of serum measures of bone turnover; second void morning fasting urine samples for the bone resorption marker (NTX) were obtained. Serum and urine samples were aliquoted and stored at −80 degrees Fahrenheit until samples were assayed at the time of collection.
P1NP levels were quantified using a radioimmunoassay [Orion Diagnostics, distributed by Immunodiagnostic Systems Inc. Fountain Hills, AZ]; both the intra-assay and the inter-assay variations were <5.5%. The OC levels were measured by homologous equilibrium radioimmunoassay (ALPCO Diagnostics, Salem, NH). The intra- and inter-assay variations for these measures were 5.6% and 5.2%, respectively. The CTX levels were quantified using an enzyme immunoradiometric assay (Nordic Bioscience, distributed by IDS, Fountain Hills, AZ); with intra-assay and inter-assay variations were <6.5% each. The urinary NTX level, corrected for creatinine was determined using an enzyme-linked immunosorbent assay (ELISA) (Osteomark, Wampole Laboratories, Princeton, NJ), with intra-assay variation of 4.2–5.2% and intra-assay variation of 4.6–7.4%. All participants' lab tests were performed in the same assay at NIH-sponsored, Harvard Catalyst Clinical Research Center (Boston, MA).
Peripheral quantitative computed tomography (pQCT)
To assess systemic changes in bone structure over time, the radius and unaffected tibia were measured at baseline, 8 and 12 weeks after an 8-week treatment period by pQCT (XCT 3000, Stratec, Germany). The precision errors for the pQCT scanned sites at our research laboratory were as followed: the distal radius (4% and 33% site) and unaffected tibia (4%, 38%, 66% site) of the cortical area (LSC 38% site = 0.45 mm2), thickness (LSC 38% site = 0.13 mm), density (LSC 38% site = 12.65 mg/cm3) and total density (LSC 38% site = 8.97 mg/cm3) [34].
Magnetic resonance imaging (MRI)
Upon enrollment to the study, all participants had MR imaging to document the presence of a stress fracture. MRI scans of the stress fracture were performed on a GE Signa 1.5 Tesla scanner with an Excite upgrade (GE Medical Systems, Milwaukee, WI). A subsequent MRI scan was conducted at 8 weeks from baseline to assess whether fracture healing would occur at 8 weeks rather than the anticipated 12 weeks [4]. A grading system for the MRI was developed to evaluate the severity of the lower-extremity stress fracture [9]. Grade 0 signified no appreciable lesions of the bone; Grades 1 and 2 indicated a mild-to-moderate periosteal edema and possible bone marrow edema; and Grades 3 and 4 showed a moderate-to-severe edema of both the periosteum and marrow. A low-signal fracture line is visible only in Grade 4 stress fractures. Fractures that demonstrated a reduction of MRI grade were considered to be healing and Grade 0 was considered healed. All MRIs were evaluated and scored quantitatively for the grade and extent of stress fracture at baseline and 8 weeks by a blinded radiologist on this study.
Adverse events
Adverse events (AEs) during the course of this study were monitored at baseline, 4 and 8 weeks, and defined as any untoward medical event that occurred or worsened since baseline. Reports of adverse events were reviewed by a Data Safety Monitoring Board.
Statistical analyses
Data were summarized using means and standard deviations. The Wilcoxon rank sum test was used to compare the treatment arms in terms of baseline characteristics, percent change in markers of bone turnover from baseline, areas under the curve for each marker, anabolic window, and mean values of DXA at baseline and pQCT structural changes from baseline. The anabolic window was defined as the difference between the area under the formation curve and the area under the resorption curve, in percent change per month units. To observe changes in MRI scores, defined as the difference between grades at baseline and at Week 8, Wilcoxon rank sum test was used. Statistical analysis was performed with SAS (version 9.3).
Results
Data were collected from April 2009 to July 2012. Of 101 women screened, 14 premenopausal women were enrolled and randomized into two treatment groups TPTD 20-µg (n = 6) or placebo (n = 8) injections (Fig. 1). One participant withdrew prior to Week 4 visit due to overseas travel. Thirteen participants were followed for the complete study period. All participants had normal serum calcium levels and HCG levels of <1 mIU/mL as tested at screening, baseline, 4 and 8 weeks. Although one participant in the control group had vitamin D deficiency at baseline [25(OH)D level of 15.8 ng/ml], no significant differences in the baseline mean and follow-up 25(OH)D levels among the TPTD- and placebo-treated premenopausal women with stress fractures were observed. At baseline of this study, there were no differences in demographics, DXA measurements, and lab results between the TPTD- and placebo-treated women (Table 1). Location of fractures includes tibia (n = 4), metatarsal (n = 4), femur (n = 3), fibula (n = 1) and calcaneus (n = 1). Locations of the three femoral stress fractures were at the following sites: 1) femoral head and neck, extending to the lesser trochanter; 2) femoral neck and intertrochanteric region near the lesser trochanter of the left femur; and 3) right femoral neck region.
Figure 1.
Subject disposition.
Table 1.
Baseline characteristics of study participants (mean ± SD)
| Baseline characteristics | Teriparatide (n = 6) | Placebo (n = 8) | P value |
|---|---|---|---|
| Age (years) | 32 ± 5.8 | 31 ± 3.4 | – |
| Weight (kg) | 57.4 ± 7.8 | 61.8 ± 13.2 | 0.75 |
| Height (cm) | 160.0 ± 3.6 | 160.4 ± 4.6 | 0.85 |
| Body mass index (kg/m2) | 22.4 ± 3.1 | 24.1 ± 4.9 | 0.7 |
| Alkaline phosphatase (U/L) | 58.3 ± 19.7 | 60.1 ± 26.3 | 0.95 |
| Serum P1NP (µg/L) | 40.1 ± 25.5 | 42.4 ± 11.9 | 0.33 |
| Serum OC (ng/mL) | 5.8 ± 5.0 | 7.9 ± 2.5 | 0.43 |
| Serum CTX (ng/mL) | 0.43 ± 0.2 | 0.54 ± 0.3 | 0.44 |
| Urine NTX/creatinine (nM/BCE) | 161.7 ± 132.1 | 356.9 ± 229.2 | 0.12 |
| Vitamin D | 31.8 ± 5.3 | 32.5 ± 8.9 | 0.75 |
| Calcium (urinary) | 8.6 ± 7.6 | 10.1 ± 5.7 | 0.56 |
| Calcium (serum) | 9.3 ± 0.4 | 9.5 ± 0.2 | 0.16 |
| Parathyroid hormone (PTH) | 28.3 ± 4.6 | 26.6 ± 9.1 | 0.33 |
| DXA spine (Z-score) | −1.2 ± 0.7 | −0.7 ± 1.3 | 0.4 |
| DXA left femoral neck (Z-score) | −0.7 ± 1.0 | −0.8 ± 1.5 | 0.94 |
| DXA right femoral neck (Z-score) | −0.6 ± 1.0 | −0.27 ±1.1 | 0.55 |
| DXA left total hip (Z-score) | −0.6 ± 0.7 | −0.26 ± 1.2 | 0.58 |
| DXA right total hip (Z-score) | −0.5 ± 0.8 | −0.09 ± 1.1 | 0.43 |
| DXA whole body (Z-score) | −0.4 ± 0.7 | 0.46 ± 1.0 | 0.12 |
Bone turnover biomarkers
Overall changes in biochemical markers of bone turnover from baseline and 8 weeks are shown in Table 2. In those treated with TPTD, serum P1NP levels steadily increased after administration of TPTD at both 4 and 8 weeks (134% ± 52%, p = 0.003 and 180% ± 99%, p = 0.005, respectively), while OC rapidly increased at 4 weeks (410% ± 726%, p = 0.03) and doubled above the baseline value at 8 weeks (859% ± 1207%, p = 0.003). At the end of treatment, all bone formation markers in participants assigned to TPTD were more than 150% above their baseline value. Placebo-treated participants showed a modest increase in P1NP and OC levels.
Table 2.
Bone turnover marker results at baseline, 4 and 8 weeks
| Bone biomarker | Time | Teriparatide group (n = 6) | Placebo group (n = 7) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | |||
| Serum ALP (IU/L) | Baseline | 58.3 | 39.0 | 85.0 | 60.1 | 36.0 | 112.0 | 0.95 |
| 4 weeks | 63.3 | 45.0 | 94.0 | 62.7 | 42.0 | 105.0 | 0.43 | |
| 8 weeks | 61.5 | 61.2 | 90.0 | 63.0 | 41.0 | 107.0 | 0.52 | |
| Serum P1NP (µg/L) | Baseline | 40.1 | 15.8 | 85.6 | 42.4 | 19.4 | 57.8 | 0.33 |
| 4 weeks | 88.5 | 47.0 | 197.2 | 43.9 | 24.2 | 62.7 | 0.003a | |
| 8 weeks | 103.1 | 61.2 | 245.1 | 47.5 | 26.1 | 76.6 | 0.005a | |
| Serum OC (ng/mL) | Baseline | 5.8 | 0.6 | 13.0 | 7.9 | 3.6 | 10.1 | 0.43 |
| 4 weeks | 12.6 | 1.8 | 24.3 | 9.2 | 5.3 | 12.8 | 0.027a | |
| 8 weeks | 16.8 | 0.9 | 30.8 | 9.6 | 6.0 | 12.7 | 0.003a | |
| Serum CTX (ng/mL) | Baseline | 0.43 | 0.26 | 0.64 | 0.54 | 0.28 | 1.28 | 0.44 |
| 4 weeks | 0.61 | 0.19 | 1.41 | 0.43 | 0.29 | 0.61 | 0.43 | |
| 8 weeks | 0.66 | 0.31 | 1.28 | 0.50 | 0.31 | 0.80 | 0.28 | |
| Urine NTX (nMBCE/mMCr) | Baseline | 161.7 | 40.0 | 371.0 | 356.9 | 94.0 | 695.0 | 0.12 |
| 4 weeks | 205.3 | 83.3 | 547.0 | 384.4 | 34.0 | 772.0 | 0.78 | |
| 8 weeks | 213.3 | 31.0 | 540.0 | 524.4 | 110.0 | 1492.0 | 0.62 | |
P1NP, N-terminal propeptide of type 1 collagen; OC, osteocalcin; CTX, C-telopeptide of type 1 collagen; urine NTX, N-telopeptide of type 1 collagen, corrected for mM creatinine.
Bone formation markers, P1NP and OC, were significantly different (p-value < 0.05) at 4 and 8 weeks.
For resorption biomarker CTX, there was a modest, but steady elevation in serum levels with TPTD (67% ± 86%) in comparison to placebo (16% ± 41%) (p = 0.28) over 8 weeks. Urinary excretion of NTX, however, showed a different pattern. Both groups showed slight elevation in NTX levels at 4 weeks, but NTX levels of the TPTD group fell below the placebo group at 8 weeks (p = 0.62). By the end of treatment, NTX levels for TPTD-treated participants were only 25% above baseline compared to placebo-treated participants who were 55% above baseline. No significant changes in resorption markers were observed during the treatment period (Table 2). The anabolic window was defined as the area between the formation and resorption curves over time on treatment. Fig. 2 depicts the anabolic window using P1NP and CTX for TPTD and placebo and indicates a significantly greater average area in those treated with TPTD versus placebo (p = 0.05).
Figure 2.
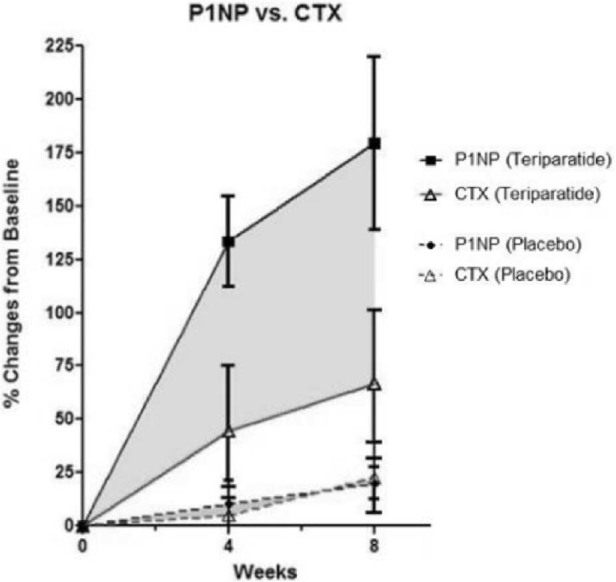
Anabolic window is the shaded area between P1NP and CTX. Comparison of the area between P1NP curve and the CTX curve in premenopausal women with stress fractures treated with Teriparatide or placebo. The graph shows a significantly larger mean area in the Teriparatide group (145.82 ± 123.0) compared to the placebo group (5.99 ± 48.4) (p = 0.05).
DXA and pQCT
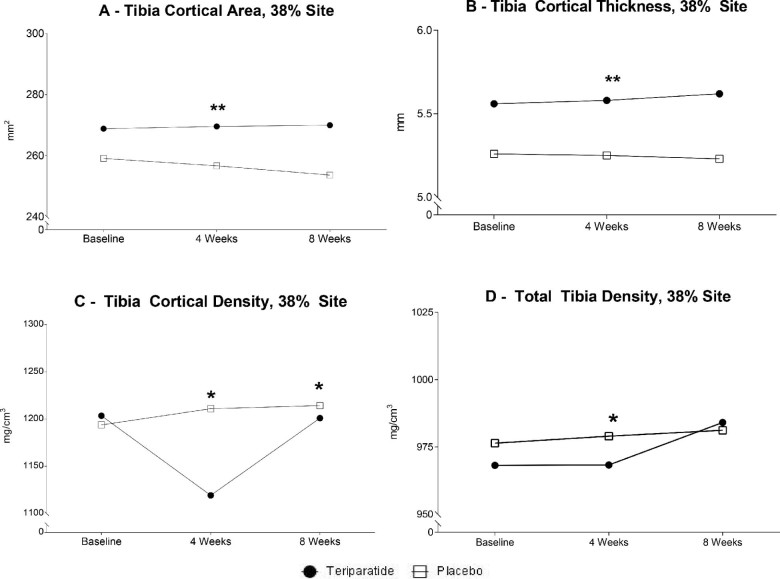
Baseline DXA measures were performed on all participants, and there were no differences between the TPTD- and placebo-treated groups. According to pQCT measures, significant relationships between groups as well as within groups were observed over time after an 8-week treatment (Fig. 3). Compared to placebo, the TPTD-treated group showed a larger tibia cortical area [median (interquartile range)] for TPTD [269.52 mm2 (232.6, 278.4)] versus placebo [256.64 mm2 (244.3, 274.8)] (p ≤ 0.01) (Fig. 3A) and cortical thickness for TPTD [5.58 mm (4.8–5.9)] versus placebo [5.25 mm (5.0, 6.0)] (p ≤ 0.01) (Fig. 3B). In the placebo group, the tibia cortical density was slightly greater at the 38% tibia site at 8 weeks [1211.10 mg/cm3 (1162.1, 1228.2)] (p = 0.01) and 12 weeks [1214.30 mg/cm3 (1167.8, 1230.0)] (p = 0.04) compared to the TPTD-group [1199.25 mg/cm3 (1166.9, 1210.4) and 1201.5 mg/cm3 (1165.9, 1211.2), respectively] (Fig. 3C). There was an unexpected, larger total tibia density at the 38% site at 8 weeks in the placebo- [979.00 mg/cm3 (914.17, 1027.0)] compared to TPTD-treated women [968.30 mg/cm3 (873.7, 995.9)] (p ≤ 0.05) (Fig. 3D). Within groups, TPTD-treated participants showed a significant increase from baseline to 8 weeks at the tibia cortical area (p ≤ 0.03) and cortical thickness (p ≤ 0.03). Placebo-treated participants demonstrated a slight significant rise in tibia cortical density (p ≤ 0.02) and total tibia density (p ≤ 0.03). There were no differences between groups at the other tibia sites (4% and 66%) or non-weight bearing distal radius (4% and 33%) during the study period.
Figure 3.
Peripheral quantitative computed tomography (pQCT) images of the 38% tibia, unaffected site at baseline, 8 and 12 weeks between teriparatide and placebo groups. Treatment was administered from baseline to 8 weeks. * Indicates = 0.05, between groups. ** Indicates = 0.05 within groups, over time.
Magnetic resonance imaging
Table 3 shows the location of the stress fractures and the MRI results of the severity of the stress fractures at baseline and following 8 weeks of TPTD vs. placebo. Fractures grades were similar between treatment and placebo groups at baseline (p = 0.21). In the TPTD-treated group, 83.3% of women had healed (Grade 0) and improved fracture repair compared to 57.1% of women in the placebo-treated group; however, these differences were not significant (p = 0.18). Of those who did not heal completely but showed improvement in fracture healing, 50% were in the TPTD-treated group, while 29% were in the placebo-treated group. Lastly, no change in bone healing was observed in 43% of participants who received placebo, while only 17% of participants who received TPTD showed no response. No significant correlation was found between baseline grade and fracture improvement, either overall according to treatment group or by absolute or proportional improvement in stress fracture healing (p > 0.13). There was no significant relationship between the grade of the stress fracture and improvement or healing.
Table 3.
MRI grade at baseline and 8 weeks
| Subject | Location of fracture | MRI baseline | MRI 8 weeks | MRI difference |
|---|---|---|---|---|
| Teriparatide | ||||
| 1 | Femur | 4 | 4 | 0 |
| 2 | Tibia | 3 | 2 | −1 |
| 3 | Tibia | 2 | 0 | −2 |
| 4 | Femur | 2 | 0 | −2 |
| 5 | Metatarsal | 4 | 2 | −2 |
| 6 | Metatarsal | 4 | 1 | −3 |
| Placebo | ||||
| 1 | Fibula | 1 | 1 | 0 |
| 2 | Tibia | 2 | 2 | 0 |
| 3 | Femur | 3 | 3 | 0 |
| 4 | Metatarsal | 1 | 0 | −1 |
| 5 | Metatarsal | 4 | 3 | −1 |
| 6 | Tibia | 2 | 0 | −2 |
| 7 | Calcaneus | 3 | 1 | −2 |
Grades 1 and 2 indicate a mild-to-moderate periosteal edema. Grades 3 and 4 showed moderate-to-severe edema of both the periosteum and marrow. A low-signal fracture line is visible in Grade 4 stress fractures. A reduction of MRI grade was considered to be healing; Grade 0 was considered healed. MRI difference was defined as the difference between MRI score at baseline and at 8 weeks.
Adverse events
There were eight reports of AEs. Six of the eight AEs were minor reports of slight bruising at the injection site, which were as expected and resolved in a few days. One subject reported a pea-sized bump below the site of stress fracture; MRI imaging of this site did not show any abnormalities. One placebo-assigned participant reported light-headedness during the day that resolved within 4 weeks of treatment.
Discussion
This prospective, placebo-controlled pilot study was designed to determine whether short-term, daily TPTD treatment, increased bone formation markers in advance of resorption markers, produced early changes in bone structure and hastened stress fracture healing. Our data did show an increase in bone formation markers P1NP and OC after daily administration of TPTD, resulting in a significant anabolic window response (p = 0.05) [35]. These rises in P1NP and OC within 1 to 3 months indicate early stimulation of bone formation activity consistent with TPTD's anabolic effects on bone [36], [37]. pQCT demonstrated a greater tibia cortical area and thickness at the 38% tibia site after 8 weeks with TPTD treatment compared to placebo. While compared to the TPTD group, the placebo group demonstrated a larger tibia cortical density and total tibia density at 8 weeks. While according to MRI, a larger percent of women with stress fractures treated with TPTD compared with placebo showed improvement in healing at 8 weeks, this study did not have sufficient power to demonstrate a significant hastening of stress fracture healing (p = 0.18). These preliminary data suggest that short-term TPTD treatment may support the improvement of fracture healing in premenopausal women with lower-extremity stress fractures.
Premenopausal women briefly treated with TPTD compared to placebo showed a rapid increase in bone formation markers P1NP by 134% and OC by 410% from baseline to 4 weeks. At 8 weeks, P1NP and OC were both 150% above baseline in the TPTD- compared to the placebo-treated women. These bone formation biomarkers were statistically significant between groups as early as 4 and 8 weeks. These results support previous findings of increases in P1NP and OC biomarkers during daily TPTD therapy among postmenopausal women and men, with potential longer-term anabolic benefits on bone mass [14], [38], [39]. After a brief 8-week TPTD treatment, our pilot study showed an immediate robust response to markers of bone formation as early as 4 weeks followed by a delayed response in bone resorption reflects the anabolic window, as described by Rubin and Bilezikian [16], [17]. The shaded area between P1NP and CTX over time reveals a significantly larger anabolic window in those treated with TPTD compared with those treated with placebo (Fig. 2). Our preliminary data suggest that brief, daily treatment of TPTD results in a robust anabolic window effect, which may support the fracture healing process in premenopausal women with lower-extremity stress fractures. A study of premenopausal women with glucocorticoid-induced osteoporosis displayed a significant rise in CTX concentration after 6 months of daily TPTD-treatment [30]. Our study showed after 8 weeks, a modest rise of CTX levels that suggests that CTX levels may increase over time. NTX levels were elevated in both TPTD and placebo groups at 4 weeks, followed by a decrease in the TPTD group but not the placebo group at 8 weeks. This may be due to a placebo-assigned participant who had high NTX levels both at baseline and at 8 weeks (669 and 1492 nM BCE/mM, respectively), which is well above the normal premenopausal range in the absence of a fracture. Although TPTD has been shown to have an effect on overall bone remodeling, there were no significant changes in bone resorption markers in our study, which may be due to the short duration of the study treatment [14], [36].
After 8 weeks of observation, our study demonstrated a significantly larger tibia cortical area and thickness in the TPTD-treated group compared to the placebo-treated group according to pQCT. Results from the Denosumab and Teriparatide Administration (DATA) study showed an increase in tibia cortical thickness after combination treatment of TPTD and denosumab but not with TPTD alone over 24 months in postmenopausal women [40]. In contrast to DATA, our pilot study showed a greater tibia cortical thickness in the TPTD group only after a brief 2-month administration compared to the placebo group [41]. An uncontrolled pilot study examining the use of TPTD in premenopausal women with IOP showed no change in cortical thickness or density but did show an increase in trabecular density and microarchitecture at the tibia after 18 months [32]. Differences between these studies and ours were the short-term study, treatment intervention of our study and the use of high resolution pQCT (HR-pQCT) versus pQCT to assess the bone structural changes. Studies have shown that pQCT provides more robust measures and correlations of cortical bones parameters at proximal scanning sites at the distal radius and tibia than HR-pQCT [42]. In addition, our data revealed an unexpected difference between the placebo-treated group compared to the TPTD-treated group, showing a greater cortical density and total tibia density in participants treated with placebo at 8 weeks. A possible explanation for this observation is that weight bearing on the unaffected lower-extremity may have resulted in larger tibia cortical and total density as more participants in the placebo groups relied on crutches. Our study did not reveal any other differences between groups at other radial or tibial sites according to pQCT during the study period. The greater tibia cortical area and thickness at the 38% tibia site in the TPTD versus placebo groups at 8 weeks suggests some potential benefit of TPTD. Larger randomized studies are necessary to reassess and validate the use of TPTD in premenopausal women, particularly in those with lower-extremity stress fractures.
To monitor fracture healing, a grading system was used to assess the severity of the lower extremity stress fractures at baseline and 8 weeks. At the end of 8 weeks, 83% in the TPTD group showed improved healing of stress fractures compared with 57% in the placebo group (p = 0.18). Of those assigned to placebo, 3 of the 7 participants (42.9%) showed no change in stress fracture healing, while only 1 participant (16.7%) of the 6 participants assigned to TPTD was unresponsive to treatment. Half of the women in the TPTD-treated group had Grade 4 stress fractures at baseline. Of these women, 2 participants showed an improvement in fracture healing (Grade 4 to 2 and Grade 4 to 1). Of the placebo-treated group, only one participant had a Grade 4 stress fracture, with slight improvement (Grade 4 to 3). Due to the variance and severity of stress fractures in our pilot study, statistical tests were used to adjust for the heterogeneity of these fractures. Results, however, showed no statistically significant difference in healing among these 13 participants, but appears to show an encouraging trend. In 102 postmenopausal women with distal radius fractures, one study demonstrated an acceleration of fracture healing with TPTD (20 µg) versus placebo (7.4 versus 9.1 weeks, respectively) [28], [43]. Although our study did not show any difference in fracture healing between groups, these studies suggest that with larger studies, TPTD is a promising potential therapy to speed up fracture healing in younger premenopausal women.
At present, there are no randomized trials exclusive to premenopausal women and daily TPTD treatment. This pilot study, therefore, is currently the only placebo-controlled trial in premenopausal women that addresses the anecdotal use of TPTD as a therapy to improve fracture healing to date. A limitation of this study is the small sample size. Although we implemented extensive recruitment efforts, enrolling study participants after an acute stress injury was a challenge since stress fractures may not be clinically evident early in the course of their presentation. In a case study of 295 military recruits, 30% of these recruits were found to have stress fractures and of those who had stress fractures of the femur, 69% were asymptomatic [5]. In another study, the time from injury to diagnosis took an average of 13.4 weeks among 320 athletes [44]. Our inclusion criteria required eligible participants to be enrolled within 4 weeks of fracture onset, which proved challenging, but necessary for the study design. We acknowledge that a small sample size may have led to increased variability in primary endpoints and limited the power to demonstrate acceleration of bone healing in response to TPTD. Although great efforts were made to extend this study and continue recruitment this was not possible form the funding agency.
Conclusion
Our pilot study shows some evidence of improvement in stress fracture healing on MRI in the TPTD- compared to the placebo-treated group; however, results were not significant at 8 weeks. These data use TPTD as a potential treatment option in premenopausal women with lower-extremity stress fractures. This pilot study demonstrated that TPTD-treated premenopausal women with lower-extremity stress fractures had a significantly greater anabolic window compared to placebo-treated women. The TPTD-treated group showed a greater tibia cortical area and thickness compared to the placebo-treated group as early as 8 weeks of treatment, while a larger tibia cortical and total tibia density were observed in the placebo-treated group. Given the potential clinical use of TPTD to accelerate fracture healing, larger prospective, placebo-controlled studies are needed to validate the effects of TPTD versus placebo in women with lower-extremity stress fractures.
Conflict of interests
The authors declare they have no conflict of interest.
Acknowledgments
This work was supported by the Department of Defense (USAMRMC Grant No. 08362001) and the Harvard Catalyst (UL1 TR001102). We greatly appreciate the generous contribution of Eli Lilly for supplying Teriparatide and placebo pens for this study and the thoughtful discussions with Colonel Rachel K. Evans, PhD PT, U.S. Department of Defense.
This research was also, in part, supported by the Steve Cobb Junior Faculty and Fellow Education Fund. We would also like to thank Kelly McInnis, D.O., who assisted in referring women with stress fractures, Nithya Setty, M.D. and Natalie Glass Ph.D. for their contributions to this study, and each of the dedicated study participants.
This research was presented at the American Society of Bone and Mineral Research (ASBMR) Annual Meeting in Baltimore, Maryland 2013 and was selected for the President's Poster Competition Award (EA).
References
- 1.Callahan L.R. Stress fractures in women. Clin Sports Med. 2000;19(2):303–314. doi: 10.1016/s0278-5919(05)70205-2. [DOI] [PubMed] [Google Scholar]
- 2.Evans R.K., Antczak A.J., Lester M., Yanovich R., Israeli E., Moran D.S. Effects of a 4-month recruit training program on markers of bone metabolism. Med Sci Sports Exerc. 2008;40(Suppl. 11):S660–70. doi: 10.1249/MSS.0b013e318189422b. [DOI] [PubMed] [Google Scholar]
- 3.Mattila V.M., Niva M., Kiuru M., Pihlajamaki H. Risk factors for bone stress injuries: a follow-up study of 102,515 person-years. Med Sci Sports Exerc. 2007;39(7):1061–1066. doi: 10.1249/01.mss.0b013e318053721d. [DOI] [PubMed] [Google Scholar]
- 4.Biberdorf C. 2004. Army lab tackles problem of military stress fractures. American Forces Information Service News Articles. [Google Scholar]
- 5.Milgrom C., Giladi M., Stein M., Kashtan H., Margulies J.Y., Chisin R. Stress fractures in military recruits. A prospective study showing an unusually high incidence. J Bone Joint Surg Br. 1985;67(5):732–735. doi: 10.1302/0301-620X.67B5.4055871. [DOI] [PubMed] [Google Scholar]
- 6.Loud K.J. Stress fracture. In: Gordon C.M., LeBoff M.S., editors. The female athelete triad: a clinical guide. Springer; New York.: 2015. pp. 131–140. [Google Scholar]
- 7.Gaeta M., Minutoli F., Scribano E., Ascenti G., Vinci S., Bruschetta D. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553–561. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 8.Moran D.S., Evans R.K., Hadad E. Imaging of lower extremity stress fracture injuries. Sports Med. 2008;38(4):345–356. doi: 10.2165/00007256-200838040-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fredericson M., Bergman A.G., Hoffman K.L., Dillingham M.S. Tibial stress reaction in runners. Correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23(4):472–481. doi: 10.1177/036354659502300418. [DOI] [PubMed] [Google Scholar]
- 10.Brewer R.B., Gregory A.J. Chronic lower leg pain in athletes: a guide for the differential diagnosis, evaluation, and treatment. Sports Health. 2012;4(2):121–127. doi: 10.1177/1941738111426115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orwoll E.S., Scheele W.H., Paul S., Adami S., Syversen U., Diez-Perez A. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 12.Zanchetta J.R., Bogado C.E., Ferretti J.L., Wang O., Wilson M.G., Sato M. Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18(3):539–543. doi: 10.1359/jbmr.2003.18.3.539. [DOI] [PubMed] [Google Scholar]
- 13.Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay R., Nieves J., Formica C., Henneman E., Woelfert L., Shen V. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 15.Lane N.E., Sanchez S., Modin G.W., Genant H.K., Pierini E., Arnaud C.D. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102(8):1627–1633. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilezikian J.P. Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep. 2008;6(1):24–30. doi: 10.1007/s11914-008-0005-9. [DOI] [PubMed] [Google Scholar]
- 17.Rubin M.R., Bilezikian J.P. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 2003;19(2):415–432. doi: 10.1016/s0749-0690(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 18.Mognetti B., Marino S., Barberis A., Martin A.S., Bala Y., Di Carlo F. Experimental stimulation of bone healing with teriparatide: histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcif Tissue Int. 2011;89(2):163–171. doi: 10.1007/s00223-011-9503-3. [DOI] [PubMed] [Google Scholar]
- 19.Alkhiary Y.M., Gerstenfeld L.C., Krall E., Westmore M., Sato M., Mitlak B.H. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34) J Bone Joint Surg Am. 2005;87(4):731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 20.Manabe T., Mori S., Mashiba T., Kaji Y., Iwata K., Komatsubara S. Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007;40(6):1475–1482. doi: 10.1016/j.bone.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Andreassen T.T., Ejersted C., Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14(6):960–968. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 22.Gomberg S.J., Wustrack R.L., Napoli N., Arnaud C.D., Black D.M. Teriparatide, vitamin D, and calcium healed bilateral subtrochanteric stress fractures in a postmenopausal woman with a 13-year history of continuous alendronate therapy. J Clin Endocrinol Metab. 2011;96(6):1627–1632. doi: 10.1210/jc.2010-2520. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz E., Steinberg D.M. 2007. Abstract: teriparatide [rhPTH(1–34)] speeds fracture healing and initiates healing of non-unions in humans. in American Society for Bone and Mineral Research 29th Annual Meeting. J Bone Miner Res 22 (Suppl 1): Honolulu, HI. p. S179. [Google Scholar]
- 24.Whyte M.P., Mumm S., Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab. 2007;92(4):1203–1208. doi: 10.1210/jc.2006-1902. [DOI] [PubMed] [Google Scholar]
- 25.Holm J., Eiken P., Hyldstrup L., Jensen J.E. Atypical femoral fracture in an osteogenesis imperfecta patient successfully treated with teriparatide. Endocr Pract. 2014;20(10):e187–90. doi: 10.4158/EP14141.CR. [DOI] [PubMed] [Google Scholar]
- 26.Chiang C.Y., Zebaze R.M., Ghasem-Zadeh A., Iuliano-Burns S., Hardidge A., Seeman E. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013;52(1):360–365. doi: 10.1016/j.bone.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho N.N.C., Voss L.A., Almeida M.O.P., Salgado C.L., Bandeira F. Atypical femoral fractures during prolonged use of bisphosphonates: short-term responses to strontium ranelate and teriparatide. J Clin Endocrinol Metab. 2011;96(9):2675–2680. doi: 10.1210/jc.2011-0593. [DOI] [PubMed] [Google Scholar]
- 28.Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25(2):404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein J.S., Klibanski A., Arnold A.L., Toth T.L., Hornstein M.D., Neer R.M. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): a randomized controlled trial. JAMA. 1998;280(12):1067–1073. doi: 10.1001/jama.280.12.1067. [DOI] [PubMed] [Google Scholar]
- 30.Langdahl B.L., Marin F., Shane E., Dobnig H., Zanchetta J.R., Maricic M. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int. 2009;20(12):2095–2104. doi: 10.1007/s00198-009-0917-y. [DOI] [PubMed] [Google Scholar]
- 31.Cohen A., Stein E.M., Recker R.R., Lappe J.M., Dempster D.W., Zhou H. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981. doi: 10.1210/jc.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama K.K., Cohen A., Young P., Wang J., Lappe J.M., Guo X.E. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: a pilot HR-pQCT study. J Clin Endocrinol Metab. 2014;99(7):2418–2425. doi: 10.1210/jc.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda F., Kurinomaru N., Hijioka A. Weekly teriparatide for delayed unions of atypical subtrochanteric femur fractures. Biol Ther. 2014;4(1):73–79. doi: 10.1007/s13554-014-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldi G., Wisniewski C.A., Setty N.G., Leboff M.S. Peripheral quantitative computed tomography: optimization of reproducibility measures of bone density, geometry, and strength at the radius and tibia. J Clin Densitom. 2011;14(3):367–373. doi: 10.1016/j.jocd.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Bilezikian J.P. Anabolic therapy for osteoporosis. Womens Health (Lond Engl) 2007;3(2):243–253. doi: 10.2217/17455057.3.2.243. [DOI] [PubMed] [Google Scholar]
- 36.Glover S.J., Eastell R., McCloskey E.V., Rogers A., Garnero P., Lowery J. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45(6):1053–1058. doi: 10.1016/j.bone.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 37.Krege J.H., Lane N.E., Harris J.M., Miller P.D. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos Int. 2014;25(9):2159–2171. doi: 10.1007/s00198-014-2646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsujimoto M., Chen P., Miyauchi A., Sowa H., Krege J.H. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011;48(4):798–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Hodsman A.B., Bauer D.C., Dempster D.W., Dian L., Hanley D.A., Harris S.T. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 40.Tsai J.N., Uihlein A.V., Burnett-Bowie S.A., Neer R.M., Zhu Y., Derrico N. Comparative effects of teriparatide, denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture, and estimated strength: the data-HRpQCT study. J Bone Miner Res. 2015;30(1):39–45. doi: 10.1002/jbmr.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spadaro J.A., Werner F.W., Brenner R.A., Fortino M.D., Fay L.A., Edwards W.T. Cortical and trabecular bone contribute strength to the osteopenic distal radius. J Orthop Res. 1994;12(2):211–218. doi: 10.1002/jor.1100120210. [DOI] [PubMed] [Google Scholar]
- 42.Lebrasseur N.K., Achenbach S.J., Melton L.J., 3rd, Amin S., Khosla S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res. 2012;27(10):2159–2169. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aspenberg P., Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010;81(2):234–236. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matheson G.O., Clement D.B., McKenzie D.C., Taunton J.E., Lloyd-Smith D.R., MacIntyre J.G. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46–58. doi: 10.1177/036354658701500107. [DOI] [PubMed] [Google Scholar]